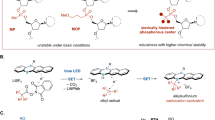
Abstract
Chemically modified nucleosi(ti)des and functional oligonucleotides (ONs, including therapeutic oligonucleotides, aptamer, nuclease, etc.) have been identified playing an essential role in the areas of medicinal chemistry, chemical biology, biotechnology, and nanotechnology. Introduction of functional groups into the nucleobases of ONs mostly relies on the laborious de novo chemical synthesis. Due to the importance of nucleosides modification and aforementioned limitations of functionalizing ONs, herein, we describe a highly efficient site-selective alkylation at the C8-position of guanines in guanosine (together with its analogues), GMP, GDP, and GTP, as well as late-stage functionalization of dinucleotides and single-strand ONs (including ssDNA and RNA) through photo-mediated Minisci reaction. Addition of catechol to assist the formation of alkyl radicals via in situ generated boronic acid catechol ester derivatives (BACED) markedly enhances the yields especially for the reaction of less stable primary alkyl radicals, and is the key to success for the post-synthetic alkylation of ONs. This method features excellent chemoselectivity, no necessity for pre-protection, wide range of substrate scope, various free radical precursors, and little strand lesion. Downstream applications in disease treatment and diagnosis, or as biochemical probes to study biological processes after linking with suitable fluorescent compounds are expected.
Similar content being viewed by others
Introduction
Nucleoside and nucleotide analogs, mimicking the natural nucleosides, represent a large family of anti-tumor and anti-virus drugs, such as FDA-approved anti-Covid-19 drug Remdesivir and Molnupiravir1,2,3. Nucleotides, such as 5′-triphosphates, are fundamental building blocks for the polymerase-mediated synthesis of nucleic acid4,5. Moreover, base-functionalized oligonucleotides are of great interest due to their applications in the field of drug discovery and chemical biology6,7. Given the importance of these biomolecules, various methods have been developed to access these compounds and their analogs8,9,10. In general, multiple synthetic steps are required for preparing these biologically active nucleosi(ti)de analogs8, including de novo synthesis via glycosylation of ribose with base, and multiple steps of chemical modification from protected nucleosides. Especially for the base-functionalized oligonucleotides synthesis9,10, solid-phase DNA synthesis based on phosphoramidite chemistry or DNA polymerases method with 5′-triphosphorylated nucleosides is always needed, which may have a compatibility issue with unnatural nucleoside monomer. Therefore, the development of efficient, mild, and water-compatible methods for the direct or late-stage functionalization of nucleosi(ti)des and oligonucleotides is highly desirable11,12, which strategy has been widely used in the modification of drug molecules, natural products, and biological macromolecules13,14,15.
C8 functionalized purines possess unique biological activities. For example, C8-arylated, alkenylated, and alkynlated analogs are important fluorescent nucleobases as conjugated scaffolds of purine structures7. Moreover, the C8-alkylated analogs exhibit antiviral or anticancer activities (Fig. 1a)16,17,18. To be more specific, 8-methyladenosine is a potent and highly selective inhibitor of the vaccinia virus, and 8-ethyl adenosine shows special inhibition against respiratory syncytial virus (RSV)16. C8-substituted guanosine triphosphate (GTP) analogs efficiently inhibit the polymerization of FtsZ and could be a promising class of novel antibiotics17, while 8-cyclopentyl-2,6-diphenyl purine is an adenosine receptor antagonist18. Hence, great efforts have been made to the synthesis of C8-alkylated purine nucleosides. As an early example, the classical transition-metal catalyzed cross-coupling strategy was presented to construct the C8-alkylpurine frames from 8-halogenopurine nucleosides (see Supplementary Fig. 7)19. Notably, Hocek group reported the direct methylation of deoxyadenosine triphosphate (dATP) via Suzuki coupling, providing the C8-Me dATP20. Moreover, the radical process, as a prevailing method for alkylation reaction, always proceeds under mild reaction conditions. However, there are few examples of radical alkylation of C8-H purines that have been reported to date (Fig. 1b)21,22,23,24. In the 1970s, Kawazoe and Pless group reported direct modification of guanosine using a classic radical approach, however, the yield is low (<30%), and substrate scope is also limited (see Supplementary Fig. 7)21,22. In 2012, Qu and Guo group successfully accomplished direct C8 radical alkylation of protected purines with cycloalkane promoted by tBuOOtBu delivering an 8-cycloalkyl purine skeleton at 140 oC23. Later, Zard and co-workers described an effective xanthate-based alkylation of the Ac group-protected guanosine derivative24. Meanwhile, very limited examples of selective functionalization at purine in DNA/RNA were reported25,26,27,28,29,30 (Fig. 1c). One approach is based on pre-functionalized oligonucleotides. In 2010, Manderville group reported the efficient C8 arylation of DNA oligonucleotides via Suzuki-Miyaura cross-coupling of C8-Br G25. In 2022, Zhou group achieved the SNAr reaction of C6-I purine using different nucleophile regents26. Another approach is late-stage functionalization of nucleic acid with non-canonical nucleosides. In 2019, Wang and Cheng group27 reported photochemical demethylation of N6-Methyladenosine (N6-mA) residues in RNA by Flavin mononucleotide (FMN), which is important in epigenetic research. Later, Balasubramanian and Gaunt group reported a selective chemical functionalization of N6-mdA in DNA28 via visible light-mediated photoredox catalysis. Direct modification of natural nucleic acid is more challenging due to the similar functional group in the nucleobases. In 2020, Saraogi group achieved selective functionalization at the N2-position of guanosine in oligonucleotide via reductive amination owing to the higher reactivity of N2 amine of guanosine than other nucleobases (adenine and cytosine)29. In the same year, He and co-workers reported fast and reversible labeling of single-stranded guanine bases in live cells using a new N3-kethoxal reagent for RNA secondary structure mapping30.
a Representative examples of bioactive C-8 alkylated nucleosides. b Selected examples of C8 alkylation of purine. c Selected examples of late-stage functionalization of purine of DNA/RNA. d Our strategy for late-stage functionalization of C-8 alkylated nucleosides, nucleotides, and RNA & DNA oligonucleotides. Me methyl, Et ethyl, tBu tert-butyl, Ac Acetyl, HMDS hexamethyldisilazane, CSA camphorsulfonic acid, DLP dilauroyl peroxide.
