Abstract
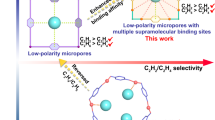
Iso-butene (iso-C4H8) is an important raw material in chemical industry, whereas its efficient separation remains challenging due to similar molecular properties of C4 olefins. The ideal adsorbent should possess simultaneous high uptakes for 1,3-butadiene (C4H6) and n-butene (n-C4H8) counterparts, endowing high efficiency for iso-C4H8 separation in adsorption columns. Herein, a sulfate-pillared adsorbent, SOFOUR-DPDS-Ni (DPDS = 4,4′-dipyridyldisulfide), is reported for the efficient iso-C4H8 separation from binary and ternary C4 olefin mixtures. The rigidity in pore sizes and shapes of SOFOUR-DPDS-Ni exerts the molecular sieving of iso-C4H8, while exhibiting high C4H6 and n-C4H8 uptakes. The benchmark Henry’s selectivity for C4H6/iso-C4H8 (2321.8) and n-C4H8/iso-C4H8 (233.5) outperforms most reported adsorbents. Computational simulations reveal the strong interactions for C4H6 and n-C4H8. Furthermore, dynamic breakthrough experiments demonstrate the direct production of high-purity iso-C4H8 (>99.9%) from C4H6/iso-C4H8 (50/50, v/v), n-C4H8/iso-C4H8 (50/50, v/v), and C4H6/n-C4H8/iso-C4H8 (50/15/35, v/v/v) gas-mixtures.
Similar content being viewed by others
Introduction
Iso-butene (iso-C4H8) is a crucial feedstock for the production of butyl rubber, tert-butanol, and methyl tert-butyl ether (MTBE), with an annual consumption exceeding 30 million tons1. Generally, the steam cracking of naphtha generates C4 hydrocarbon mixtures containing 30~60% 1,3-butadiene (C4H6), 10~20% n-butene (n-C4H8), and 10~30% iso-C4H82. While, the stringent purity requirement for polymer-grade iso-C4H8 (>99.5%) necessitates mandatory purification processes3. In industry, C4H6 is removed from C4 hydrocarbons in high extractive distillation towers (more than 110 trays) at harsh conditions of 3 bar and 323~393 K4. Furthermore, thermal-derived methods are inadequate for separating n-C4H8 and iso-C4H8 due to their subtle difference in boiling points (0.6 °C, Supplementary Table 2)3. The high-purity iso-C4H8 is obtained through the cracking of MTBE, the reaction product of iso-C4H8 fraction in C4 mixtures and methanol using strong acidic ion exchange resins as catalysts2. The excessive consumption of organic solvents and energy in traditional separation methods emphasizes the need for an energy- and cost-efficient strategy for iso-C4H8 purification from C4H6 and n-C4H8.
Physisorption utilizing porous adsorbents, e.g., zeolites and metal-organic frameworks (MOFs)5,6,7,8,9,10,11,12,13, shows great promise in various challenging gas separations, including C2H2/C2H4, CO2/C2H2, and n-C4H10/iso-C4H1014,15,16,17,18,19,20,21,22. The development of advanced adsorbents capable of recognizing the subtle differences in molecular shapes, sizes, and properties among C4 counterparts remain a formidable challenge (Supplementary Fig. 1 and Supplementary Table 2). Namely, zeolite DD3R exhibited unsatisfactory adsorption capacities (0.832 mmol g−1 for C4H6), resulting in low separation efficiency15. In recent examples, adsorbents indiscriminately adsorbed C4 components with unsaturated bonds through open metal sites and high-polar pillars, causing significant co-adsorption and low iso-C4H8 recovery in fixed-bed columns23,24. The ideal adsorbent for iso-C4H8 purification from C4 olefins should possess high simultaneous adsorption capacities for both C4H6 and n-C4H8 while preventing the co-adsorption of iso-C4H8 in order to achieve optimal separation efficiency (Fig. 1a). To date, achieving complete molecular sieving of iso-C4H8 remains challenging for MOF adsorbents1,24. For example, Prof. Eddaoudi’s group reported Y-fum with a suitable aperture size of ~4.7 Å exhibited molecular sieving capabilities for iso-C4H10 and iso-C5H12 from their corresponding n-paraffins25. The successful synthesis of this type of adsorbent remains a formidable challenge, which is rarely reported thus far26,27,28.
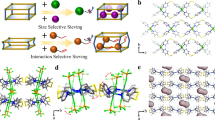
Schematic illustration of a adsorption behaviors on ideal adsorbents and b structure changes of adsorbents linked by linear and angular pillars after activation; c Building blocks (Ni2+, SO42−, and DPDS organic ligand) and local coordination environment of metal atoms; d SOFOUR-DPDS-Ni structure along the X-axes.
Sulfate anions (SO42-) possessing abundant lone pair electrons facilitate the formation of coordination bonds with metal ions29. Zaworotko et al., reported the first sulfate-pillared hybrid ultra-microporous adsorbent (SOFOUR-1-Zn) in 202130. Our group has recently demonstrated the exceptional molecule recognition ability of SO42- groups in a sulfate-pillared adsorbent (SOFOUR-TEPE-Zn, TEPE = 1,1,2,2-tetra(pyridin-4-yl) ethene), which exhibited a benchmark selectivity of 16,833 for C2H2/CO2 separation31. For separation purposes, the rotation of linear pillars (e.g., SiF62−, TiF62−, GeF62−) in anion-pillared adsorbents induced by interactions with adsorbates may compromise the yield and purity of iso-C4H8 due to its potential entrance and co-adsorption20,24. Moreover, the densely packed pore channels resulting from layer-to-layer sliding after solvent removal may lead to high gate-opening pressures, causing poor adsorption capacity and limited diffusion rate (Fig. 1b)32,33. In sharp contrast, the inflexibility of tetrahedral SO42- anions enables the preservation of pore shapes and sizes during activation and adsorption processes34, thereby conferring limited iso-C4H8 adsorption/diffusion in adsorbents.
