Abstract
The cavity inside fullerene C60 provides a highly symmetric and inert environment for housing atoms and small molecules. Here we report the encapsulation of formaldehyde inside C60 by molecular surgery, yielding the supermolecular complex CH2O@C60, despite the 4.4 Å van der Waals length of CH2O exceeding the 3.7 Å internal diameter of C60. The presence of CH2O significantly reduces the cage HOMO-LUMO gap. Nuclear spin-spin couplings are observed between the fullerene host and the formaldehyde guest. The rapid spin-lattice relaxation of the formaldehyde 13C nuclei is attributed to a dominant spin-rotation mechanism. Despite being squeezed so tightly, the encapsulated formaldehyde molecules rotate freely about their long axes even at cryogenic temperatures, allowing observation of the ortho-to-para spin isomer conversion by infrared spectroscopy. The particle in a box nature of the system is demonstrated by the observation of two quantised translational modes in the cryogenic THz spectra.
Similar content being viewed by others
Introduction
On its discovery in 19851 it was recognised that fullerene C60 contains an almost spherical 3.7 Å diameter cavity which is capable of encapsulating other species. The implantation of noble gas atoms into C60 under extreme conditions and in very low yields followed2,3. A great advance has been the development of molecular surgery, involving the opening of the fullerene cages by chemical reactions, insertion of a small molecule or an atom into each cage, and subsequent closure by further reactions. The procedure has recently been reviewed4. Systems synthesised this way include H2@C605,6,7, He@C607,8, H2O@C606,9, Ne@C607, HF@C6010, CH4@C6011, Kr@C6012, and Ar@C6013.
The encapsulation of atoms and molecules inside C60 provides a unique environment for the encapsulated species. The fullerene cages isolate the encapsulated molecules from each other, preventing intermolecular bonding and causing the guest molecules to behave, to some extent, as if they are in the rarefied gas phase, even at cryogenic temperatures in the solid state, allowing detailed study of quantum effects14,15,16. At low temperatures, the tight confinement leads to translational quantisation, the study of which can be used to determine potential energy surfaces17. Ortho- and para spin isomers are observed for H2@C6018,19 and H2O@C6020,21,22,23. The interconversion of the spin isomers influences macroscopic properties such as the dielectric constant22. The quantised rotational and translational states of the confined molecules interact through rotation-translation coupling24,25. Unconventional confinement-induced spin-spin 0J-couplings between endohedral 3He and the cage 13C nuclei have been observed26. Endohedral fullerenes are a frequent target for theoretical calculations and the provision of experimental data on them provides an important benchmark, particularly for non-covalent interactions27,28,29,30.
Formaldehyde CH2O is often regarded as a model polyatomic system and has been subject to intense spectroscopic, theoretical, and computational study31. Monomeric formaldehyde has two hydrogens with nuclear spin I = 1/2, giving rise to two nuclear spin isomers with total nuclear spin I = 1 and 0, denoted ortho- and para-formaldehyde32. The interconversion of the two spin isomers has been studied in the gas phase by using selective UV laser photolysis to destroy one of the spin isomers leaving a non-equilibrium mixture33,34,35. The mechanism and rate of ortho-para interconversion has been investigated theoretically36,37. The ortho/para ratio of formaldehyde has been used to estimate the temperature in a variety of interstellar environments38,39,40. However, as far as we know, the spin-isomer conversion of formaldehyde has never been observed experimentally in the condensed phase.
C60 cages provide the ideal nano-container for the study of monomeric formaldehyde molecules at low temperature. However, at first sight, formaldehyde seems to be too big to fit inside C60. A formaldehyde molecule has a longest van der Waals dimension of 4.38 Å parallel to the CO bond, considerably larger than the 3.7 Å diameter internal space of C60. Although formaldehyde has been successfully accommodated in the bowl-like cavities of open-cage fullerenes41,42, the chemical closure of the open fullerene cages, with complete encapsulation of the formaldehyde molecules, has not been achieved.
We now report the successful encapsulation of formaldehyde (CH2O) molecules inside closed C60 cages. The compound CH2O@C60 displays some remarkable physical properties, including the spin-isomer conversion of the formaldehyde guest molecules in the cryogenic solid state, the spatial quantisation of the encapsulated molecules, confinement-induced internuclear couplings between the host and the guest nuclei, and the modulation of the fullerene electronic structure upon accommodation of the oversized guest molecule.
Results & discussion
Synthesis of CH2O@C60 and isotopologues
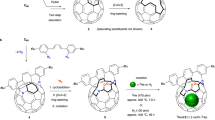
Murata and co-workers42 inserted CH2O through the 17-membered orifice of 1 using high temperature and pressure (trioxane, 150 °C, 8000 atm, 35% incorporation) and observed that the CH2O escaped at room temperature. Reducing one of the carbonyls on the orifice to an alcohol, prevented the escape of CH2O. Density Function Theory (DFT) calculations indicated that the high temperatures and pressures used to insert formaldehyde into the sulfide 1 should not be necessary (Supplementary Table 6). Calculations also showed that the activation energy for loss of CH2O from the sulfoxide 2 is 13 kJ mol−1 higher than from the sulfide 1 suggesting that our previously reported11 method of photochemical orifice contraction of 2 might be possible without substantial loss of CH2O. Passing a stream of formaldehyde gas, generated by pyrolysis of paraformaldehyde43, into a solution of sulfide 1 in THF at 50 °C gave CH2O@1 with 70% incorporation of the endohedral molecule. THF was removed under vacuum at room temperature and the residue dissolved in toluene and filtered through a short column of silica to remove paraformaldehyde. Immediate oxidation by addition of a cold solution of dimethyldioxirane11 gave the sulfoxide CH2O@2 with 70% filling, the remainder being H2O@2 with <5% empty 2 (Fig. 1). The CH2O protons appeared as a singlet at δ = −0.95 ppm in the 1H NMR, and could be compared with the endohedral H2O signal at −10.5 ppm. The filling could also be assessed from one of the alkene protons in CH2O@2 which was separated from the equivalent H2O@2 and 2 signals (Supplementary Section 1.1). The half lives for loss of CH2O from CH2O@1 were measured as 7.9 h at 40 °C and 70 h at 25 °C, whereas from CH2O@2 it was 35 h at 55 °C (Supplementary Fig. 17, Tables 1 and 2).
Sulfide 1 is 70% filled with formaldehyde (CH2O) using a solution of the monomer, then oxidised to the sulfoxide 2 before photochemically induced loss of sulfur monoxide (SO) gave the orifice contracted bis-hemiacetal 3. Phosphine and phosphite induced deoxygenative ring closures to give CH2O@5 followed by a thermal extrusion reaction gave CH2O@C60. The route for labelled materials CD2O@C60 and 13CH2O@C60 were identical except that the initial filling was carried out by heating 1 with paraformaldehyde in a sealed tube which gave only 25% incorporation of formaldehyde.