Despite the above-mentioned efforts, deficiencies in the alkylation procedures have yet to be overcome, which include harsh conditions, lengthy steps, limited substrate scope, and poor product diversity. In particular, fewer methods are available for the selective functionalization at the C8 site of G in RNA/DNA oligonucleotides. In connection with our continuous interest in the late-stage functionalization of drug molecules, natural products, and biomacromolecules31,32, we wish to report our investigations toward the direct alkylation at the C8 site of nucleosi(ti)de analogs via catechol-promoted photo-mediated Minisci reaction with alkylboronic acids and their derivatives (Fig. 1d). Moreover, late-stage alkylation at the C8-site of G in RNA/DNA oligonucleotides has also been achieved, which would have the potential to be further applied in drug development and biological studies.
Results
Reaction development
We commenced our research by investigating the C8-alkylation of unprotected guanosine 1a under several reported reaction conditions (see Supplementary Fig. 17)23,24,33. However, the desired C8-Et guanosine 3a was obtained in 23% yield only under the condition developed by Monlander group with EtBF3K 2a as radical precursors: [MesAcr (5 mol%), (NH4)2S2O8 (2.0 equiv.), and TFA (1.0 equiv.) in MeCN/H2O (1/1) under white light (85 W) irradiation at room temperature] (Table 1, entry 1)33. These results further demonstrated the challenge of late-stage functionalization of unprotected guanosine. Then, extensive conditions were screened to optimize the reaction. Neither changing the photocatalyst nor solvent gave detectable improvement. (see Supplementary Tables 3 and 4 full optimization). When the loading of alkylating reagent increased from 2 equiv. to 4 equiv., the yield increased to 37% (Table 1, entry 2). However, no better results were obtained after other condition screenings (see Supplementary Table 5 for full optimization). The oxidative potential of RBF3K is lower than the corresponding RB(OH)2, and RBPin, Gouverner, and Davis group reported that the addition of catechol would in situ-generate boronic acid catechol ester derivative (BACED) reagents with reduced oxidative potential to promote the formation of alkyl radicals34,35,36. Indeed, a much higher yield was obtained when catechol was used as an additive in our reaction (Table 1, entry 3). Moreover, ethylboronic acid (EtB(OH)2, 2a) acting as the radical precursor, which is much cheaper than EtBF3K and not compatible with the reported condition33, proceeded well with a similar yield (87%, Table 1, entry 4). Whereas, by using other additives such as phenol, hydroquinone, and ethylene glycol, the desired alkylated product was in much lower yields (Table 1, entries 5-7). Other analogs with an electron-donating group of catechol present the same effect (see Supplementary Table 7 for full optimization). Further control experiments proved that catechol and oxidant are essential for this transformation (Table 1, entries 8, 9). This reaction can also deliver the desired product in 74% yield in the absence of trifluoroacetic acid (TFA), which could be further applied to the acid-sensitive substrates (Table 1, entry 10). Because the RB(OH)2 or RBF3K with special functional groups are not commercially available, and need to be synthesized from the corresponding RBpin, so RBpin as radical precursors were tested to expand the range of substrate adaptability, which may avoid the additional functional group transformations. Adding methylboronic acid (MeB(OH)2), in situ generating the EtB(OH)2 from the EtBPin, delivered the desired product 3a in high yield (Table 1, entry11)37. This is facilitated by the fact that our protocol, with MeB(OH)2 as the substrate, is essentially free of conversion (see Supplementary Table 8). Further control experiments proved that catechol and MeB(OH)2 are essential for this transformation (Table 1, entries 12, 13).
In the presence of radical scavenger 2,2,6,6-tetramethylpiperidinooxy (TEMPO), the alkylation reaction was completely inhibited and the radical-trapping byproduct 2j-1 was isolated in 44% yield (see Supplementary Fig. 21). Based on observed results and the previous reports34, a possible radical mechanism was proposed (see Supplementary Fig. 22). Initially, alkylboronic acids and their derivative reacted with catechol to deliver the boronic acid catechol ester derivative (BACED) with reduced oxidative potential. This in situ formed BACED reagent advantageously generated RCH2• radical via Mesacr-catalyzed SET way, which is further reacted with S1 from protonated 1a with or without TFA to provide the C8-alkylated intermediate S2. The aminium radical intermediate S2 via direct HAT event with the sulfate anion, delivered the nucleoside intermediate S3. Finally, the intermediate S3 via subsequent deprotonation delivered the target alkylated nucleoside product 3a.
Reaction scope
With the optimized reaction conditions in hand, we set out to study the scope of the reaction concerning alkyl boron radical precursors, including RB(OH)2, RBF3K, and RBPin (Fig. 2). Due to the inaccessibility of some RB(OH)2 and RBF3K, different radical sources were tested. Gratifyingly, various primary alkyl radical precursors with different lengths were found to be suitable substrates, providing the desired products (3a–3g) in yields of 50-83%. Notably, the yields of products (3a and 3c) were much lower in the absence of catechol, which further indicates that catechol is necessary for this reaction.
aCondition A: Guanosine (1.0 equiv.), alkylboronic acid (4.0 equiv.), MesAcr (5 mol%), (NH4)2S2O8 (2.0 equiv.), TFA (1.0 equiv.) and catechol (1.0 equiv.) in MeCN: H2O (1: 1, 0.1 M) on 0.2 mmol scale; irradiated by 85 W white light at RT for 16 h; isolated yield. bCondition B: Alkyltrifluoroborate (2.0 equiv.) was used instead of alkylboronic acid. c Condition C: alkylpinacolyl boronate ester (2.5 equiv.) was used instead of alkylboronic acid, adding methylboronic acid (5.0 equiv.). Boc, t-Butyloxy carbony.