Herein, we report a SO42−-anion pillared adsorbent, SOFOUR-DPDS-Ni (DPDS = 4,4′-dipyridyldisulfide), for efficient iso-C4H8 separation from C4 olefins. The adsorption isotherms demonstrate simultaneous high uptakes of C4H6 and n-C4H8 while almost excluding iso-C4H8. Notably, the C4H6 adsorption capacity reaches a high value of 1.15 mmol g−1 at 0.001 bar (1000 ppm), indicating its potential for removing trace amounts of C4H6 from C4 olefin mixtures. The Henry’s selectivity is exceptional with values of 2321.8 and 233.5 for C4H6/iso-C4H8 and n-C4H8/iso-C4H8 at 298 K. Computational simulations confirm that C4H6 and n-C4H8 exhibit strong interactions with SO42− pillars and pyridyl rings through multiple C-H∙∙∙O, C-H∙∙∙C, and C-H∙∙∙H interactions. Charge bias analysis reveals the charge shifts of positive H atoms in C4H6 and n-C4H8 to negative states, while O atoms in SO42- pillars shift towards positive potential. Furthermore, dynamic breakthrough experiments demonstrate the direct production of high-purity iso-C4H8 (>99.9%) from binary and ternary components mixtures.
Results
Structure characterization
The reaction of NiSO4·6H2O and DPDS in methanol solutions at room temperature yielded light blue powder of SOFOUR-DPDS-Ni with the chemical formula of Ni(DPDS)2SO4 (Fig. 1c, see methods for details). Note that the synthesis must be conducted under anhydrous conditions as H2O molecules possess a significantly stronger coordination ability compared to SO42− anions, and may therefore occupy the coordination sites for Ni2+ ions35. Despite numerous attempts, high-quality single crystals of SOFOUR-DPDS-Ni could not be obtained for single crystal X-ray diffraction analysis. The Rietveld refinements of powder X-ray diffraction (PXRD) data revealed that the as-synthesized SOFOUR-DPDS-Ni crystallizes in the orthorhombic crystal system with specific cell parameters of a = 10.5377, b = 14.2957, c = 19.8289 (Supplementary Fig. 2 and Supplementary Table 8). The well matched PXRD and simulated XRD patterns confirmed the high phase-purity of bulk SOFOUR-DPDS-Ni (Supplementary Fig. 3). Each Ni2+ ion was coordinated with four pyridyl N atoms from four independent DPDS ligands in a distorted octahedral environment, forming one-dimensional chains of [Ni(DPDS)2]n (Supplementary Fig. 4). These chains were further pillared by two SO42− anions in the axial direction, generating two-dimensional (2D) layers of [SOFOUR-DPDS-Ni]n with the sql topology. Adjacent 2D layers are assembled into three-dimensional structures via multiple π-π stacking interactions among pyridyl rings and p-π interactions between S atoms and pyridyl rings (Fig. 1d)36. The pore sizes of resulting intralayer and interlayer channels were measured to be 3.3 × 4.5 and 3.6 × 3.7 Å2, respectively (Supplementary Fig. 5).
Thermogravimetric analysis (TGA) revealed the removal of guest molecules at 383 K, and demonstrated structural stability up to 473 K under N2 atmosphere (Supplementary Fig. 6). The permanent porosity of activated SOFOUR-DPDS-Ni was probed by CO2 gas sorption isotherms at 195 K, and the Brunauer-Emmett-Teller (BET) specific surface area was calculated to be 270 m2 g−1 with a total pore volume of 0.15 cm3 g−1 (Supplementary Fig. 7). Whereas, the N2 adsorption in SOFOUR-DPDS-Ni was limited at 77 K. Based on the Horvath-Kawazoe model, the experimental pore size distributions exhibited a centered pore size of approximately 4.7 Å (Supplementary Fig. 8), which was consistent with the dimensions of intralayer cavities (5.3 × 4.5 × 5.8 Å3) derived from the simulated crystal structure. The pore sizes of SOFOUR-DPDS-Ni were larger than the kinetic diameters of C4H6 (4.31 Å) and n-C4H8 (4.46 Å), yet smaller than that of iso-C4H8 (4.84 Å), suggesting the potential molecular sieving of iso-C4H8 from C4H6 and n-C4H8 counterparts. Additionally, the adsorption isotherms exhibited a high C2H2 uptake (2.87 mmol g−1) and the moderate CO2 uptake (1.36 mmol g−1) at 298 K and 1.0 bar (Supplementary Fig. 9). The C2H2/CO2 separation performance was considerably inferior compared to the molecular sieving effect on SO42−-pillared SOFOUR-TEPE-Zn31.
The PXRD pattern remained unchanged after activation, indicating the rigidity of SOFOUR-DPDS-Ni (Supplementary Fig. 3). Due to the presence of angular SO42− pillars connecting adjacent 2D layers, the obtained unparallel stacking effectively prevented layer-to-layer sliding after removal of guest molecules (Fig. 1b). Additionally, no gate-opening or step-wise adsorption behavior was observed in the measured adsorption isotherms of C4H6 and n-C4H8 at different temperatures (Supplementary Fig. 10). The excellent chemical stability was demonstrated by its ability to withstand soaking in various organic solvents for one week, hot water at 60 °C for 2 h, and exposure to air for 13 months (Supplementary Fig. 11). The ultimate elemental analysis demonstrated that the element composition of each element corresponded well with the theoretical formula of SOFOUR-DPDS-Ni (C20H16N4O4S5Ni, Supplementary Table 1). For instance, the measured content ratio of N/S (0.39) was closely matched to the theoretical value (0.35). The scanning electron microscopy (SEM) image revealed a block morphology of SOFOUR-DPDS-Ni (Supplementary Fig. 12). Fourier transform infrared spectroscopy (FT-IR) spectra exhibited characteristic peaks corresponding to stretching vibrations for Ni-O at 493.2 cm−1 and S-O in SO42− at 1058.3 cm−1. Detailed discussions regarding FT-IR results are presented below Supplementary Fig. 13.