Photochemical SO extrusion from CH2O@2 to give the hemiacetal CH2O@3 was achieved by stirring CH2O@2 in a solvent mixture of THF / Toluene/ acetic acid (10% v/v aq.) using 3 × 100 W yellow LED lights for 18 h at 55 °C11,12. The use of THF instead of our previously reported CH3CN allowed higher concentrations to be used. This combination furnished a mixture of products H2O@3 and CH2O@3 in 36% yield. Since the hemiacetal 3 is rather unstable the mixture was reacted with triphenylphosphine to give CH2O@4 and H2O@4 in 82% yield with 35% filling of CH2O. Allowing for the change in filling factors the overall yields for CH2O@4 and H2O@4 can be estimated as 15% and 64% respectively. For comparison previously reported yields over these steps of CH4@4, Ar@4 and Kr@4 were 13%, 23% and 21%, although an improved procedure using a more powerful light source was used in the current method. The NMR of CH2O@4 showed a singlet for endohedral CH2O at δ = 0.80 ppm (cf. H2O@4 at δ = −8.80 ppm). On some occasions unreacted CH2O@2 could be isolated during the purification of CH2O@3 and was found to have an increased filling factor indicating that the drop in filling factor of CH2O@4 is mostly due to inhibition of the closure steps by endohedral CH2O rather than loss of CH2O from CH2O@2. Although leaving the photochemical reaction on for longer gave increased filling of CH2O in 3, the yield dropped substantially due to further photochemical reactions of the desired product so it was less productive.
As an aside, CO2@2 (81% filled) was easily prepared by exposure of solid 1 to 69 atm CO2 at 100 °C for 18 h followed by DMDO oxidation44. Exposure of CO2@2 to the photochemical orifice contraction conditions gave no CO2@3 indicating that the large size of the endohedral molecule is stopping the desired reaction.
Finally, the orifice of CH2O@4 was closed back to the C60 cage using same conditions to those given in our earlier reports (Fig. 1)7. A combined sample of CH2O@4 from several preparations was used with 25% filling of CH2O, and a mixture of C60, H2O@C60 and CH2O@C60 was obtained in 46% yield over last two steps. Recirculating preparative HPLC on a Cosmosil Buckyprep® column indicated the composition to be H2O@C60 (80%), C60 (4.5%) and CH2O@C60 (15.5%) and allowed pure CH2O@C60 to be isolated. It is noteworthy that the separation of CH2O@C60 from C60 on the Buckyprep® HPLC column is considerably greater than that known for other atomic or molecular endofullerenes (e.g. ratio of retention times CH2O@C60: C60 is 1.081compared with 1.054 for CH4@C60: C6011). Allowing for the filling factors the yield for CH2O@C60 can be estimated as 29% (cf. 52% for the H2O@C60 + C60). Since it is not possible for CH2O to leave the cage of 4 or 5 the drop in filling factor must be due to strong inhibition of the closure steps by the endohedral molecule. We were able to isolate the known by-product 645,46,47 which had a substantially increased CH2O filling factor of 37%. The isolated CH2O@C60 could be further purified by sublimation (550 °C, 10−5 bar) without decomposition.
We also made the labelled species 13CH2O@C60 and CD2O@C60. The high cost of the starting labelled paraformaldehydes precluded the use of a large excess as in the synthesis of the unlabelled material above. Instead the labelled paraformaldehyde (100 mg) and sulfide 1 (500 mg) were sealed in a 1/4” diameter × 10 cm long stainless steel tube and heated at 100 °C for 3 h. Work-up and DMDO oxidation as above gave 13CH2O@2 or CD2O@2 in high yields with ~25% filling. Photochemical orifice contraction and PPh3-induced ring closure, as above, gave 13CH2O@4 and CD2O@4 with ~15% filling, the rest being H2O@4 (~80%) with ~5% 4. 13CH2O@4 was closed to 13CH2O@C60 as above, HPLC indicating the final material was around 5% filled. Pure 13CH2O@C60 was isolated by recycling HPLC and displayed a doublet (J = 173 Hz) at 3.80 ppm (toluene-d8) in the proton NMR and triplet (J = 173 Hz) at 197.9 ppm in the 13C NMR. CD2O@C60 was made by the same method and displayed a singlet at δ = 3.76 ppm (referenced to PhCH2D at 2.09 ppm) in the deuterium NMR.
Crystal structure of CH2O@C60
A crystal of the nickel(II) octaethylporphyrin/benzene solvate of CH2O@C60 was obtained and subjected to X-ray crystallography. Unconstrained attempts at a structure solution gave an unreasonably short C-O bond. Treating the CH2O molecule as a rigid fragment with idealised geometry and refining its orientation in space against the observed electron density gave a satisfactory solution (R factor 4.97%) (Fig. 2a). The large thermal ellipsoid on oxygen is one indication of high mobility of the endohedral CH2O at 100 K. To gain greater insight the electron density due to the CH2O molecule was calculated from the difference between that observed for the entire structure in the X-ray, and that calculated from a model containing all the atoms except the CH2O. The residual electron density maps (Fig. 2b–d) show 2 maxima corresponding to a favoured orientation of the C-O bond axis. DFT calculations on the structure (Supplementary Fig. 27) predict the distances of the oxygen and carbon of the CH2O molecule from the centroid of the C60 (shown as a green dot in Fig. 2a, c) to be 0.86 and 0.34 Å respectively confirming the identification of these atoms in Fig. 2a. Figure 2c shows the oxygen atom distributed over a wide arc. There is no evidence that CH2O introduces a distortion of the cage compared with the published structure of the C60 complex48 that is measurable within the resolution of the crystallographic experiment.
Recorded at 100 K with CCDC deposition number 2126579 (R1 = 0.050). a Thermal ellipsoids drawn at 50% probability, hydrogens, except on the CH2O, are omitted for clarity. b Representation showing the observed electron density at the CH2O location. Electron density surface drawn at the 2.1 e Å3 level. c and d Orthogonal views of difference electron density at the centre of the C60 cage (contour levels drawn at approximately 0.9 e Å3 level). The centroid of the cage carbons is shown as a green sphere.
UV-vis and electrochemical studies on CH2O@C60
The UV-vis absorption spectra of CH2O@C60 (Fig. 3a) showed the longest wavelength absorption peak to be red-shifted by ~6 nm from C60. This shift corresponds to an energy change of −18 meV. Using a recent comparison of calculations to experimental values49, the change in the Highest Occupied Molecular Orbital (HOMO) – Lowest Unoccupied Molecular Orbital (LUMO) gap is estimated to be 37% larger than this, i.e. −25 meV. Both Differential Pulse Voltametery (DPV) (Fig. 3b) and Cyclic Voltametery (CV) (Supplementary Fig. 18) show a decrease in the first 4 reduction potentials of CH2O@C60 by ~30 mV relative to C60 (Supplementary Table 4). The change in the 1st reduction potential indicates that the LUMO level is lowered in energy by 30 meV. Hence UV-vis and electrochemical studies indicate a reduction in the LUMO energy of 30 meV and increase in the HOMO energy of 5 meV of C60 upon encapsulation of CH2O. The incorporation of MeCN into the open fullerene 1 has recently been reported to lower its 1st oxidation potential by 40 meV50. Photoelectron spectroscopy of [H2O@C60]- indicates the electron affinity of H2O@C60 to be 8.8 meV higher than C6051 although no difference in UV spectra or cyclic voltammetry was observed9.
a Long wavelength part of UV-vis spectra of C60 and CH2O@C60 in toluene. b Differential Pulse Voltametry of C60 and CH2O@C60 showing first four reductions relative to ferrocene (Fc/Fc+) at 0 V. The fullerenes were dissolved in a 4:1 mixture of toluene and acetonitrile containing 0.1 M Bu4N.BF4 as electrolyte. The cell contained a 3 mm diameter glassy carbon working electrode, a 1 cm2 sheet of platinum as the counter electrode and a silver wire pseudo-reference electrode. Ferrocene was added as an internal standard.