Alkylboronic acids and derivatives possessing diverse functional groups such as alkenyl (2h), aromatic scaffolds (2i and 2j), tert butoxy substituted (2k), ester (2l), alkynyl (2m), and azide (2n) could smoothly participate in this C8 alkylation, delivering the corresponding products (3h-3n). Notably, the products 3m and 3n with an alkyne or azide handle could be further functionalized via click reaction for biological application. As seen in 3o-3u, the alkylation reactions of secondary alkylboronic acid derivatives typically proceeded well in good to excellent yield under standard conditions. Acyclic substituted alkylboronic acids afforded the corresponding alkylated products (3o and 3p) in the yields of 80% and 72%, respectively, whereas 3p was obtained as a mixture of diastereoisomers (d.r. = 1:1). The presence of cyclic structures, including cyclobutane (3q), cyclopentane (3r), cyclohexane (3s), oxane (3t), and piperidine (3u) rings, were found to be amenable to deliver the desired products in 50–80% yields. However, no desired product 3v was obtained in the case of the cyclopropyl system, probably due to the destabilization of the resulting cyclopropyl radical38. Additionally, we were pleased to find that the tertiary alkylboronic acids and derivatives gave desired products (3w-3z) in good to excellent yields (56–82%). It should be mentioned that this reaction cannot tolerate MeB(OH)2, ArB(OH)2, and other alkyl boron radical precursors (3-1-3-4, see Supplementary Table 13), which are also challenging substrates in this type of Minisci reactions33.
Subsequently, a wide array of purine-based nucleosides was then explored (Fig. 3). Purine nucleosides with artificial ribose containing 2-fluoro, OMe, and O-methoxyethyl (MOE) substitution on the ribose moiety which were widely used in antisense oligonucleotides (ASOs) and small interfering RNAs (siRNAs) drug discovery39,40,41 proceeded well to give the corresponding C8-alkylated products (4b-4d) with high yields. Satisfying results were also obtained from Ac protected substrate (4e, 72%); Due to its acid-sensitivity, 2’,3′-O-isopropylidene guanosine 3f afforded the desired product in good yield (4f, 64%) in the absence of TFA. However, no desired product 4g was obtained using deoxyguanosine (dG) as a substrate, resulting in the ribose-cleaved byproduct 4g’. We reason that the glycosidic bond in dG is less stable than G under this condition, as radical hydrogen atom transfer (HAT) may take place at the 2’ position of ribose ring42. Decreasing the reactivity of ribose ring with the electron-withdrawing group could avoid the cleavage of the ribose. Fortunately, Ac group-protected deoxyguanosine (1h) was found to be a competent reaction partner for the current alkylation reaction, providing 4h in high yield. The broad substrate scope and promising functional group compatibility of this reaction encouraged us to evaluate their utility for late-stage modification of purine-based drug molecules, such as Penciclovir, Ganciclovir, Acyclovir (anti-HSV agents)43 and Valganciclovir (anti-HIV agents) all progressed well to give the corresponding alkylation products with good to high yields (4i-4l, 52–90%). Meanwhile, alkylation of adenosine analogs was also investigated; however, the reactivity of simple adenosine is much lower than the G, providing mixed products with poor selectivity between the C2 and C8 positions. 2-fluoro- and 2-chloro substitutions blocking the C2 position were evaluated, and both gave products with good yields (4n and 4o, 52% and 61%). However, no alkylation product 4p was generated when employing C2-NH2 substituted adenosine analog.
aGeneral condition: Guanosine (1.0 equiv.), alkylboronic acid (4.0 equiv.), MesAcr (5 mol%), (NH4)2S2O8 (2.0 equiv.), TFA (1.0 equiv.) and catechol (1.0 equiv.) in MeCN: H2O (1: 1, 0.1 M) on 0.2 mmol scale; irradiated by 85 W white light at R.T. for 16 h; isolated yield. bNo TFA was used. cRibose-cleaved byproduct 4 g’ was obtained in 71% yield. dUridine (1.0 equiv.), alkylboronic acid (4.0 equiv.), (NH4)2S2O8 (2.0 equiv.), MgCl2 (2.0 equiv.) and catechol (1.0 equiv.) in DMSO: H2O (1: 1, 0.1 M) on 0.2 mmol scale; irradiated by 36 W blue LED at RT for 24 h. HSV herpes simplex virus, HIV human immunodeficiency virus.
With this radical alkylation method in hand, we turned our attention to the C5 alkylation of uridine. C5 alkylated uridines are also important due to their application in drug development44, such as C5-Et substituted drug molecular Edoxudine. Although various biological C5 alkylated uridines were prepared based on classic methods45,46,47,48 (see Supplementary Fig. 8), direct C5 alkylation of uridine is still desirable due to the high-efficiency transformation. However, only a 3% yield of the desired product 6a was obtained under the standard condition (see Supplementary Table 9). Then, dimethyl sulfoxide (DMSO)/H2O solvent system improved the yield to 40%, and additive MgCl2 instead of TFA further improved the yield to 45%. As shown in Fig. 3, a range of uridine nucleoside analogs was then investigated, affording the desired product (6a-6e) in moderate yields under the optimized condition. Alkyl chains carrying an ethyl, propyl, or isopropyl group can be successfully installed at the C5 site of the uridine moieties in moderate yields (6f-6h, 32-49%).
Having established the applicability of our methodology to a variety of purine nucleosides, we turned our attention to the modification of complex nucleotides (Fig. 4). Because of their lipophilicity, metabolic stability, binding selectivity, and bioabsorption characteristics, dinucleotide motifs are widely used in pharmaceuticals for anti-virus and anti-tumor drug development49,50,51,52,53,54. Moreover, dinucleotide (UpG) was identified as an efficient “initiator dinucleotide” RNA polymerases at the 5′ terminus of a transcript54. However, limited examples of late-stage functionalization of dinucleotides were reported due to the reactivity and selectivity issues. To our delight, the protocol has been effective in a range of dinucleotide scaffolds (Fig. 4a). Notably, alkylation of dinucleotides selectively occurred at the C8 site of the guanine subunit to give the desired alkylated products in good to excellent yields (8a-8e, 41–80%), without effecting other bases (uracil, thymine, cytosine, and adenine). For example, GpU and GpA dinucleotide with multiple reactive sites provided the single C8-Et desired products 8a and 8d. We reason that G is much more reactive than U and A in the nucleoside (4a vs 4m in Fig. 3), as single U and A afforded trace or less product, so the selectivity could be achieved in these dinucleotide substrates. Meanwhile, dinucleotide holding two guanine subunits (GpG, 7e), was selectively alkylated at both the C8 sites of guanine in 41% yield. Dinucleotides or oligonucleotides containing phosphorothioate (PS) linkage, with better chemical and biological stability55 provided the corresponding products 8f and 8f’ in good yields. Moreover, the protocol is also applicable to dGpT and dTpG, providing the desired product 8g and 8h, despite the fact that unprotected dG decomposed under this condition. We reason that the phosphate group has an electron-withdrawing effect similar to Ac group (4h in Fig. 3), making the glycoside bond of deoxyguanosine more stable under this condition. Since modified cyclic dinucleotides (CDNs) are promising stimulators of interferon genes (STING) receptor agonizts in cancer immunotherapy56, we also applied our method for late-stage modification of C-di-GMP, and di-alkylated C-di-GMP 8i was successfully obtained in 53% yield. These promising results indicate that this method could be applied in the late-stage C8–H alkylation of G/dG in the RNA/DNA oligonucleotides.