Gas adsorption and separation behaviors
Single-component gas adsorption isotherms of C4H6, n-C4H8, and iso-C4H8 were collected on SOFOUR-DPDS-Ni (Fig. 2a and Supplementary Fig. 10). The adsorption capacity for C4H6 and n-C4H8 was measured to be 1.68 and 1.48 mmol g−1 at 298 K and 1.0 bar, respectively. In contrast, iso-C4H8 was almost completely excluded with a negligible uptake of only 0.17 mmol g−1. The kinetic adsorption curve disclosed that C4H6 reached adsorption equilibrium within 5 min, while n-C4H8 required a longer time of ~30 min to reach equilibrium (Fig. 2b). Whereas, iso-C4H8 exhibited negligible adsorption even after an extended exposure of 35 min. The kinetic adsorption capacity for C4H6 and n-C4H8 was determined to be 1.47 and 1.17 mmol g−1 at 0.5 bar, which were comparable to their static adsorption uptakes. The diffusion time constant (D/r2) was calculated to be 1.29 × 10−3 s−1 for C4H6 and 2.72 × 10−4 s−1 for n-C4H8, suggesting a faster diffusion rate for C4H6 in SOFOUR-DPDS-Ni (Supplementary Fig. 14). Furthermore, molecular dynamic (MD) simulations demonstrated that the diffusion coefficient of C4H6 (4.82 × 10−11) was faster than that of n-C4H8 (1.02 × 10−11) at 298 K (Supplementary Figs. 15 and 16). The kinetic separation selectivity of 4.7 for C4H6/n-C4H8 indicated a limited kinetic contribution to the apparent selectivity. Benefiting from the molecular sieving effect of iso-C4H8, uptake ratios were utilized as an intuitive measure of selectivity for separating C4 olefins. At 1.0 bar and 298 K, the uptake ratios of C4H6/iso-C4H8 and n-C4H8/iso-C4H8 on SOFOUR-DPDS-Ni were calculated to be 9.9 and 8.7, surpassing most top-ranking adsorbents including TMOF-1 (8.2 and 5.4)1, ZU-52 (5.7 and 5.0)24, and SIFSIX-3-Ni (4.7 and 4.7)24 (Fig. 2c and Supplementary Table 6). In comparison, we measured the adsorption isotherms of C4 olefins on SOFOUR-1-Zn and SOFOUR-TEPE-Zn at 298 K (Supplementary Fig. 17). SOFOUR-1-Zn adsorbed comparable uptakes for n-C4H8 (0.72 mmol g−1) and iso-C4H8 (0.55 mmol g−1). Meanwhile, negligible n-C4H8 and iso-C4H8 uptakes (<0.13 mmol g−1) were observed on SOFOUR-TEPE-Zn. These inferior performances on SO42−-pillared adsorbents further highlighted the advantages of pore environments of SOFOUR-DPDS-Ni.
a Pure-component isotherms at 298 K and b kinetic adsorption curves at 298 K and 0.5 bar for C4H6, n-C4H8, and iso-C4H8; c Comparison of uptake ratios of C4H6/iso-C4H8 and n-C4H8/iso-C4H8 at 1.0 bar; d Adsorption isotherms in logarithmic form for C4H6, n-C4H8, and iso-C4H8 at 298 K; e Comparison of uptakes of C4H6 and n-C4H8 at 0.01 bar; f Comparison of Henry’s selectivity for C4H6/iso-C4H8 and n-C4H8/iso-C4H8.
Remarkably, SOFOUR-DPDS-Ni exhibited high adsorption capacity of 1.36 mmol g−1 for C4H6 and 1.25 mmol g−1 for n-C4H8 at a low pressure of 0.01 bar (Fig. 2d). At even lower pressure of 0.001 bar (1000 ppm), the C4H6 uptake unprecedently reached 1.15 mmol g−1, suggesting its potential removal of trace C4H6 from C4 olefin mixtures. The adsorption affinities for C4H6 and n-C4H8 were further confirmed by the isosteric heats of adsorption (Qst) using the Clausius-Clapeyron equation37 (Supplementary Fig. 18). Specifically, the Qst was calculated to be 77 kJ mol−1 and 38 kJ mol−1 for C4H6 and n-C4H8 at near zero coverage, respectively (Supplementary Fig. 19). The high uptakes of both C4H6 and n-C4H8 established a new benchmark for iso-C4H8 separation, outperforming most adsorbents including ZU-3224, TMOF-1 1, and Ni-gallate38 (Fig. 2e). Moreover, the Henry’s constant of C4H6 and n-C4H8 was calculated to be 50.87 and 5.11 mmol g−1 kPa−1, which were significantly higher than that of iso-C4H8 (0.022 mmol g−1 kPa−1). As a result, the Henry’s selectivity for C4H6/iso-C4H8 and n-C4H8/iso-C4H8 on SOFOUR-DPDS-Ni reached high values of 2321.8 and 233.5 at 298 K, respectively. As shown in Fig. 2f, these values were much higher than other reported adsorbents such as TMOF-1 (519.2 and 93.2)1, ZU-33 (228.7 and 2.4)24, and ZU-52 (75.2 and 48.9)24 (Supplementary Table 7).