NMR studies on CH2O@C60
Detailed NMR experiments were performed at 16.45 T using a 23 mM solution of CH2O@C60 in 1,2-dichlorobenzene-d4 (ODCB-d4), degassed by bubbling with nitrogen gas for 10 min. The 1H chemical shift of the CH2O@C60 peak is 3.75 ppm. The 13C spectrum of CH2O@C60 shows a 1:2:1 triplet at 197.22 ppm with a J-coupling of |1JCH | = 173.49 ± 0.09 Hz corresponding to the 13C peak of CH2O@C60 (Fig. 4a). The triplet collapses to a single peak when applying 1H decoupling. Polarisation transfer from 1H to 13C using an INEPT sequence with inter-pulse delay τ/2 = 1.44 ms ≃ |4JHC | −1 gives an antiphase 13C spectrum with enhanced outer peaks and a missing central peak, as expected52.
Taken prior to complete removal of H2O@C60 and C60 and sublimation, 23 mM in ODCB-d4 at 16.45 T and 298 K (a–e). a showing the CH2O triplet 13C spectrum, a proton decoupled 13C{1H} spectrum and a non-refocused INEPT spectrum with inter-pulse delay of 1.44 ms. b expansion of the 13C spectrum around 143ppm, showing the 13C signals for CH2O@C60, H2O@C60 and empty C60. c 13C (CH2O@C60) NMR signal amplitude modulation following the J-modulated spin-echo sequence shown in the figure [90(13C) – delay – 180(13C, 1H) – delay – Acquire (13C)], acquired with 16 transients. Fitting the modulation gives |0JHC | = 70.6 ± 0.3 mHz. d and e Inversion-recovery curves for the T1 spin-lattice relaxation time constant of CH2O nuclei in CH2O@C60 for 1H and 13C (central line) respectively. f 13C T1 of the central line of 13C-labelled CH2O in 13CH2O@C60, measured at 16.45 T by inversion recovery as a function of sample temperature, in a 1 mM solution in toluene-d8. The error bars represent Standard Error estimates in the fitted T1 values.
A single peak is observed for the cage 13C of CH2O@C60 at 143.49 ppm, which is shifted by +0.684 ppm with respect to that of empty C60 (Fig. 4b). The shift is substantially greater than that observed for CH4@C60 (+0.52 ppm)11. This conforms to the general finding that formation of an endofullerene leads to a change in the cage 13C chemical shift which increases with the size of the encapsulated species.
J-couplings between the nuclei of atoms which are not linked by a sequence of covalent bonds have been denoted 0J, where the superscript zero indicates the absence of a covalent linkage26. A 0JHeC coupling has been detected by a small splitting of the 13C resonance of the helium endofullerene 3He@C6026. Neither the 13C cage peak of the CH2O@C60, nor the 1H peak of the majority 12CH2O@C60 isotopologue, display a resolved spectral splitting. Nevertheless, the presence of an unresolved 0JHC-coupling is clearly indicated by an oscillation of the signal amplitude for the CH2O@C60 cage sites as a function of the evolution interval τEV, in a heteronuclear J-modulated spin-echo experiment (Fig. 4c). Since each 13C nucleus of the CH2O@C60 cage is coupled to the two 1H nuclei of CH2O, the modulation of the NMR signal amplitude (the integral of the signal) oscillates at twice the J-coupling frequency. Fitting the modulation gives an estimated 0J-coupling |0JHC | = 70.6 ± 0.3 mHz.
The solution-state 1H T1 spin-lattice relaxation time constant of the endohedral CH2O protons was measured by inversion recovery to be 30.65 ± 0.04 s, in ODCB-d4 at 16.45 T and 298 K (Fig. 4d). It was only slightly shorter (28.0 ± 0.8 s at 298 K) in the 13CH2O@C60 isotopologue (Supplementary Fig. 21). This indicates that the relaxation rate contribution of the 1H−13C dipole-dipole coupling mechanism is very small. The long 1H relaxation times are consistent with high rotational mobility for the endohedral molecule, which diminishes the contribution to relaxation from dipole-dipole and chemical shift anisotropy mechanisms.
A similarly long 1H T1 relaxation time of 30.8 ± 0.8 s was observed for free monomeric CH2O in a THF solution (Supplementary Fig 24). In contrast, the formaldehyde 1H relaxation in the open-cage system CH2O@2 was observed to be much faster: T1 = 2.31 ± 0.03 s for CH2O@2 in ODCB-d4 (Supplementary Fig. 23). The large difference between the 1H T1 for the formaldehyde protons in CH2O@C60 and CH2O@2 is attributed to the low-symmetry confining environment in the latter case, which partially couples the formaldehyde orientation to that of the open-cage fullerene. Hence the long rotational correlation time of the open fullerene cage leads to a short 1H T1 for the endohedral CH2O in CH2O@2. This effect is absent for the symmetrical CH2O@C60 complex, where the rotational motion of the formaldehyde guest is strongly decoupled from that of the fullerene host.
In contrast to the 1H relaxation, the 13C T1 relaxation time constant for the endohedral formaldehyde in CH2O@C60 is surprisingly short: 214 ± 13 ms for natural abundance 13CH2O@C60 in ODCB-d4 at 298 K (Fig. 4e) and 204 ± 4 ms for 13C-labelled 13CH2O@C60 in toluene-d8 at 297 K (Fig. 4f). The 13C T1 value for 13C-labelled 13CH2O@C60 decreases only slightly as the magnetic field is increased (223 ± 7 ms at 9.4 T, 204 ± 3 ms at 14.1 T). This indicates that chemical shift anisotropy provides a relatively weak 13C relaxation mechanism in this case. However, the 13C T1 has a strong inverse relationship on the sample temperature (Fig. 4f). This is indicative of a strong spin-rotation contribution to the T1−1 rate constant for the formaldehyde 13C nuclei. Strong spin-rotation relaxation is typical for nuclei of molecules in the gas phase53. This reinforces the general observation that molecules encapsulated in C60 behave, in many respects, as if they are in the gas phase.
The strong spin-rotation relaxation of the 13C nuclei of formaldehyde, and the weakness of the same mechanism for the 1H nuclei, demands explanation. Experimental measurements of the spin-rotation tensors of isolated CH2O molecules54,55 indicate that the Frobenius norm of the 13C spin-rotation tensor is ~26 times larger than that of the 1H spin-rotation tensors. Since the relaxation rate constant induced by the spin-rotation mechanism, under plausible assumptions, is proportional to the square of the Frobenius norm of the relevant tensor56, this explains the large ratio of the 13C to 1H T1-1 relaxation rate constants. A more detailed theory will require consideration of the anisotropic rotational motion of the formaldehyde inside the cage, the different orientations of the 13C and 1H spin-rotation tensors relative to the molecular reference frame, the inclusion of the dipole-dipole and chemical shift anisotropy relaxation mechanisms, and the possible influence of the fullerene cage on the spin-rotation interaction tensors of formaldehyde. This study is in progress and will be described elsewhere.
The 13C T1 relaxation time constant for the cage 13C of CH2O@C60 was measured to be 17.42 ± 0.06 s in ODCB-d4 at 298 K (Supplementary Fig. 22). This behaviour is unremarkable and is similar to that observed for empty C6057. The relaxation is attributed to a chemical shift anisotropy mechanism driven by rotational diffusion of the C60 cages, which is not significantly affected by the endohedral molecule.