a C-H alkylation of dinucleotide and cyclic dinucleotide substrates. aGeneral condition: dinucleotides or nucleotides (1.0 equiv.), alkylboronic acid (4.0 equiv.), MesAcr (5 mol%), (NH4)2S2O8 (2.0 equiv.), TFA (1.0 equiv.) and catechol (1.0 equiv.) in MeCN: H2O (1: 1, 0.1 M) on 0.06 mmol scale; irradiated by 10 W blue LED at ~10 °C for 24 h; isolated yield. bNo TFA was used, and the reaction time was shortened to 5 hours. cRun on 0.04 mmol scale. b C-H alkylation of nucleotide substrates. dIrradiated by 36 W blue LED at r.t. for 16 h. eIrradiated by 85 W white light at r.t. for 16 h. 10 mM NH4HCO3 (aq.) was used instead of H2O, and No TFA was used. ei-PrB(OH)2 was used instead of EtB(OH)2 as C8-Et-GTP cannot be separated from GTP.
Nucleotides, such as 5′-mono- and 5′-polyphosphorylated nucleosides play important functional and structural roles in biological systems4,5. Direct modification of nucleotides remains rare as nucleoside 5′-phosphate, including triphosphates, are stable only in slightly alkaline solution (pH ≈ 8), and very sensitive under acidic conditions. As seen in Fig. 4b, guanosine monophosphate (GMP) provided the desired product 8j in good yield (LC-MS yield 91%, isolated yield 52%). However, guanosine diphosphate (GDP), and GTP afforded the corresponding C8-alkylated products (8k and 8l) with low yield, along with phosphodiester bond hydrolyzed byproduct under the standard condition due to the acidic reaction condition. Since 5′-polyphosphorylated nucleosides is not stable in the acidic system, reaction condition without TFA may improve the yield. However, no better results were obtained in the absence of TFA, as this system after reaction is still very acidic (the pH value was 1.97, see Supplementary Table 12). Finally, this reaction proceeded well with moderate to good yields using the 10 mM NH4HCO3 as solvent (pH value was 2.72 after reaction, see Supplementary Table 12). In this condition, regrettably, we did not find a satisfactory method to prevent hydrolysis of 8 l. It is worth noting that this method requires only one-step to obtain GTP analogs with C8 substitution, which efficiently inhibits FtsZ polymerization, in comparison, the previous method required four steps17.
Applications of the methodology
To verify the practicability of this reaction manifold, the reaction of compounds 1a and 2a was scaled up to 6 mmol, and the desired product 3a was obtained in 90% yield (1.6 g) after 24 h, opening the possibility of achieving a successful scale-up for further application (Fig. 5a). Moreover, 3a was further alkylated at the site of C2-NH2 by reductive amination, which is already applied for the modification of oligonucleotides29. It was able to obtain the target compound 10 in an excellent yield of 81%. Meanwhile, the reductive amination at the N2 site afforded alkylated 9, followed by our C8-H radical reaction resulted in a high yield of the double alkylation product 10. Modified nucleoside acids play an important role in the drug development and bio-imaging field. The compound 3l with an alkyne handle could be bioconjugated with Zidovudine57 which has an azide group via click reaction58, resulting in the formal dinucleotide 11 in high yield (Fig. 5b). Additionally, compound 3m also can be conjugated with coumarin 12 having azide group, affording the fluorescent compound 13 in 81% yield. In the same way, compound 3n with an azide handle was successfully conjugated with biotin-alkyne 14 via click reaction.
Alkylation of RNA/DNA oligonucleotides
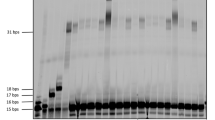
After the successful application of this method to the C8-alkylation of single G and various dinucleotides, our attention then focused on the late-stage functionalization of RNA and DNA oligonucleotides (Tables 2 & 3). Although high selectivity between the G base and other bases was demonstrated in the dinucleotides system (Fig. 4a), the reactivity problem may exist as the substrate concentration of oligonucleotides reaction was much dilute than the normal reaction condition (mM vs M)59. Indeed, no desired product was obtained using the guanosine as a substrate under the condition of normal concentration of the oligonucleotides (100 nmol, 0.2 mM) (see Supplementary Table 17, entry 1). By simultaneously increasing the equivalents of ethylboronic acid, catechol, (NH4)2S2O8, TFA, and MesAcr (see Supplementary Table 17, entries 2-4), a much better result was obtained with a yield of 89% for the target product under the condition with 400 times of above reagents compared with the original condition). Due to the stability of oligonucleotides under relatively high concentration TFA and solubility in the water system, the equivalents of the aforementioned compounds were reduced while still keeping the reactivity (see Supplementary Table 17, entries 5-21 for full optimization). Finally, quantitative yield (99%) was achieved with the full conversion of starting material under the condition of alkylboronic acid (400.0 equiv.), MesAcr (50 mol%), (NH4)2S2O8 (200.0 equiv.), TFA (50.0 equiv.), and catechol (100.0 equiv.) in MeCN/H2O (0.25 mL/0.25 mL) (see Supplementary Table 17, entry 10, Table 2, entry 1). Notably, catechol was demonstrated to be essential for this reaction (see Supplementary Table 17, entry 18). Furthermore, several dinucleotides containing with G also proceeded well under this dilute reaction (Table 2, entries 2-4), affording the mono C8-alkylation of G products despite using the excess of ethylboronic acid (400 equiv.). With this optimized methodology in hand, we set up to test the application of RNA oligonucleotides. Firstly, ONs 1-3 (6 nt) with a guanine base in different sites (internal, or 5′, 3′ terminal position) and without adenine base in the sequence delivered the desired product in high yield (Table 2, entries 5-7), and the product and reactive site were confirmed by high-resolution mass spectrometry (HRMS), matrix-assisted laser desorption/ionization Fourier transform mass spectrometry (MALDI-FTMS) (see Supplementary Figs. 87–91) and nuclease digestion method (see Supplementary Figs. 92 and 93). Then, ON 4 containing both guanine and adenine also proceeded with high selectivity for C8 guanine in 78% yield, and no poly-substituted products were observed (Table 2, entry 8). ON 5 switching the position of A and G also afforded the desired product in high yield (Table 2, entry 9). ONs 6-8 with 7-9 nt resulted in good yields (Table 2, entries 10-12). Finally, different alkyl groups, such as n-Pr, azide chain, cyclobutyl, and cyclopentyl, were successfully introduced to the C8 G in the ON 5 (Table 2, entries 13-16). Since there are rare examples of C8-alkylated oligonucleotides that have been reported, these various C8-alkylated oligonucleotides may have applications in the area of nucleic acid drugs, such as ASO, siRNA, and aptamer.