Simulation studies
Grand Canonical Monte Carlo (GCMC) and first-principles dispersion-corrected density function theory (DFT-D) simulations were employed to elucidate the adsorption mechanism of C4 olefins on SOFOUR-DPDS-Ni. The distribution densities of C4H6 and n-C4H8 were investigated via GCMC simulations at 0.01 bar and 1.0 bar, both gas molecules were adsorbed in the intralayer spaces near SO42- pillars (Fig. 3a, b). It is noteworthy that the distribution densities of C4H6 and n-C4H8 were nearly identical at pressures of 0.01 and 1.0 bar (Supplementary Figs. 21 and 22), which aligned with their early adsorption saturation at low pressures in SOFOUR-DPDS-Ni. Moreover, four C4H6 or n-C4H8 molecules were adsorbed per unit cell, their uptakes were accordingly calculated to be 1.68 mmol g−1 at 1.0 bar, which were closely matched to their experimental uptakes.
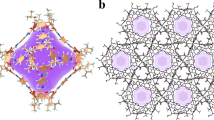
Distribution density for a C4H6 and b n-C4H8 in SOFOUR-DPDS-Ni at 1.0 bar; Adsorption binding site for c C4H6 and d n-C4H8 in SOFOUR-DPDS-Ni; Charge density difference plots of e C4H6-loaded and f n-C4H8-loaded structure; Illustration of diffusion pathway and corresponding energy levels g C4H6 and h n-C4H8; i Comparison of diffusion energy barriers for C4H6, n-C4H8, and iso-C4H8.
DFT-D simulations revealed two favorable adsorption sites for C4H6 in the interlayer cavities of SOFOUR-DPDS-Ni, which were captured by SO42− anions through multiple C-H∙∙∙O interactions with distances of 2.41–2.98 Å and pyridine rings through multiple van der Walls interactions of C-H∙∙∙C and C-H∙∙∙H with distances of 2.77–3.10 Å (Fig. 3c and Supplementary Fig. 23a). The calculated static binding energy (∆E) for Site I and Site II were estimated to be 78.3 and 80.6 kJ mol−1, respectively (Supplementary Fig. 24). Due to the conformational changes of n-C4H8, four favorable adsorption sites for n-C4H8 were identified, while the resulting interactions were comparable to those of C4H6. Specifically, n-C4H8 molecules strongly interacted with SO42− anions through multiple C-H∙∙∙O interactions with distances of 2.43–2.98 Å and pyridine rings through multiple van der Walls interactions of C-H∙∙∙C and C-H∙∙∙H with distances of 2.24–2.95 Å (Fig. 3d and Supplementary Fig. 23b–d). The calculated ∆E values for the four binding sites were 72.8, 58.3, 64.6, and 71.2 kJ mol−1, respectively (Supplementary Fig. 24). Furthermore, gas-loaded structures were subjected to charge transfer analysis, with the blue and yellow surfaces indicating charge accumulation and depletion, respectively. Upon adsorption, the initial positively charged H atoms in C4H6 and n-C4H8 were shifted to strong negative potentials, while O atoms in SO42- pillars shifted to positive potential (Fig. 3e, f). In addition, charge transfer also occurred between the adsorbed guests and the pyridine rings (Supplementary Fig. 25). Therefore, the strong interaction forces between [SOFOUR-DPDS-Ni]n layers (i.e., SO42− pillars and pyridyl rings) and adsorbed guests (i.e., C4H6 and n-C4H8) stabilized the framework of SOFOUR-DPDS-Ni, thereby endowing it with rigidity during C4 adsorptions.
The energy levels associated with the diffusions processes of C4H6, n-C4H8, and iso-C4H8 into SOFOUR-DPDS-Ni framework were further investigated. The rate-determining step was attributed to the largest energy input required for the energy barrier of transition-states (TS) between surface adsorption TS (II) and diffusion into pores TS (III). The diffusion energy barrier of C4H6 in SOFOUR-DPDS-Ni was determined to be 0.358 eV, indicating its facile transport through interlayer channels (Fig. 3g). The diffusion energy barrier increased to 0.927 eV for n-C4H8, attributing to its larger steric hindrance caused by the methyl group (Fig. 3h). In contrast, the overwhelming diffusion energy barrier for iso-C4H8 (2.712 eV) suggested inhibited diffusions thus causing the negligible adsorption amount (Fig. 3i and Supplementary Fig. 26).
Transient breakthrough experiments
Transient breakthrough experiments were conducted to evaluate the practical separation performances of SOFOUR-DPDS-Ni for C4 mixtures39, which exhibit varying component proportions due to diverse streams from different stream cracking processes (Supplementary Fig. 27). Here, C4 olefin gas-mixtures of C4H6/iso-C4H8 (50/50, v/v), n-C4H8/iso-C4H8 (50/50, v/v), and C4H6/n-C4H8/iso-C4H8 (50/15/35, v/v/v) were selected as representative gas-mixtures for simulating the actual separation process. For C4H6/iso-C4H8 mixture with a flow rate of 1.0 mL min−1, iso-C4H8 was eluted first at the outlet of packed column, while C4H6 was not detected until 57.6 min (Fig. 4a). The kinetic adsorption capacity of C4H6 on SOFOUR-DPDS-Ni was calculated to be 1.48 mmol g−1, which closely approximated its static uptake of 1.59 mmol g−1 at 0.5 bar. High-purity iso-C4H8 (>99.9%) could be collected at the outlet with a productivity of 1.20 mmol g−1. Furthermore, high-purity C4H6 (>99.0%) could be collected from 14.4 min during the desorption process with a productivity of 1.17 mmol g−1 using He purge at 5.0 mL min−1 and 343 K (Supplementary Fig. 28). The efficient separation of C4H6/iso-C4H8 could also be achieved at a slower flow rate of 0.5 mL min−1 with a comparable kinetic C4H6 uptake of 1.51 mmol g−1. Similarly, for n-C4H8/iso-C4H8 gas-mixture, iso-C4H8 immediately broke through the column, followed by n-C4H8 at 39.6 min at 1.0 mL min−1 (Fig. 4b). The dynamic uptake of n-C4H8 was determined to be 1.09 mmol g−1, which was 76.8% of its static adsorption uptake (1.42 mmol g−1) at 0.5 bar. High-purity iso-C4H8 (>99.9%) effluent could be obtained with a productivity of 0.85 mmol g−1. While high-purity n-C4H8 (>99.0%) could also be collected during the desorption process with a productivity of 0.74 mmol g−1 (Supplementary Fig. 29). The kinetic uptake of n-C4H8 increased to 1.28 mmol g−1 (90.1% of static uptake) at 0.5 mL min−1, indicating the slower diffusion rate of n-C4H8 compared to that of C4H6.