IR and THz spectroscopy of CH2O@C60
Ortho/para interconversion of CH2O@C60
Spin-isomer conversion in CH2O@C60 was studied by a temperature jump method. The sample was allowed to equilibrate at 20 K and then rapidly cooled to 5 K. A series of IR spectra were taken at different times after the cooling. Subtraction of the spectrum measured two hours after the temperature jump from the spectra taken at earlier times gives a series of difference spectra in which the ortho-CH2O@C60 peaks are positive, while para-CH2O@C60 peaks are negative. The inset in Fig. 5 shows the difference spectra for a peak at 1235–1280 cm-1, tentatively assigned as the ν6 (B2) vibration. The points in Fig. 5 show the integrals of two different spectral regions as a function of time, one attributed to the para spin isomer (blue squares), and one attributed to the ortho spin isomer (black dots). The time constant for the ortho-para conversion is estimated to be approximately 13 min at 5 K.
Change of para and ortho species signal amplitude, Δs, measured at the 1255 cm−1 ro-vibrational band of CH2O@C60 after the temperature jump from 20 K to 5 K. The data points are the normalised line areas integrated between 1235 and 1255 cm−1 for the ortho spin isomers (black dots) and between 1255 and 1280 cm−1 for the para spin isomers (blue squares) of CH2O@C60. The para signal grows with a time constant of 12.4 ± 0.6 min, while the ortho signal decays with a time constant of 13.3 ± 0.4 min at 5 K.
Quantised translational modes
At temperatures below ~50 K, the far-infra-red (THz) spectrum of CH2O@C60 shows two sharp lines at 167 cm-1 and 231 cm−1 (Fig. 6). These lines do not appear in the far-IR spectra of C6058 nor He@C60 (Supplementary Fig. 25). Both peaks get weaker with increasing temperature and have a similar temperature dependence, implying that they correspond to transitions starting from the ground state (inset to Fig. 6). Furthermore, a comparison of spectra taken at different times after cooling shows that the 167 cm−1 peak has an unresolved structure, with components belonging to different spin isomers (Supplementary Fig. 26). These properties prove that the two peaks are indeed due to the endohedral CH2O@C60 molecules. However, the 167 and 231 cm−1 lines do not correspond to vibrations of free CH2O because all vibration frequencies of free CH2O are above 1000 cm−131. Also, it is unlikely that the observed lines are rotational transitions of formaldehyde, because there are no rotational transitions of free CH2O, starting from thermally populated low-lying states, in this spectral range59. We assign the 167 cm−1 and 231 cm-1 lines to translational transitions, i.e. transitions between the particle-in-a-box quantum levels of the confined CH2O molecules. Translational peaks of this type have been observed previously for noble gas endofullerenes17 and for H2O@C6020. We provisionally attribute the 231 cm-1 peak to translation along the long axis of the molecule, where the confinement is particularly tight, and the 167 cm-1 peak to translation perpendicular to the long axis of the molecule. A more detailed analysis of the THz and IR spectra of CH2O@C60 will be given elsewhere.
Absorption coefficient as a function of wavenumber, between 5 K and 80 K. The translational modes are observed at 166.8 cm−1 and 231.1 cm−1. Inset: temperature dependence of the 167 cm−1 (crosses) and 231 cm−1 (open dots) integrated absorption peak area s, normalised to the peak area at 5 K, after subtracting the 80 K spectrum.
Theoretical studies CH2O@C60
Some initial theoretical studies on CH2O@C60 were carried out using Density Functional Theory (DFT) to compare with the experimental results described above. Calculations were carried out with Gaussian 09 revision D1.0160 using the B3LYP functional61,62 with the Grimme D3 empirical dispersion correction using Beck–Johnson damping63,64 and the cc-pVTZ basis set65 with a superfine integration grid and tight criteria for convergence. We concentrate on differences in properties of the C60 cage caused by incorporation of endohedral CH2O, and on the translational modes resulting from its encapsulation.
In the minimised structure the C-O axis of the CH2O aligns with the centre of the bond between 5 and 6 member rings of the cage (Fig. 7) although structures where it is aligned with the centre of the bond between two 6 member rings is only 0.1 kJ mol-1 (1 meV) higher in energy (Supplementary Fig 19). The calculated parameters below for the second conformer are given in the Supplementary. Comparing the minimised structure with that of C60, calculated using the same method, shows that the diameter of the cage measured along the C-O axis has increased by 3.8 pm (Supplementary Tables 7 and 8). The diameter in the plane of the CH2O decreased by 0.2 pm, and perpendicular to the CH2O plane decreased by 1.5 pm. The mean diameter increases by 0.6 pm.
The positions of the HOMO and LUMO of CH2O@C60 are strongly influenced by the presence of the endohedral molecule (Fig. 7). The LUMO energy of CH2O@C60 is calculated to be 35 meV lower than C60 which compares well to the 30 mV reduction in the first reduction potential found electrochemically (Supplementary Table 9). The HOMO is little changed (+3.5 meV) giving a calculated HOMO-LUMO gap for CH2O@C60 38 meV smaller than C60 (cf. 25 meV reduction estimated experimentally from the difference in optical band gaps above). The excitation energy to the 1st excited states of CH2O@C60 and C60 were calculated using TD-DFT (B3LYP-D3/cc-pVTZ) and the former found to be 23.8 meV smaller (Supplementary Table 11) corresponding to a 6.9 nm increase wavelength of the longest wavelength absorption in the UV-vis spectra, in excellent agreement with that observed (6 nm change).
Frequency calculations on the minimised structure of CH2O@C60 gave three translational modes. That corresponding to movement along the long axis of CH2O is at 233 cm−1, surprisingly good agreement with the observed peak at 231 cm−1 (Supplementary Table 10). Calculations also give translational modes in the plane of the CH2O at 202 cm−1, and perpendicular to that plane at 175 cm−1, compared to the observed single peak at 167 cm−1. DFT neglects the delocalised wave nature of nuclei, treating them as fixed points (Born-Oppenheimer approximation) whereas the observation of ortho and para hydrogen spin isomers due to rotation about the C-O axis indicate that the hydrogen nuclei are delocalised, even at cryogenic temperatures, so the observation of a single peak for translation perpendicular to the C-O axis is reasonable.
The 13C chemical shift of the cage carbons in CH2O@C60 was calculated using the Gauge-Independent Atomic Orbital (GAIO) method to be 0.80 ppm greater than that of empty C60 (Supplementary Tables 12 and 13). This is in rough agreement with the observed 0.684 ppm increase in chemical shift of the cage 13C upon encapsulation of CH2O. Since the DFT calculations are performed at 0 K in the gas phase, a difference to room temperature liquid state NMR data is to be expected. Encapsulation of CH2O by C60 may change the 13C chemical shift of the cage carbons by at least two separate mechanisms: (i) direct interactions with the electrons and nuclei of the guest molecule, and (ii) expansion of the cage geometry upon accommodation of the guest, which in turn modifies the electronic structure of the cage and hence the 13C chemical shift. The relative importance of these contributions was assessed as follows: First, calculations were performed on empty C60, but fixing the geometry of the C60 cage to that of CH2O@C60 (as described above). In this case, the calculated 13C chemical shift of the cage carbons was found to be 0.35 ppm greater than that of empty C60 with the energy-minimised geometry. Second, calculations were performed on CH2O@C60, but fixing the geometry of the C60 cage to that of empty C60. In this case, the calculated 13C chemical shift of the cage carbons was 0.45 ppm greater than that of empty C60 with the energy-minimised geometry. We conclude that the direct interactions with the guest molecule, and the geometry changes in the C60 cage, both contribute to the observed 0.684 ppm change in the 13C chemical shift of the cage carbons in CH2O@C60, relative to that of C60.