Meanwhile, dinucleotide dGpT and dTpG could also proceed well under the modified condition [short reaction time (5 h), lower temperature (0 °C), and without TFA] (Table 3, entries 1, 2). For this protocol, the aforementioned condition is needed to avoid the glycoside bond breaking in the reaction, since ethylboronic acid, catechol, (NH4)2S2O8, TFA, and MesAcr are far excess. Then, DNA oligonucleotides with different sequences and lengths were also tested. ONs 9 and 10 with G in the 5′ and 3′ sites of the sequence, the generation of by-products was observed, and the yield of the target product was relatively less (Table 3, entries 3, 4). While ON 12 with internal dG in the sequences provided the desired product in 80% yield with staring material recovered (Table 3, entry 6). Gratefully, ONs 11, 13-17 with different sequences and lengths afforded the desired product in good yields (Table 3, entries 5, 7-11).
We have carried out the experiment with DNA oligonucleotides ON 18 (5′-dTGTCGC-3′) containing two guanines as substrate (Table 4, entry 1). It was found that monosubstituted product 16a and disubstituted product 17a can be obtained with yields of 33% and 67%, respectively. Next, we used RNA oligonucleotides containing two guanines and one adenine (5′-CGAUGU-3′) as substrates (Table 4, entry 2). The results showed two monosubstituted products 16b were generated, with a total yield of 33%, and a disubstituted product 17b was generated, with a yield of 56%.
Previous late-stage cross-coupling reactions on oligonucleotides strongly depend on the sequence and secondary structure, showing the differences between internal and terminal position and single- and double-stranded DNA60,61,62. From our preliminary results (see Supplementary Table 22 and Supplementary Fig. 82 for more detail), both chains were found to have ethylated products generated. The G on these two strands is in no special position, both in internal and terminal position, which might mean that this method can be directly used for modifying dsDNA without being affected by its pairing. However, the cosolvent and lack of salts may not support double-stranded hybridization, and it is possible that the strands are reacting in single-stranded form.
Since click chemistry has been widely used in nucleic acids for labeling, ligation, cyclization and other applications. We also conducted the application of modified oligonucleotide via click chemistry (Fig. 6). Firstly, an oligonucleotide (18) with azide group was obtained from ON 5 in 31% yield, after separation with pre-HPLC, followed by click reactions to conjugate a biotin-alkyne 14 to afford the oligonucleotide 19 in 41% yield.
Discussion
Modified nucleosides, nucleotides, and oligonucleotides are important in the fields of medicinal chemistry, chemical biology, biotechnology, and nanotechnology. The introduction of functional groups into the nucleobases of these molecules mostly relies on the laborious de novo chemical synthesis, and late-stage functionalization of nucleosi(ti)des and oligonucleotides have been developed in recent years. There are successful examples of C8-arylation, alkenylation, and alkynlation of purines of oligonucleotides using a cross-coupling approach. In contrast, there are rare methods for selective alkylation at the C8 site of G in RNA/DNA oligonucleotides, which prevents further application of these alkylated nucleosi(ti)des and oligonucleotides. Here, we have achieved C8-alkylation of nucleosides, nucleotides, and oligonucleotides via mild Minisci radical reaction. We believe that the introduction of various alkyl groups at the C8 position of guanine is a good supplement to the previous aryl/alkenyl/alkynyl substitution, these alkylated nucleosi(ti)des and oligonucleotides will have potential application in the field of nucleic acid.
With our methods, a wide array of readily accessible primary, secondary, and tertiary alkylboronic acids and derivatives were successfully introduced to the C-8 unprotected guanine and analogs in just one-step (Figs. 1 and 2). In addition, C5 alkylation of uridine was also achieved in moderate yield via modified methods (Fig. 2). The limitation of our method is that methyl and phenyl groups cannot be introduced using current conditions. We believe that this practical alkylation method for the modification of nucleosides will be useful in medical chemistry, especially for medicinal chemists to quickly set up nucleoside compound libraries for drug discovery.
Our method also demonstrates its application for the late-stage modification of nucleotides, such as dinucleotides and CDNs. Since de novo synthesis of CDNs requires multi-steps, setting up CDNs libraries for drug screening is time and resource-consuming. Our method can directly modify the CDN (like C-di-GMP in Fig. 4), and has the potential to quickly provide a CDNs library for screening. Moreover, our methods can easily introduce alkyl group to the C-8 position of nucleoside triphosphates, which cannot be easily achieved using previous methods (like GTP in Fig. 4). C-8 modification will result in altering purine geometry to the nonstandard syn conformation, which strongly hinders its ability to undergo base pairing, only 8-substituted purine derivatives bearing small groups, like amino, bromo, or methyl are generally good substrates for DNA polymerases. In contrast, 8-Ph-dATP is too bulky for the polymerase to accept as a substrate25. Due to the hard accessibility of NTPs bearing other alkyl groups, fewer studies were reported on these functionalized oligonucleotides. After preparation of alkyl functionalized NTPs using our one-step method, we will study their compatibility with DNA/RNA polymerase and further application.