Moreover, efficient separation of a ternary gas-mixture containing C4H6/n-C4H8/iso-C4H8 (50/15/35, v/v/v) at 1.0 mL min−1 was successfully achieved using SOFOUR-DPDS-Ni as well. Figure 4c depicted that iso-C4H8 was immediately detected, while C4H6 and n-C4H8 were retained in the column for 46.8 min and 43.2 min, respectively. Their competitive adsorptions will simultaneously occupy the available adsorption sites and inevitably influence their adsorption uptakes. High-purity iso-C4H8 (>99.9%) could be directly collected with a productivity of 0.71 mmol g−1 during a time period of 43.2 min. The reusability of SOFOUR-DPDS-Ni was validated through three successive breakthrough cycles using binary and ternary C4 gas-mixtures (Supplementary Figs. 30–35). The cycling breakthrough points and curve shapes were almost overlapped, indicating consistent separation performance. Furthermore, the kinetic adsorption capacity for C4H6 and n-C4H8 also remained consistent at 0.5 and 1.0 mL min−1 throughout the cycling process (Fig. 4d and Supplementary Fig. 36). Furthermore, the PXRD patterns of SOFOUR-DPDS-Ni remained change after cycled adsorption tests and breakthrough experiments, thereby indicating its exceptional structural stability (Supplementary Fig. 39).
Discussion
In summary, we successfully synthesized the sulfate-pillared adsorbent, SOFOUR-DPDS-Ni, for efficient separation of iso-C4H8 from C4 olefin mixtures. Simultaneous high uptakes for C4H6 and n-C4H8 of 1.68 and 1.48 mmol g−1 at 298 K and 1.0 bar were achieved on SOFOUR-DPDS-Ni, while exhibiting size-sieving effect for iso-C4H8. At low pressures of 0.01 bar, SOFOUR-DPDS-Ni exhibited high adsorption capacity of 1.36 mmol g−1 for C4H6 and 1.25 mmol g−1 for n-C4H8. Notably, at an ultralow pressure of 0.001 bar (1000 ppm), the unprecedented C4H6 adsorption capacity of 1.15 mmol g−1 indicated the potential for trace C4H6 removal. Therefore, the benchmark uptake ratio and Henry’s selectivity for C4H6/iso-C4H8 (9.9 and 2321.8) and n-C4H8/iso-C4H8 (8.7 and 233.5) surpassed most top-ranking adsorbents. GCMC and DFT-D simulations demonstrated the adsorption sites and separation mechanisms. Breakthrough experiments confirmed the practical iso-C4H8 separation performances of SOFOUR-DPDS-Ni from binary and ternary C4 olefins mixtures. The high-purity iso-C4H8 (99.9%) could be directly collected, meanwhile high-purity C4H6 (99.0%) and n-C4H8 (99.0%) could also be obtained during desorption processes.
Methods
Materials
All reagents and solvents were obtained from commercial sources and used without further purification. Nickel sulfate hexahydrate (NiSO4·6H2O, 99.0%, Aladdin), 4,4’-dipyridyl disulfide (C10H8N2S2, 98.0%, Xiya Reagent), and methanol (CH4O, anhydrous, 99.9%, Aladdin). 1,3-butadiene (C4H6, 99.9%), n-butene (n-C4H8, 99.9%), iso-butene (iso-C4H8, 99.9%), N2 (99.999%), He (99.999%), and mixed gas-mixtures of C4H6/iso-C4H8 (50/50, v/v), n-C4H8/iso-C4H8 (50/50, v/v), and C4H6/n-C4H8/iso-C4H8 (50/15/35, v/v/v) were purchased from Nanchang Jiangzhu Gas Co., Ltd (China).
Synthesis of SOFOUR-DPDS-Ni
The material was designated as SOFOUR-DPDS-Ni, with a chemical formula of Ni(DPDS)2SO4. NiSO4·6H2O (0.2 mmol, 0.0526 g) was added to a solution of 4-DPDS (0.4 mmol, 0.0881 g) in 20 mL MeOH and stirred at room temperature for 24 h. SOFOUR-DPDS-Ni was obtained as a light blue powder and washed with 100 mL MeOH, followed by drying for 6 h at room temperature.
Details for Rietveld refinement
We applied the EXPO2014 software to conduct the Rietveld refinement, the 2θ range of 5~60° was used for the refinement. Chebyshev (Background Function) and Pseudo-Voigt (Peak Shape Functions) were applied to refine the structure until the Rwp value converged and the overlay of the observed with refined profiles showed good agreement. Unit cell parameters and fitting reliability are listed in Supplementary Table 8, and we have deposited the CIF in the CCDC database with an identifier number of 2260840.