In summary, formaldehyde CH2O was successfully encapsulated into C60 despite the large nominal size of formaldehyde relative to the C60 cavity. The large size of the CH2O significantly inhibited several orifice contraction steps. The isotopically labelled materials 13CH2O@C60 and CD2O@C60 were also made.
A significant perturbation of the electronic structure of C60 is observed in CH2O@C60. UV-vis spectroscopy shows a 6 nm red shift in the longest wavelength absorption, and electrochemistry a 30 meV lowering of the 1st reduction potential on incorporation of CH2O into C60. However, X-ray crystallography did not show a significant change in C60 geometry.
The solution-state NMR spectroscopy of CH2O@C60 displays a confinement-induced 70 mHz J-coupling between the cage 13C nuclei and the formaldehyde 1H nuclei. The spin-lattice relaxation behaviour is very unusual, with the 13C nuclei of formaldehyde relaxing about 150 times faster than the 1H nuclei. The rapid 13C relaxation is attributed to a strong spin-rotation relaxation mechanism, associated with unusually free rotation.
The tightly confined CH2O molecules display spatial quantisation, with two translational modes observed in the far-infra-red, at 167 cm−1 and 231 cm−1. Despite this tight confinement, the encapsulated formaldehyde molecules rotate freely about their long axes. Conversion between the ortho and para nuclear spin isomers is observed at low temperatures by infra-red spectroscopy, with a spin-isomer conversion time constant of ~13 min at 5 K. To the best of our knowledge, this is the first time that the spin-isomer conversion of formaldehyde has been observed in a condensed phase.
DFT calculations on C60 and CH2O@C60 predict that incorporating the endohedral species distorts the cage by +3.8 pm along the long axis of the CH2O, although the average diameter, which might be expected to be observed for a freely rotating endohedral molecule, only increases by 0.6 pm. Calculations are consistent with the experimentally observed changes in the electrochemical reduction potentials, the UV absorption spectra, and the 13C chemical shift of the cage carbons. The frequencies of the translational modes of the confined CH2O molecules predicted by DFT calculations are in reasonable agreement with the experimental results.
Methods
Synthesis
General methods
Toluene was freshly distilled from sodium benzophenone ketal under argon. MeCN was freshly distilled from CaH2 under argon. Technical grade 1-chloronaphthalene (≥85%) was filtered through a short column of activated Al2O3 and distilled under argon at reduced pressure (117 °C, 13 mbar). Triisopropyl phosphite was distilled over sodium at reduced pressure (67 °C, 14 mbar). Dimethyldioxirane was prepared according to the published procedure43. C60 (98%) was purchased from Solaris Chem Inc. All other reagents or solvents were used as received from commercial suppliers. Open-cage fullerene 1 was prepared according to the published methods7,9.
CH2O@2
Finely ground sulfide 1 (438 mg, 386 mmol) was dissolved in dry, oxygen-free THF (40 mL) in a round bottom flask with an inlet tube connected to a round bottom flask containing excess paraformaldehyde (8.15 g) and p-toluene sulfonic anhydride (2.09 g), and heated to 50 °C. The flask containing paraformaldehyde was heated using a hot-air gun and the monomeric formaldehyde produced was bubbled through the sulfide 1 solution for 30 min using a flow of N2 gas43. After cooling to room temperature, THF was removed by rotary evaporation and the crude CH2O containing sulfide 1 was dissolved in toluene and quickly purified using column chromatography (SiO2 eluted with a 90:8:2 mixture of toluene:EtOAc: AcOH).
To a stirred solution of the mixture of CH2O@1 and H2O@1 + 1 in toluene (80 mL) at 0 °C, was added dimethyldioxirane (10.0 mL of a 98.0 mM solution in acetone, 1.00 mmol) rapidly using an ice-chilled syringe. The resulting mixture was stirred at 0 °C for 10 min. before removal of the cooling bath and stirring for 1 h. Solvents were removed in vacuo to give the title compound as a crude brown powder containing CH2O@2 and H2O@2 + 2 which was used directly in the next step without purification (403 mg, 0.341 mmol, 88%). The fillings in 2 was determined by NMR as 70% CH2O@2 and 30% H2O@2 + 2.
13CH2O@2 and CD2O@2
Sulfide 1 (478 mg, 0.421 mmol) was finely ground with 13C paraformaldehyde (99 mg) and loosely packed in a 1/4” stainless steel tube. The tube was sealed at both the ends using Swagelok caps and kept in preheated oven at 105 °C for three hours. After cooling to room temperature the tube was opened and the crude material quickly dissolved in toluene (40 ml). The crude solution was passed through a silica column using 90:8:2 (Toluene: EtOAc: AcOH) as an eluent. The purified material was concentrated on rotary evaporator and dissolved in toluene (40 mL). To this solution was added dimethyldioxirane (10.0 mL of a 98.2 mM solution in acetone, 1.00 mmol) rapidly using an ice-chilled syringe. The resulting mixture was stirred at 0 °C for 10 min. before removal of the cooling bath and stirring for 1 h. Solvents were removed in vacuo to give the mixture of 13CH2O@2 and H2O@2 + 2 as a brown powder which was used directly in the next step without purification (470 mg, 0.398 mmol, 95%). The filling of 13CH2O@2 was determined as 24% by integrating the alkene peaks of 13CH2O@2 and H2O@2 + 2 in the 1H NMR spectrum.
The same method was used to synthesise CD2O@2 using tetraketone 1 (430 mg, 0.379 mmol), deuterated paraformaldehyde (98 mg), dimethyldioxirane (10.0 mL of a 98.2 mM solution in acetone, 1.00 mmol) and Toluene (40 mL). The mixture of CD2O@2 and H2O@2 + 2 was obtained as a brown powder which was used directly in the next step without purification (383 mg, 0.324 mmol, 86%). The filling of CD2O@2 was determined as 25% by integrating alkene peaks in the 1H NMR of CD2O@2 and H2O@2 + 2.
CH2O@4
A purpose-built reaction vessel (Supplementary Fig. 29) was charged with mixture of CH2O@2 and H2O@2 (380 mg, 0.322 mmol) and the apparatus placed under an atmosphere of argon. THF (250 mL, degassed), AcOH (50 mL of a degassed 10% v/v aqueous solution) and toluene (250 mL) were added, and the resulting mixture was vigorously stirred under irradiation with a 3 × 100 W yellow (590–595 nm) LED lamp, for 18 h at 55 °C. Solvents were then removed in vacuo. Purification by rapid, repeat column chromatography (SiO2 eluted with a 90:8:2 mixture of toluene:EtOAc:AcOH; then SiO2 eluted with 2% AcOH in toluene) gave mixture of CH2O@3 and H2O@3 as a dark brown solid (130 mg, 0.115 mmol, 36%) which was used directly in the next step.