Since C and T are not reactive under this radical condition, meanwhile, U and A are much lower reactive compared with G, so selective late-stage modification of G in the RNA oligonucleotides was successfully achieved. Although simple dG is not compatible with the current condition due to the ribose-cleavage, 3′ and 5′ phosphate groups lower the electron density of the ribose ring to prevent the side reaction of ribose-cleavage, so direct alkylation of DNA oligonucleotides could also be accomplished. In the future, one major application of this method for the modification of DNA/RNA oligonucleotides is in the area of nucleic acid drugs, such as ASO, siRNA and aptamer. Due to the selectivity and compatibility with PS in the oligonucleotides, various alkyl groups could be introduced to these oligonucleotides containing less G (one or two), and further studies of the effects of these alkylated modifications on the stability, activity, and toxicity in nucleic acid drugs will be carried out. Although the selective function of G between different bases can be achieved in the oligonucleotides, the selective function of specific G in the sequence with many G is still challenging under the current method. If specific G in the oligonucleotides bearing many G (more than 3) need to be alkylated, solid-phase synthesis would be a more suitable approach using alkylated monomers. Another application of this method would be the bioconjugation of the oligonucleotides for labeling and cross-linking with other biomacromolecules63.
In conclusion, we have successfully developed a visible light-mediated catechol-assisted direct C8 alkylation of the guanines in guanosine (together with its congeners), guanosine-containing dinucleotides, and oligonucleotides. This robust methodology can also be applied to the late-stage functionalization of drugs and some fragile biomolecules, such as GMP, GDP, and GTP. The realization of site-selective post-functionalization of 20 oligonucleotides indicates its potential application values in the areas of medicinal science, chemical biology, biotechnology, and nanotechnology other than organic chemistry.
Methods
General procedure for the C8–H alkylation of nucleoside
To a 10 mL Schlenk tube containing a Teflon stir bar was charged with nucleoside substrates (0.2 mmol, 1.0 equiv.), alkylboronic acids or alkyltriuoroborate (0.8 mmol, 4.0 equiv.), MesAcr (0.01 mmol, 0.05 equiv.), (NH4)2S2O8 (0.4 mmol, 2.0 equiv.), catechol (0.2 mmol, 1.0 equiv.), 1.0 mL CH3CN, 1.0 mL H2O, and trifluoroacetic acid (0.1 mmol, 1.0 equiv.) sequentially. The Schlenk tube was sealed with a rubber plug and taped, utilizing the freeze-pump-thaw (FPT) method for deaeration. The reaction system was exposed to an 85 W white light, monitoring the progress of the reaction by thin layer chromatography (TLC) (DCM/MeOH = 5/1) or liquid chromatography-mass spectrometry (LC-MS). The mixture was concentrated under reduced pressure, and the crude product was purified by silica gel flash column chromatography (C18 Spherical silica) using MeOH/H2O as an eluent to give the pure product.
General procedure for the C8–H alkylation of complex nucleotide substrates and RNA/DNA Oligonucleotides
First, preparing aqueous solutions of the complex nucleotide substrates (0.01 mM), alkylboronic acids (4.0 mM), (NH4)2S2O8 (2.0 mM), and trifluoroacetic acid (0.5 mM), as well as the acetonitrile solutions of MesAcr (0.005 mM) and catechol (1.0 mM). Then, utilizing a suitable pipette gun, sequentially, to take 210 µL H2O, 190 µL MeCN (ensuring the total amount of H2O and acetonitrile is 250 mL respectively), 10 µL of complex nucleotide substrates solution (0.10 µmol, 1 equiv.), 10 µL of alkylboronic acids solution (40 µmol, 400 equiv.), 10 µL of (NH4)2S2O8 solution (20 µmol, 200 equiv.), 20 µL of MesAcr solution (0.050 µmol, 0.5 equiv.), 40 µL of catechol solution (10 µmol, 100 equiv.), and 10 µL of trifluoroacetic acid solution (5.0 µmol, 50 equiv.) into a 5 mL Schlenk tube containing a Teflon stir bar, sealed with a rubber plug, and taped, utilizing the FPT method for deaeration, liquid nitrogen for freeze, and argon as an inert gas (see Supplementary Information for specific analysis procedure). Pay attention to safety when using this system, and keep the Schlenk Line in the state of blowing argon when thawing, which is to maintain atmospheric pressure and an inert gas atmosphere. The time from mixing various solutions and solvents to freezing and vacuuming should not exceed ~10 minutes. Then, the reaction system was exposed to an 85 W white light. The yield and recovery of the reaction are detected by LC-MS (see Supplementary Information for specific analysis method). The operation steps for RNA/DNA Oligonucleotide are basically the same as those above, while diethyl pyrocarbonate-treated water (DEPC-treated Water) (RNA enzyme and DNA enzyme free) was used instead of H2O, as well as dissolving the sub-packed RNA/DNA Oligonucleotide (100 nmol) with 90 µL (30 µL × 3 times) DEPC water.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Details about materials and methods, experimental procedures, mechanistic studies, and NMR spectra are available in the Supplementary Information.
References
Shelton, J. et al. Metabolism, biochemical actions, and chemical synthesis of anticancer nucleosides, nucleotides, and base analogs. Chem. Rev. 116, 14379–14455 (2016).
Seley-Radtke, K. L. & Yates, M. K. The evolution of nucleoside analogue antivirals: a review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antivir. Res. 154, 66–86 (2018).
Yates, M. K. & Seley-Radtke, K. L. The evolution of antiviral nucleoside analogues: a review for chemists and non-chemists. Part II: Complex modifications to the nucleoside scaffold. Antivir. Res. 162, 5–21 (2019).
Roy, B., Depaix, A., Périgaud, C. & Peyrottes, S. Recent trends in nucleotide synthesis. Chem. Rev. 116, 7854–7897 (2016).
Hollenstein, M. Nucleoside triphosphates-building blocks for the modification of nucleic acids. Molecules 17, 13569–13591 (2012).
Xu, W., Chan, K. M. & Kool, E. T. Fluorescent nucleobases as tools for studying DNA and RNA. Nat. Chem. 9, 1043–1055 (2017).
Gillingham, D., Geigle, S. & von Lilienfeld, O. Properties and reactivity of nucleic acids relevant to epigenomics, transcriptomics, and therapeutics. Chem. Soc. Rev. 45, 2637–2655 (2016).