Characterizations
Powder X-ray diffraction (PXRD) analysis of powder samples was carried out on a PANalytical Empyrean Series 2 diffractometer with Cu Kα radiation ((λ = 1.540598 Å), which operated at 40 kV, 40 mA and a scan speed of 0.0167°, a scan time of 15 s per step and 2θ ranging from 5 to 60° at room temperature. The thermogravimetric analysis (TGA) data were obtained on a NETZSCH Thermogravimetric Analyzer (STA2500) from 25 to 800 °C with a heating rate of 20 °C min−1 under an N2 atmosphere. The contents of C, H, N, and S elements were determined by an Elementar Vario MICRO elemental analyzer with CHNS measurement mode. The SEM images were recorded on a Thermo Scientific Apreo 2C scanning electron microscope with an accelerated voltage of 10 kV. The Fourier transfer infrared spectroscopy (FT-IR) was tested by NICOLET FT-IR spectrometer (iS50 FI-IR), and the resolution was 4 cm−1, the number of scans was 32, and the test wave number was 400–4000 cm−1.
Gas adsorption measurements
Single-component isotherms of C4H6, n-C4H8, and iso-C4H8 were measured up to 1 bar at 283 and 298 K on Micromeritics 3Flex adsorption apparatus (Micromeritics Instruments, USA). The kinetic adsorptions of C4H6, n-C4H8, and iso-C4H8 were obtained on Intelligent Gravimetric Analyzer (IGA−100, HIDEN), and the pressure rise rate is 200 mbar min−1. About 100 mg powder samples were evacuated under high vacuum (<5 μm of Hg) at 70 °C for 12 h before adsorption measurement, and the free space of the system was measured by using helium gas. Liquid nitrogen and dry ice were used for adsorption isotherms at 77 K and 195 K, the pore size distribution was calculated based on CO2 adsorption isotherms at 195 K.
Transient breakthrough experiments
The breakthrough experiments were implemented in a stainless-steel column (4.6 mm inner diameter ×200 mm) manually packed with the weight of 0.9853 g activated SOFOUR-DPDS-Ni. The column was first purged with a He flow (10 mL min−1) at room temperature for 10 h before breakthrough tests. The mixtures of C4H6/iso-C4H8 (50/50, v/v), n-C4H8/iso-C4H8 (50/50, v/v), and C4H6/n-C4H8/iso-C4H8 (50/15/35, v/v/v) was conducted at a flow rate of 0.5 and 1.0 mL min−1, respectively. The outlet gas from the column was monitored using gas chromatography (Panna A91 Plus GC) for continuous sampling gas analysis, and an attached mass flow controller (Seven Star, MC-2SCCM-D, D07 series) was used to control the gas flow rate. After the breakthrough tests, the columns packed with samples were regenerated by purging He gas of 10 mL min−1 at 70 °C for 8 h. For the C4 mixtures of C4H6/iso-C4H8 (50/50, v/v) and n-C4H8/iso-C4H8 (50/50, v/v), desorption process was carried out in order to obtain high-purity C4H6 and n-C4H8 after the breakthrough experiment. The desorption process was conducted under He gas flow rate of 5.0 mL min−1 at 70 °C.
Structural stability tests
The activated samples of about 100 mg were placed in 20 mL vials containing 10 mL different solvents for 7 days, treated in hot water of 60 °C for 2 h, exposed air for thirteen months, respectively. The treated samples were washed with 100 mL MeOH and dried at room temperatures, and then characterized by PXRD measurements to determine whether the sample retains structural integrity.
Grand Canonical Monte Carlo (GCMC) calculations
All the GCMC simulations were performed in Materials Studio package. The framework, C4H6, and n-C4H8 were considered to be rigid during the simulation. The charges for atoms of the SOFOUR-DPDS-Ni and gas components were derived from the Mulliken method. The simulations adopted the fixed pressure task, Metropolis method in sorption module, and the universal force field (UFF). The interaction energy between the adsorbed molecules and the framework was computed through Lennard-Jones 6−12 (LJ) potentials. The cutoff radius was chosen 15.5 Å and the electrostatic interactions were handled using the Ewald summation method. The loading steps and the equilibration steps were 1 × 107, the production steps were 1 × 107.
Density Functional Theory calculations
First-principles density functional theory (DFT) calculations were performed using the Materials Studio’s CASTEP code. All calculations were conducted under the generalized gradient approximation (GGA) with Perdew–Burke–Ernzerhof (PBE). A semiempirical addition of dispersive forces to conventional DFT was included in the calculation to account for van der Waals interactions. The total energy coverage within 0.01 meV atom−1. The optimization process commenced with refine structures of the synthesized materials. The charge transfer analysis on gas-loaded structures was calculated using “Electron density difference” in properties of CASTEP module. Single point energy calculations using Dmol3 module. To obtain the binding energy, an isolated gas molecule placed in a cell unit (with the same cell dimensions as the MOF crystal). The static binding energy was calculated by the equation:
The energy barrier calculation method
The energy barrier calculations were carried out using the Dmol3 module in Materials Studio. The unit cells were optimized until the force acting between atoms was below 0.002 Ha Å−1 with SCF convergence of 10−6. The Global orbital cutoff was 5.2 Å. The diffusion of guest molecules was studied by determining the transition state energies using the climbing nudged elastic band (cNEB) method. Firstly, the surface model and the host structure would be optimized using the refine structures as initial geometries with full structural relaxation. The isolated guest molecules (C4H6, n-C4H8, and iso-C4H8) were placed in unit cell and relaxed as references. Next, the guest molecules were introduced onto the host surface and different locations in the channel pore of the host structure, respectively, followed by a full structural relaxation. Then the optimized configurations of the lowest energy were utilized for the subsequent analysis and calculation. The transition state search calculations were used to capture the transition states with guest transport between the two energy minimum configurations from the host surface to channels.