The mixture of CH2O@3 and H2O@3 obtained above (130 mg, 0.115 mmol) was transferred to the RBF and was back filled with N2 and triphenylphosphine (310 mg, 1.18 mmol) and dry toluene was added under N2 and the resulting mixture stirred for 72 h at reflux. After cooling to room temperature, solvents were removed in vacuo. Purification by column chromatography (SiO2 eluted with a gradient of 1:1 hexane:toluene → toluene) gave mixture of CH2O@4 and H2O@4, as a brown/black solid (104 mg, 0.0945 mmol, 82%). 35% filling of CH2O@4 was determined by comparison of integrals in the experimental 1H NMR spectrum.
The labelled materials 13CH2O@4 and CD2O@4 were prepared in the same way.
Synthesis of CH2O@C60
Into a dry flask containing 25% filled CH2O@4 (rest H2O@4 + 4) (210 mg, 0.191 mmol) was added toluene (25 mL) and dry, distilled P(OPri)3 (760 μL, 3.22 mmol). The solution was heated to reflux in the dark for 16 h. The reaction mixture was poured directly onto a SiO2 column and eluted with toluene to collect a purple band. Removing the solvent in vacuo afforded a mixture of CH2O@5 and H2O@5 (22% filling of CH2O measured by NMR) which was immediately dissolved in 1-chloronaphthalene (43 mL) and transferred to a Young’s tube containing N-phenyl maleimide (88 mg, 0.51 mmol). The solution was degassed under dynamic vacuum for 10 min, put under N2 atmosphere and sealed. The flask was immersed into a preheated metal heating block at 255 °C and stirred for 40 h. After cooling to room temperature, the solution was flushed through a SiO2 column packed with toluene, collecting the required product as a purple band, followed by a red band containing the side product 6. The side product CH2O@6 + H2O@6 was further purified using hexane: EtOAc 90:10 to give 39 mg of 37% filled CH2O@6, see data for the same in 1.4. After removing the bulk of the toluene in vacuo the remaining toluene and 1-chloronaphthalene were distilled off under vacuum (<1 torr). The crude product was purified by preparative HPLC (2 × 20 mm × 250 mm) Cosmosil™ Buckyprep columns in series eluting with toluene at a flow rate of 10 mL min−1 to remove traces of 1-chloronapthalene to give a mixture of C60 (4.5%), H2O@C60 (80%) and CH2O@C60 (15.5%), 66 mg, 46%, 0.088 mmol (ratios from HPLC trace). The mixture again subjected to recycling HPLC using the same conditions (retention time after 7 cycles: C60: 220.3 min; H2O@C60: 228.9 min CH2O@C60: 238.3 min) to give CH2O@C60 (>99% filled, remainder H2O@C60, 7.1 mg, 0.0093 mmol, 20% based on the estimated amount of CH2O@4 in the starting material).
The labelled materials 13CH2O@C60 and CD2O@C60 were prepared in the same way.
CO2@Sulfoxide
Finely grounded 1 (402 mg, 0.354 mmol) was put into a stainless steel tube (100 mm, 13.2 mm o.d., 5.2 mm i.d) with SITEC® high-pressure fittings, and loosely plugged with glass wool. The tube was heated at 180 °C for 2 h at < 1 torr. After cooling to room temperature the tube was filled with CO2 to 50 bar using a SITEC® 750.01 hand-operated pressure intensifying syringe. The reactor was then heated to 100 °C and maintained at this temperature for 18 h, with a stable internal pressure of 58–59 atm. The reactor was cooled after 18 h and pressure was released slowly, the tube was taken out and material was collected as dark red solid which was directly taken for DMDO oxidation. To a stirred solution of CO2@1 in toluene (40 mL) at 0 °C, was added dimethyldioxirane (10 mL of a 98.2 mM solution in acetone, 1.0 mmol) rapidly using an ice-chilled syringe. The resulting mixture was stirred at 0 °C for 10 min. before removal of the cooling bath and stirring for 1 h, during which time the mixture warmed to room temperature. Solvents were removed in vacuo to give the title compound as a crude brown powder (381 mg, 0.319 mmol, 90%) which was used directly in the attempted photochemical orifice contraction without purification. The filling factor was determined by comparison of integrals in the experimental 1H NMR spectrum.
Density functional theory calculations
Binding constants and activation energies for entry/loss of endohedral species into sulfide 1 and sulfoxide 2 were determined using model structures 1a and 2a in which the 6-tert-butylpyridyl groups were replaced by methyl substituents. Calculations were carried out using Gaussian 0960, using either the B3LYP functional61,62 with Grimme D3 empirical dispersion correction using Beck–Johnson damping63,64, or the M06-2X functional66 with cc-pVDZ65 basis set to locate minimum energy and transition state structures and to characterise them through frequency calculations. The cc-pVTZ65 basis set with an ultrafine integration grid was used to calculate electronic energies and to correct for Basis Set Superposition Error using the counterpoise method67. Thermal corrections to the electronic energy to give the free energy (G) at 298 K and 1 atm were derived from frequency calculations at the cc-pVDZ level using the Gaussian freqchk utility68. The frequencies were not scaled and low frequency modes were not removed. The values from B3LYP are used in the paper, those from MO6-2X (also widely used in the published literature for calculations on endohedral open fullerenes) are given for comparison in Supplementary Tables 5 and 6.
Calculations on CH2O@C60 were carried out using the B3LYP functional61,62 with Grimme D3 empirical dispersion correction using Beck–Johnson damping63,64, and the cc-pVTZ basis set65 with a superfine integration grid. Minimum energy structures were located using the tight convergence criteria and characterised with frequency calculations. Details on geometry, orbital energy levels, excited state energies, predicted 13C NMR shifts and calculated vibrations and rotations are given in Supplementary Tables 7−13.
X-ray crystallography
Dark orange plate-shaped crystals of the nickel(II) octaethylporphyrin/benzene solvate of CH2O@C60 were obtained from benzene by slow evaporation. A suitable crystal with dimensions 0.08 × 0.08 × 0.05 mm3 was selected and mounted on a MITIGEN holder with silicon oil on a ROD, Synergy Custom system, HyPix diffractometer. The crystal was kept at a steady T = 103(1) K during data collection. The structure solved and the space group P−1 (# 2) determined with the ShelXT 2014/5 solution programme69 using dual methods and by using Olex2 1.5-alpha70 as the graphical interface. The model was refined with ShelXL 2016/670,71 using full matrix least squares minimisation on F2 constraining the CH2O molecule to idealised geometry.
Voltammetry of CH2O@C60
Solutions of ~ 3 mg of C60 or ~1.5 mg CH2O@C60 were prepared in 5 mL of a 4:1 mixture of toluene and acetonitrile containing 0.1 M Bu4N.BF4 as electrolyte. The cell contained a 3 mm diameter glassy carbon working electrode, a 1 cm2 sheet of platinum as the counter electrode and a silver wire pseudo-reference electrode. Cyclic Voltammetry (CV) and Differential Pulse Voltammetry (DPV) were carried out using an AUTOLAB PG204 potentiostat at room temperature using a scan rate of 50 mV/s. After acquiring initial scans of each component, 0.1 mL of a 3 mg/mL solution of ferrocene in toluene was added as an internal reference and further scans acquired.