Chemical synthesis of nucleoside analogues. (eds. Merino, P.) (John Wiley & Sons, 2013).
Oligonucleotide Synthesis: methods and application. (Springer Science & Business Media, 2005).
McKenzie, L. K., El-Khoury, R., Thorpe, J. D., Damha, M. J. & Hollenstein, M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 50, 5126–5164 (2021).
Yuan, Y. & Wang, W. Post-synthetic chemical functionalization of oligonucleotides. Curr. Org. Synth. II 9, 463–493 (2014).
Ito, Y. & Hari, Y. Synthesis of nucleobase-modified oligonucleotides by post-synthetic modification in solution. Chem. Rec. 22, e202100325 (2022).
Moir, M., Danon, J. J., Reekie, T. A. & Kassiou, M. an overview of late-stage functionalization in today’s drug discovery. Expert. Opin. Drug. Discov. 14, 1137–1149 (2019).
Hong, B., Luo, T. & Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 6, 622–635 (2020).
Lechner, V. M. et al. Visible-light-mediated modification and manipulation of biomacromolecules. Chem. Rev. 122, 1752–1829 (2021).
Van Aerschot, A. A. et al. Antiviral activity of C-alkylated purine nucleosides obtained by cross-coupling with tetraalkyltin reagents. J. Med. Chem. 36, 2938–2942 (1993).
Läppchen, T. et al. Probing FtsZ and tubulin with C8-substituted GTP analogs reveals differences in their nucleotide binding sites. Chem. Biol. 15, 189–199 (2008).
Chang, L. C. W. et al. 2,6-Disubstituted and 2,6,8-trisubstituted purines as adenosine receptor antagonists. J. Med. Chem. 49, 2861–2867 (2006).
Hirota, K., Kitade, Y., Kanbe, Y. & Maki, Y. Convenient method for the synthesis of C-alkylated purine nucleosides: palladium-catalyzed cross-coupling reaction of halogenopurine nucleosides with trialkylaluminums. J. Org. Chem. 57, 5268–5270 (1992).
Cahová, H. et al. Synthesis of 8-bromo-, 8-methyl- and 8-phenyl-dATP and their polymerase incorporation into DNA. Org. Biomol. Chem. 6, 3657–3660 (2008).
Maeda, M., Nushi, K. & Kawazoe, Y. Studies on chemical alterations of nucleic acids and their components—VII: C-alkylation of purine bases through free radical process catalyzed by ferrous ion. Tetrahedron 30, 2677–2682 (1974).
Pless, R., Dudycz, L., Stolarski, R. & Shugar, D. Purine nucleosides and nucleotides unequivocally in the syn conformation: guanosine and 5′-GM P with 8-tert-Butyl and 8-(α-hydroxyisopropyl) substituents. Z. Naturforsch. C. J. Biosci. 33, 902–907 (1978).
Xia, R., Niu, H. Y., Qu, G. R. & Guo, H. M. CuI controlled C–C and C–N bond formation of heteroaromatics through C (sp3)–H activation. Org. Lett. 14, 5546–5549 (2012).
Revil-Baudard, V. L., Vors, J. P. & Zard, S. Z. Xanthate-mediated incorporation of quaternary centers into heteroarenes. Org. Lett. 20, 3531–3535 (2018).
Omumi, A., Beach, D. G., Baker, M., Gabryelski, W. & Manderville, R. A. Postsynthetic guanine arylation of DNA by Suzuki−Miyaura cross-coupling. J. Am. Chem. Soc. 133, 42–50 (2010).
Xie, Y. et al. 6-iodopurine as a versatile building block for RNA purine architecture modifications. Bioconjug. Chem. 33, 353–362 (2022).
Xie, L. J. et al. Identification of flavin mononucleotide as a cell‐active artificial N6‐methyladenosine RNA demethylase. Angew. Chem. Int. Ed. 58, 5028–5032 (2019).
Nappi, M., Hofer, A., Balasubramanian, S. & Gaunt, M. J. Selective chemical functionalization at N6-methyladenosine residues in DNA enabled by visible-light-mediated photoredox catalysis. J. Am. Chem. Soc. 142, 21484–21492 (2020).
Bhoge, B. A., Mala, P., Kurian, J. S., Srinivasan, V. & Saraogi, I. Selective functionalization at N2-position of guanine in oligonucleotides via reductive amination. Chem. Comm. 56, 13832–13835 (2020).
Weng, X. et al. Keth-seq for transcriptome-wide RNA structure mapping. Nat. Chem. Biol. 16, 489–492 (2020).
Zhao, Q. et al. Palladium-catalyzed C–H olefination of uridine, deoxyuridine, uridine monophosphate and uridine analogues. RSC Adv. 12, 24930–24934 (2022).
Wang, H.-N., Bag, R., Karuppu, S., Chen, G. & Xiao, G. C-H functionalization‐based strategy in the synthesis and late-stage modification of drug and bioactive molecules. In: Handbook of CH‐functionalization (eds. Maiti, D.). https://doi.org/10.1002/9783527834242.chf0217. (John Wiley & Sons, 2022).
Matsui, J. K., Primer, D. N. & Molander, G. A. Metal-free C–H alkylation of heteroarenes with alkyltrifluoroborates: a general protocol for 1°, 2° and 3° alkylation. Chem. Sci. 8, 3512–3522 (2017).
Josephson, B. et al. Light-driven post-translational installation of reactive protein side chains. Nature 585, 530–537 (2020).
Pillitteri, S., Ranjan, P., Van der Eycken, E. V. & Sharma, U. K. Uncovering the potential of boronic acid and derivatives as radical source in photo(electro)chemical reactions. Adv. Synth. Catal. 364, 1643–1665 (2022).
Corcé, V., Ollivier, C. & Fensterbank, L. Boron, silicon, nitrogen and sulfur-based contemporary precursors for the generation of alkyl radicals by single electron transfer and their synthetic utilization. Chem. Soc. Rev. 51, 1470–1510 (2022).
Hinkes, S. P. A. & Klein, C. D. P. Virtues of volatility: a facile transesterification approach to boronic acids. Org. Lett. 21, 3048–3052 (2019).
Genossar, N. et al. Ring-opening dynamics of the cyclopropyl radical and cation: the transition state nature of the cyclopropyl cation. J. Am. Chem. Soc. 144, 18518–18525 (2022).