The energy barrier was determined using the following:
where E(Transition State) is the transition energy, E(Initial State) is the energy of the optimized host-guest structure where guests were introduced onto the host surface.
Calculation of selectivity
The single-component adsorption isotherms of C4 hydrocarbons were correlated by the Langmuir model at low pressure of 0–5 kPa. The Langmuir model was defined as:
where, q is the adsorbed amount of the pure component i (mmol g−1), p is the pressure of the bulk gas at equilibrium (kPa), qm is the saturated adsorption capacities (mmol g−1), b is the affinity parameters of the pure component (kPa−1).
To estimate the separation selectivity of SOFOUR-DPDS-Ni, Henry’s selectivity (αij) was developed and applied, which reflected separation selectivity at low pressure about 0–5 kPa. The Henry’s selectivity (αij) based on equilibrium alone can be calculate from the ratio of Henry’s constants, H=qm×b. The selectivity was defined by the following equation:
Isosteric heat of adsorption
The experiment isosteric heat of adsorption for C4H6 and n-C4H8 were calculated using the data at 283 K and 298 K, which was calculated by the Clausius–Clapeyron equation and was defined as:
where Qst (kJ mol−1) represents the adsorption heat of gas molecular, P (mmHg) and T (K) represent the pressure and temperature, respectively, and R is the universal gas constant. Here, the adsorption heat of each component was determined precisely according to the virial fitting parameters of single-component adsorption isotherms measured at 283 and 298 K, which was calculated as follows:
where the N (mg g−1) is the adsorption amount, and m and n determine the number of items required to precisely fit the adsorption isotherms.
Calculation of kinetic adsorption
The diffusional time constants (D’, D/r2) were calculated by the short-time solution of the diffusion equation assuming a step change in the gas-phase concentration, clean beds initially and micropore diffusion control:
where t (s) is the time, Mt (mmol g−1) is gas uptake at time t, Me (mmol g−1) is the gas uptake at equilibrium, D (m2 s−1) is the diffusivity and r (m) is the radius of the equivalent spherical particle. The slopes of Mt/Me versus t1/2 are derived from the fitting of the plots at 0.5 bar and 298 K, and the pressure rise rate is 200 mbar min−1.
Data availability
All data supporting the finding of this study are available within this article and its Supplementary Information. Crystallographic data for the structure in this article have been deposited at the Cambridge Crystallographic Date Center under deposition nos. CCDC 2260840 (SOFOUR-DPDS-Ni). Correspondence and requests for materials should be addressed to J.W.
References
Cui, J. et al. Geometry control of adsorption sites in sulfonate-pillared hybrid ultramicroporous materials for efficient C4 olefin separations. Chem. Eng. J. 425, 130580 (2021).
Gehre, M., Guo, Z., Rothenberg, G. & Tanase, S. Sustainable separations of C4-hydrocarbons by using microporous materials. ChemSusChem 10, 3947–3963 (2017).
Cui, J. et al. Efficient separation of n‐butene and iso‐butene by flexible ultramicroporous metal‐organic frameworks with pocket‐like cavities. Chem. Asian J. 14, 3572–3576 (2019).
Liao, P., Huang, N., Zhang, W., Zhang, J. & Chen, X. Controlling guest conformation for efficient purification of butadiene. Science 356, 1193–1196 (2017).
Adil, K. et al. Gas/vapour separation using ultra-microporous metal–organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev. 46, 3402–3430 (2017).
Belmabkhout, Y. et al. Hydrocarbon recovery using ultra-microporous fluorinated MOF platform with and without uncoordinated metal sites: I- structure properties relationships for C2H2/C2H4 and CO2/C2H2 separation. Chem. Eng. J. 359, 32–36 (2019).
Mohideen, M. I. H. et al. Upgrading gasoline to high octane numbers using a zeolite-like metal–organic framework molecular sieve with ana-topology. Chem. Commun. 54, 9414–9417 (2018).
Ding, Q. et al. Exploiting equilibrium-kinetic synergetic effect for separation of ethylene and ethane in a microporous metal-organic framework. Sci. Adv. 6, eaaz4322 (2020).
Shan, W. et al. New class of type III porous liquids: a promising platform for rational adjustment of gas sorption behavior. ACS Appl. Mater. Interfaces 10, 32–36 (2018).
Xing, H., Wang, T., Zhou, Z. & Dai, Y. Novel brønsted-acidic ionic liquids for esterifications. Ind. Eng. Chem. Res. 44, 4147–4150 (2005).
Cui, X. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016).
Xing, H., Wang, T., Zhou, Z. & Dai, Y. The sulfonic acid-functionalized ionic liquids with pyridinium cations: acidities and their acidity–catalytic activity relationships. J. Mol. Catal. A Chem. 264, 53–59 (2007).
Cui, X. et al. Ultrahigh and selective SO2 uptake in inorganic anion‐pillared hybrid porous materials. Adv. Mater. 29, 1606929 (2017).
Tijsebaert, B. et al. Liquid phase separation of 1-butene from 2-butenes on all-silica zeolite RUB-41. Chem. Commun. 21, 2480 (2008).
Zhu, W., Kapteijn, F., Moulijn, J. A. & Jansen, J. C. Selective adsorption of unsaturated linear C4 molecules on the all-silica DD3R. Phys. Chem. Chem. Phys. 2, 1773–1779 (2000).
Palomino, M. et al. Pure silica ITQ-32 zeolite allows separation of linear olefins from paraffins. Chem. Commun. 12, 1233–1235 (2007).