NMR studies on CH2O@C60
Detailed NMR experiments were performed at 16.45 T, carried out using a Bruker Ascend 700 NB magnet fitted with a Bruker AVANCE NEO console and Bruker TCI prodigy 5 mm liquids cryoprobe. An approx. 23 mM solution of CH2O@C60 in 1,2-dichlorobenzene-d4 (ODCB-d4) was prepared by dissolving 14 mg of powdered CH2O@C60 (86.7% filling) in 0.8 mL of the solvent. The solution was then filtered and then degassed by bubbling nitrogen gas through the solution for 10 min. 1H and 13C NMR spectra were referenced to the solvent chemical shifts (1,2-dichlorobenzene-d4), 1H = 6.93 ppm and 13C = 127.19 ppm7. All chemical shifts have confidence limits of ±0.01 ppm. NMR measurements on 13C-labelled 13CH2O@C60 were performed at 16.45 T on a 1 mM solution in toluene-d8, degassed by bubbling with nitrogen gas for 10 min.
IR and THz spectroscopy of CH2O@C60
A sample of CH2O@C60 which had been purified to 100% filling by recirculating HPLC was sublimed (550 °C, 10-5 bar) before the measurements. The sublimed powder was pressed into pellet 3 mm in diameter and with thickness d = 0.355 mm. IR spectroscopy measurements were carried out on a Bruker Vertex 80 v spectrometer. Between 30 and 600 cm−1 a 4 K bolometer detector and above 600 cm−1 a mercury cadmium telluride detector with glowbar light source were used. Instrument resolution was 0.3 cm−1. The sample pellet was mounted inside a vacuum-tight sample chamber filled with helium exchange gas and attached to a cold finger of a continuous flow cryostat. The absorption coefficient was calculated from the intensities transmitted through the sample and through a 3 mm diameter reference hole.
Data availability
Supplementary material containing spectroscopic and analytical data and copies of 1H and 13C NMR spectra of synthesised compounds; Kinetic data on loss of CH2O from 1 and 2, and CO2 from 2; Additional plots and data from UV, voltametry, NMR and IR studies, and energies and measurements of species from DFT calculations are provided in the supplementary information file. Cartesian coordinates from DFT calculations are provided in the source data file. Crystallographic data for the structure reported in this article has been deposited at the Cambridge Crystallographic Data Centre under deposition no. CCDC 2126579. This data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge ■CB2 1EZ, UK; fax: +44 1223 336033. All other data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature 318, 162–163 (1985).
Saunders, M., Cross, R. J., Jiménez-Vázquez, H. A., Shimshi, R. & Khong, A. Noble gas atoms inside fullerenes. Science 271, 1693–1697 (1996).
Cross, R. J., Khong, A. & Saunders, M. Using cyanide to put noble gases inside C60. J. Org. Chem. 68, 8281–8283 (2003).
Bloodworth, S. & Whitby, R. J. Synthesis of endohedral fullerenes by molecular surgery. Commun. Chem. 5, 121 (2022).
Komatsu, K., Murata, M. & Murata, Y. Encapsulation of molecular hydrogen in fullerene C60 by organic synthesis. Science 307, 238–240 (2005).
Krachmalnicoff, A., Levitt, M. & Whitby, R. An optimised scalable synthesis of H2O@C60 and a new synthesis of H2@C60. Chem. Commun. 50, 13037–13040 (2014).
Hoffman, G. et al. A solid state intramolecular Wittig reaction enables efficient synthesis of endofullerenes including Ne@C60, 3He@C60 and HD@C60. Angew. Chem., Int. Ed. 60, 8960–8966 (2021).
Morinaka, Y., Tanabe, F., Murata, M., Murata, Y. & Komatsu, K. Rational synthesis, enrichment 13C NMR spectra of endohedral C60 and C70 encapsulating a helium atom. Chem. Commun. 46, 4532–4534 (2010).
Kurotobi, K. & Murata, Y. A single molecule of water encapsulated in fullerene C60. Science 333, 613–616 (2011).
Krachmalnicoff, A. et al. The dipolar endofullerene HF@C60. Nat. Chem. 8, 953–957 (2016).
Bloodworth, S. et al. First synthesis and characterization of CH4@C60. Angew. Chem. Int. Ed. 58, 5038–5043 (2019).
Hoffman, G. et al. Synthesis and 83Kr NMR spectroscopy of Kr@C60. Chem. Commun. 58, 11284–11287 (2022).
Bloodworth, S. et al. Synthesis of Ar@C60 using molecular surgery. Chem. Commun. 56, 10521–10524 (2020).
Levitt, M. H. & Horsewill, A. J. Nanolaboratories: physics and chemistry of small-molecule endofullerenes. Philos. Trans. R. Soc. Ser. A 371, 20130124 (2013).
Levitt, M. H. Spectroscopy of light-molecule endofullerenes. Philos. Trans. R. Soc. Ser. A 371, 20120429 (2013).
Rõõm, T. et al. Infrared spectroscopy of small-molecule endofullerenes. Philos. Trans. R. Soc. Ser. A 371, 20110631 (2013).
Bacanu, G. R. et al. Experimental determination of the interaction potential between a helium atom and the interior surface of a C60 fullerene molecule. J. Chem. Phys. 155, 144302 (2021).
Murata, Y., Komatsu, K., Guldi, D. M., Lawler, R. G. & Turro, N. J. A photochemical on-off switch for tuning the equilibrium mixture of H2 nuclear spin isomers as a function of temperature. J. Am. Chem. Soc. 133, 14232–14235 (2011).
Turro, B. N. J. et al. Demonstration of a chemical transformation inside a fullerene. the reversible conversion of the allotropes of H2@C60. J. Am. Chem. Soc. 130, 10506–10507 (2008).
Shugai, A. et al. Infrared spectroscopy of an endohedral water in fullerene. J. Chem. Phys. 154, 124311 (2021).
Beduz, C. et al. Quantum rotation of ortho and para-water encapsulated in a fullerene cage. Proc. Natl Acad. Sci. USA 109, 12894 (2012).
Meier, B. et al. Electrical detection of ortho–para conversion in fullerene-encapsulated water. Nat. Commun. 6, 1 (2015).
Mamone, S. et al. Nuclear spin conversion of water inside fullerene cages detected by low-temperature nuclear magnetic resonance. J. Chem. Phys. 140, 194306 (2014).
Xu, M., Sebastianelli, F., Bačić, Z., Lawler, R. & Turro, N. J. H2, HD, and D2 inside C60: coupled translation-rotation eigenstates of the endohedral molecules from quantum five-dimensional calculations. J. Chem. Phys. 129, 06413 (2008).
Mamone, S. et al. Rotor in a cage: infrared spectroscopy of an endohedral hydrogen-fullerene complex. J. Chem. Phys. 130, 081103 (2009).
Bacanu, G. R. et al. An internuclear J-coupling of 3He induced by molecular confinement. J. Am. Chem. Soc. 142, 16926–16929 (2020).
Dolgonos, G. A. & Peslherbe, G. H. Encapsulation of diatomic molecules in fullerene C60: implications for their main properties. Phys. Chem. Chem. Phys. 16, 26294–26305 (2014).
Saroj, A., Ramanathan, V., Kumar Mishra, B., Panda, A. N. & Sathyamurthy, N. Improved estimates of host‐guest interaction energies for endohedral fullerenes containing rare gas atoms, small molecules, and cations. Chem. Phys. Chem. 23, e202200413 (2022).
Bacic, Z. Perspective: accurate treatment of the quantum dynamics of light molecules inside fullerene cages: translation-rotation states, spectroscopy, and symmetry breaking. J. Chem. Phys. 149, 100901 (2018).
Cioslowski, J. Electronic structure calculations on endohedral complexes of fullerenes: reminiscences and prospects. Molecules 28, 1384 (2023).