Crooke, S. T., Baker, B. F., Crooke, R. M. & Liang, X. Antisense technology: an overview and prospectus. Nat. Rev. Drug Discov. 20, 427–453 (2021).
Setten, R. L., Rossi, J. J. & Han, S. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18, 421–446 (2019).
Wan, W. B. & Seth, P. P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 59, 9645–9667 (2016).
Burrows, C. J. & Muller, J. G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 98, 1109–1152 (1998).
Human herpesviruses: biology, therapy, and immunoprophylaxis. (eds. A. Arvin et al.) (Cambridge University Press, 2007).
Herdewijn, P. A. M. M. 5-Substituted-2′-deoxyuridines as anti-HSV-1 agents: synthesis and structure activity relationship. Antivir. Chem. Chemother. 5, 131–146 (1994).
Bergstrom, D. E. & Ogawa, M. K. C-5 substituted pyrimidine nucleosides. 2. Synthesis via olefin coupling to organopalladium intermediates derived from uridine and 2’-deoxyuridine. J. Am. Chem. Soc. 100, 8106–8112 (1978).
Hassan, M. E. Photochemical synthesis of 5-alkylpyrimidine nucleosides. Rec. Trav. Chim. Pays-Bas. 105, 30–32 (1986).
Larock, R. C., Wang, Y., Dong, X. & Yao, T. Synthesis of C-5 substituted nucleosides via palladium-catalyzed coupling of dienes and amines. Tetrahedron 61, 11427–11439 (2005).
Wang, D.-C., Xia, R., Xie, M.-S., Qu, G.-R. & Guo, H.-M. Synthesis of cycloalkyl substituted purine nucleosides via a metal-free radical route. Org. Biomol. Chem. 14, 4189–4193 (2016).
Zlatev, I. et al. Phosphoramidate dinucleosides as hepatitis C virus polymerase inhibitors. J. Med. Chem. 51, 5745–5757 (2008).
Priet, S. et al. 3′-Deoxy phosphoramidate dinucleosides as improved inhibitors of hepatitis C virus subgenomic replicon and NS5B polymerase activity. J. Med. Chem. 53, 6608–6617 (2010).
Weinreich, J. et al. Cytostatic activity of the duplex drug linking 2′-deoxy-5-fluorouridine (5FdU) with 3′-C-ethynylcytidine (ECyd) against gastric adenocarcinoma cell lines. Invest. New Drugs 29, 1294–1302 (2011).
Eicher, C. et al. Effect of duplex drugs linking 2′-deoxy-5-fluorouridine (5-FdU) with 3′-C-ethynylcytidine (ECyd) on hepatoblastoma cell lines. Pediatr. Surg. Int. 29, 121–127 (2013).
Schott, S. & Brüning, A. Induction of apoptosis in cervical cancer cells by the duplex drug 5-FdU–ECyd, coupling 2′-deoxy-5-fluorouridine and 3′-C-ethinylcytidine. Gynecol. Oncol. 135, 342–348 (2014).
Krause, A., Hertl, A., Muttach, F. & Jäschke, A. Phosphine-free stille-migita chemistry for the mild and orthogonal modification of DNA and RNA. Chem. Eur. J. 20, 16613–16619 (2014).
Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 24, 374–387 (2014).
Vavřina, Z. et al. Design, synthesis, and biochemical and biological evaluation of novel 7-deazapurine cyclic dinucleotide analogues as sting receptor agonists. J. Med. Chem. 65, 14082–14103 (2022).
Van Poecke, S. et al. 3′-[4-Aryl-(1,2,3-triazol-1-yl)]−3′-deoxythymidine analogues as potent and selective inhibitors of human mitochondrial thymidine kinase. J. Med. Chem. 53, 2902–2912 (2010).
Fantoni, N. Z., El-Sagheer, A. H. & Brown, T. A hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 121, 7122–7154 (2021).
Wang, J. et al. Kinetically guided radical-based synthesis of C(sp3)-C(sp3) linkages on DNA. Proc. Natl. Acad. Sci. 115, E6404–E6410 (2018).
Sýkorová, V., Tichý, M. & Hocek, M. Polymerase synthesis of DNA containing iodinated pyrimidine or 7‐deazapurine nucleobases and their post‐synthetic modifications through the Suzuki‐Miyaura cross‐coupling reactions. Chembiochem. 23, 1–8 (2021).
Walunj, M. B. & Srivatsan, S. G. Nucleic acid conformation influences postsynthetic Suzuki–Miyaura labeling of oligonucleotides. Bioconjug. Chem. 31, 2513–2521 (2020).
Walunj, M. B., Tanpure, A. A. & Srivatsan, S. G. Post-transcriptional labeling by using Suzuki–Miyaura cross-coupling generates functional RNA probes. Nucleic Acids Res. 46, e65 (2018).
Ivancová, I., Leone, D.-L. & Hocek, M. Reactive modifications of DNA nucleobases for labelling, bioconjugations, and cross-linking. Curr. Opin. Chem. Eng. 52, 136–144 (2019).
Acknowledgements
This work is supported by the Natural Science Foundation of Qinghai Province (2023-ZJ-917M, G.C.), the National Natural Science Foundation of China (NSFC) (Grant no. 22001165 and 22271186, G.C.) and Shanghai Jiao Tong University (SJTU). We thank professor Juntao Ye (SJTU) for the helpful discussion. We also thank Dr. Raghunath Bag (SJTU) and Dr. Karuppu Selvaraj (SJTU) for their editorial advice on manuscript preparation.
Author information
Authors and Affiliations
Contributions
Y.G. and G.C. conceived and directed the project. R.X., W.L., Y.Z., G.X., Q.Z., Y.H. and Y.L. performed the experiments and analyzed the data. G.C. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
A patent application by G.C., Y.G., W.L., R.X., G.X. and Y.L. detailing part of this research was filed through the Patent Office of the People’s Republic of China (June 2022). The other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Michal Hocek, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, R., Li, W., Ge, Y. et al. Late-stage guanine C8–H alkylation of nucleosides, nucleotides, and oligonucleotides via photo-mediated Minisci reaction. Nat Commun 15, 2549 (2024). https://doi.org/10.1038/s41467-024-46671-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46671-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.