Jiao, J., Liu, H., Bai, D. & He, Y. A chemically cross-linked NbO-Type metal–organic framework: cage or window partition. Inorg. Chem. 55, 3974–3979 (2016).
Lange, M. et al. Structural flexibility of a copper-based metal–organic framework: sorption of C4-hydrocarbons and in situ XRD. J. Mater. Chem. A. 2, 8075–8085 (2014).
Luna-Triguero, A. et al. Improving olefin purification using metal organic frameworks with open metal sites. ACS Appl. Mater. Interfaces 10, 16911–16917 (2018).
Liu, Y. et al. Two novel 4,6-connected anion-pillared metal-organic frameworks for simultaneous separation of C3 and C4 olefins. Chem. Eng. Process. 172, 108768 (2022).
Assen, A. H. et al. Kinetic separation of C4 olefins using Y-fum-fcu-MOF with ultra-fine-tuned aperture size. Chem. Eng. J. 413, 127388 (2021).
Zhang, Z. et al. Efficient splitting of trans-/cis-olefins using an anion-pillared ultramicroporous metal–organic framework with guest-adaptive pore channels. Engineering 11, 80–86 (2022).
Barnett, B. R. et al. Thermodynamic separation of 1-butene from 2-butene in metal–organic frameworks with open metal sites. J. Am. Chem. Soc. 141, 18325–18333 (2019).
Zhang, Z. et al. Sorting of C4 olefins with interpenetrated hybrid ultramicroporous materials by combining molecular recognition and size-sieving. Angew. Chem. Int. Ed. 56, 16282–16287 (2017).
Assen, A. H. et al. Ultra‐tuning of the rare‐earth fcu‐MOF aperture size for selective molecular exclusion of branched paraffins. Angew. Chem. Int. Ed. 54, 14353–14358 (2015).
Zhang, Z., Tan, B., Wang, P., Cui, X. & Xing, H. Highly efficient separation of linear and branched C4 isomers with a tailor‐made metal–organic framework. AIChE J. 66, e16236 (2020).
Du, S. et al. Ultramicroporous carbons featuring sub‐Ångstrom tunable apertures for the selective separation of light hydrocarbon. AIChE J. 67, e17285 (2021).
Wang, L. et al. Stepwise engineering the pore aperture of a cage‐like MOF for the efficient separation of isomeric C4 paraffins under humid conditions. Angew. Chem. Int. Ed. 135, e202218596 (2023).
Luo, J. et al. Self‐assembly of three CdII ‐ and CuII ‐ containing coordination polymers from 4,4′‐dipyridyl disulfide. Eur. J. Inorg. Chem. 2003, 3623–3632 (2003).
Sensharma, D. et al. The first sulfate‐pillared hybrid ultramicroporous material, SOFOUR‐1‐Zn, and its acetylene capture properties. Angew. Chem. Int. Ed. 61, e202116145 (2022).
Liu, X. et al. Engineering pore environments of sulfate‐pillared metal‐organic framework for efficient C2H2/CO2 separation with record selectivity. Adv. Mater. 35, 2210415 (2023).
Wang, J. et al. Fine pore engineering in a series of isoreticular metal-organic frameworks for efficient C2H2/CO2 separation. Nat. Commun. 13, 200 (2022).
Shen, J. et al. Simultaneous interlayer and intralayer space control in two-dimensional metal−organic frameworks for acetylene/ethylene separation. Nat. Commun. 11, 6259 (2020).
Zheng, F. et al. A robust two–dimensional layered metal–organic framework for efficient separation of methane from nitrogen. Sep. Purif. Technol. 281, 119911 (2022).
Cordes, D. B., Hanton, L. R. & Spicer, M. D. Six-coordinated Cd(II) centers as four- or six-connected nodes in coordination polymer networks containing bis(4-pyridyl)amine. Cryst. Growth. Des. 7, 328–336 (2007).
Zheng, F. et al. Strengthening intraframework interaction within flexible MOFs demonstrates simultaneous sieving acetylene from ethylene and carbon dioxide. Adv. Sci. 10, 2207127 (2023).
Zhang, Y., Yang, L., Wang, L., Duttwyler, S. & Xing, H. A microporous metal‐organic framework supramolecularly assembled from a Cu II dodecaborate cluster complex for selective gas separation. Angew. Chem. Int. Ed. 58, 8145–8150 (2019).
Chen, J. et al. Adsorptive separation of geometric isomers of 2-butene on gallate-based metal–organic frameworks. ACS Appl. Mater. Interfaces 12, 9609–9616 (2020).
Yang, L. et al. A single‐molecule propyne trap: highly efficient removal of propyne from propylene with anion‐pillared ultramicroporous materials. Adv. Mater. 30, 1705374 (2018).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 22322807, 22168023, 22308142, and 22268029) and the Natural Science Foundation of Jiangxi Province (No. 20224ACB204003).
Author information
Authors and Affiliations
Contributions
J.L. and J.W. conceived the project, designed the research, and co-wrote the manuscript. J.L. carried out the materials synthesis, adsorption experiments, transient breakthrough measurement, and computational simulations. H.X. carried out computational simulations. H.S. conducted the IAST and Qst calculations. X.L. and Y.P. conducted dynamic adsorption experiments. L.W. and P.W. collected the PXRD data. Z.W.Z. and Z.D. conducted stability testing. Z.Y.Z., J.C. and S.C. validated and visualized the results. Z.L.Z., S.D., and J.W. contributed to the discussion of the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Youssef Belmabkhout, Andreas Schneemann and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, J., Xiong, H., Shuai, H. et al. Molecular sieving of iso-butene from C4 olefins with simultaneous high 1,3-butadiene and n-butene uptakes. Nat Commun 15, 2222 (2024). https://doi.org/10.1038/s41467-024-46607-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-46607-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.