Clouthier, D. J. & Ramsay, D. A. The spectroscopy of formaldehyde and thioformaldehyde. Ann. Rev. Phys. Chem. 34, 31–58 (1983).
Bunker, P. R. & Jensen, P. Molecular Symmetry and Spectroscopy 2nd edn (NRC Research Press, 2006)
Schramm, B., Bamford, D. J. & Moore, C. B. Nuclear spin state conservation in photodissociation of formaldehyde. Chem. Phys. Lett. 98, 305–309 (1983).
Peters, G. & Schramm, B. Nuclear spin state relaxation of formaldehyde in mixtures with inert gases. Ber. Bunsenges. Phys. Chem. 102, 1837–1864 (1998).
Bechtel, C., Elias, E. & Schramm, B. F. Nuclear spin symmetry state relaxation in formaldehyde. J. Mol. Struct. 741, 97–106 (2005).
Curl, R. F.Jr, Kasper, J. V. V. & Pitzer, K. S. Nuclear spin state equilibration through nonmagnetic collisions. J. Chem. Phys. 46, 3220–3228 (1967).
Chapovsky, P. L. Nuclear spin conversion in formaldehyde. J. Mol. Struct. 599, 337–345 (2001).
Dickens, J. E. & Irvine, W. M. The formaldehyde ortho/para ratio as a probe of dark cloud chemistry and evolution. Astrophys. J. 518, 733 (1999).
Guzmán, V. V. et al. H2CO ortho-to-para ratio in the protoplanetary disk HD 163296. Astrophys. J. 864, 170 (2018).
Tudorie, M. et al. Nuclear spin conversion of formaldehyde in protostar environments induced by non reactive collisions. Astron. Astrophys. 453, 755–759 (2006).
Chen, C.-S., Kuo, T.-S. & Yeh, W.-Y. Encapsulation of formaldehyde and hydrogen cyanide in an open-cage fullerene. Chem. Eur. J. 22, 8773–8776 (2016).
Futagoishi, T., Murata, M., Wakamiya, A. & Murata, Y. Encapsulation and dynamic behavior of methanol and formaldehyde inside open-cage C60 derivatives. Angew. Chem. Int. Ed. 56, 2758–2762 (2017).
Schlosser, M. & Coffinet, D. α-Substitution plus carbonyl olefination via β-oxido phosphorus ylides (SCOOPY) reactions. Stereoselectivity of allyl alcohol synthesis via betaine ylides. Synthesis 7, 380–381 (1971).
Futagoishi, T., Murata, M., Wakamiya, A. & Murata, Y. Trapping N2 and CO2 on the sub-nano scale in the confined internal spaces of open-cage C60 derivatives: isolation and structural characterization of the host–guest complexes. Angew. Chem. Int. Ed. 54, 14791–14794 (2015).
Hashikawa, Y. & Murata, Y. C2-insertion into a fullerene orifice. Chem. Commun. 59, 1645–1648 (2023).
Hoffman, G. Synthesis of Atomic and Molecular Endohedral Fullerenes. PhD Thesis, Univ. Southampton (2021).
Light, M. E., Hoffman, G. & Whitby, R. J. CSD Communication Deposition number 1877860 (2023).
Hao, Y., Wang, Y., Spree, L. & Liu, F. Rotation of fullerene molecules in the crystal lattice of fullerene/porphyrin: C60 and Sc3N@C80. Inorg. Chem. Front. 8, 122–126 (2021).
Sworakowski, J. How accurate are energies of HOMO and LUMO levels in small-molecule organic semiconductors determined from cyclic voltammetry or optical spectroscopy?. Synth. Metal 235, 125–130 (2018).
Huang, G., Ide, Y., Hashikawa, Y., Hirose, T. & Murata, Y. CH3CN@ open‐C60: an effective inner‐space modification and isotope effect inside the nano‐sized flask. Chem. Eur. J. 29, e202301161 (2023).
Zhu, G. et al. Probing the interaction between the encapsulated water molecule and the fullerene cages in H2O@C60 and H2O@C59N. Chem. Sci. 9, 5666–5671 (2018).
Morris, G. A. & Freeman, R. Enhancement of nuclear magnetic resonance signals by polarization transfer. J. Am. Chem. Soc. 101, 760–762 (1979).
McClung, R. E. D. Spin-rotation relaxation theory. In eMagRes https://doi.org/10.1002/9780470034590.emrstm0524 (2007).
Flygare, W. H. & Weiss, V. W. 13C spin—rotation interaction and magnetic shielding at the carbon and oxygen nuclei in formaldehyde. J. Chem. Phys. 45, 2785–2792 (1966).
Kukolich, S. G. Proton magnetic shielding tensors from spin-rotation measurements on formaldehyde and ammonia. J. Am. Chem. Soc. 97, 5704–5707 (1975).
Spiess, H. W. Rotation of molecules and nuclear spin relaxation. In Dynamic NMR Spectroscopy. NMR Basic Principles and Progress / Grundlagen und Fortschritte Vol 15 (Springer, Berlin, 1978).
Bacanu, G. R. et al. Fine structure in the solution state 13C-NMR spectrum of C60 and its endofullerene derivatives. Phys. Chem. Chem. Phys. 22, 11850–11860 (2020).
Bini, R., Salvi, P. R. & Schettino, V. The far-infrared spectrum of crystalline fullerene C60. J. Phys. Chem. 97, 10580–10584 (1993).
Carter, S., Handy, N. C. & Demaison, J. The rotational levels of the ground vibrational state of formaldehyde. Mol. Phys. 90, 729–738 (1997).
Gaussian 09, Revision D.01, Frisch, M. J., et al. Gaussian 09, Revision D.01 (Gaussian, Inc., Wallingford, CT, 2013).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 37, 785–789 (1988).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Dunning, T. H. Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Boys, S. F. & Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 19, 553–566 (1970).
Ochterski, J. W. Thermochemistry in Gaussian https://gaussian.com/thermo/ (2000).
Sheldrick, G. M. Crystal structure refinement with ShelXL. Acta Cryst. C71, 3–8 (2015).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. Olex2: a complete structure solution, refinement and analysis program. J. Appl. Cryst. 42, 339–341 (2009).
Sheldrick, G. M. ShelXT-integrated space-group and crystal-structure determination. Acta Cryst. A71, 3–8 (2015).
Acknowledgements
This research was supported by the EPSRC (UK) under Grant Nos. EP/P009980/1 and EP/T004320/1 (MHL and RJW), the Estonian Ministry of Education, personal research funding PRG736 (UN), and the European Regional Development Fund, Project No. TK134 (UN). RJW acknowledges the use of the IRIDIS High Performance Computing Facility at the University of Southampton. We thank James W. Whipham for discussions.
Author information
Authors and Affiliations
Contributions
VKV and ESM performed synthetic experiments; GRB and MS performed NMR experiments; TJ, AS and UN performed IR / THz experiments. RJW performed DFT theoretical studies; MEL solved the crystal structure. RJW, MHL and TR designed and supervised the project. All authors contributed to writing the manuscript and have given approval to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Nazario Martín and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vyas, V.K., Bacanu, G.R., Soundararajan, M. et al. Squeezing formaldehyde into C60 fullerene. Nat Commun 15, 2515 (2024). https://doi.org/10.1038/s41467-024-46886-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46886-5
This article is cited by
-
Quantum scattering of icosahedron fullerene C60 with noble-gas atoms
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.