Abstract
One-step adsorption separation of C2H4 from ternary C2 hydrocarbon mixtures remains an important and challenging goal for petrochemical industry. Current physisorbents either suffer from unsatisfied separation performance, poor stability, or are difficult to scale up. Herein, we report a strategy of constructing multiple supramolecular binding sites in a robust and scalable MOF (Al-PyDC) for highly efficient one-step C2H4 purification from ternary mixtures. Owing to suitable pore confinement with multiple supramolecular binding sites, Al-PyDC exhibits one of the highest C2H2 and C2H6 uptakes and selectivities over C2H4 at ambient conditions. The gas binding sites have been visualized by single-crystal X-ray diffraction studies, unveiling that the low-polarity pore surfaces with abundant electronegative N/O sites provide stronger multiple supramolecular interactions with C2H2 and C2H6 over C2H4. Breakthrough experiments showed that polymer-grade C2H4 can be separated from ternary mixtures with a maximum productivity of 1.61 mmol g−1. This material can be prepared from two simple reagents using a green synthesis method with water as the sole solvent, and its synthesis can be easily scaled to multikilogram batches. Al-PyDC achieves an effective combination of benchmark separation performance, high stability/recyclability, green synthesis and easy scalability to address major challenges for industrial one-step C2H4 purification.
Similar content being viewed by others
Introduction
Purification of olefins such as ethylene (C2H4) accounts for 0.3% of global energy consumption, highlighting as one of seven most important chemical separations1. As the largest-volume product of the chemical industry, the worldwide production of C2H4 has exceeded 200 million tons per year in 2020 and will continuously increase in the future. At present, polymer-grade C2H4 is mainly produced by energy-intensive separation of downstream C2 hydrocarbon gas mixtures during the steam cracking process2. Acetylene (C2H2) is first removed through catalytic hydrogenation using noble-metal catalysts under high temperatures and pressures, and then ethane (C2H6) is separated by energy-intensive cryogenic distillation3. These high energy footprints associated with C2H4 purification have pushed the development of cost- and energy-efficient separation technologies to a level of utmost importance.
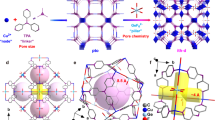
Adsorption separation technology based on porous materials has been demonstrated to be a promising technology to replace traditional cryogenic distillation and thus to fulfill the energy-efficient separation economy4,5,6,7,8,9. Highly efficient separation of C2H4 from binary C2H2/C2H4 and C2H6/C2H4 mixtures has been well addressed by various materials including metal−organic frameworks (MOFs)10,11,12,13,14,15,16,17,18,19, covalent organic frameworks (COFs)20, zeolites and so on21,22,23. Amongst them, microporous MOFs have shown particular promise for gas separations because of the powerful tunability on pore size and functionality24,25,26,27,28,29,30. Compared with separation of binary mixtures, simultaneous removal of C2H2 and C2H6 from ternary C2 mixtures would be more desirable to directly obtain polymer-grade C2H4, which can simplify the separation process to result in large energy saving. However, the simultaneous separation of C2H2 and C2H6 impurities from C2H4 remains a daunting challenge for classical physisorbents, since all the physical properties of C2H4 molecule lie between C2H2 and C2H6 (Supplementary Table 1)31, 32. Owing to the higher quadrupole moment and acidity of C2H2 over C2H4, the preferential adsorption of C2H2 over C2H4 with high selectivities can be generally achieved by MOFs with highly polar groups (e.g., open metal sites and fluoridated anion pillars)33,34,35,36,37,38,39,40,41; however, these kinds of materials bind more strongly with C2H4 over C2H6 to result in the selective adsorption of C2H4 over C2H6 (Fig. 1). Given that C2H6 has a higher polarizability than C2H4 (44.7 × 10−25 vs 42.52 × 10−25 cm3), the selective adsorption of C2H6 over C2H4 is commonly favored by MOFs with nonpolar/inert pore surfaces (e.g., aromatic or aliphatic moieties), wherein soft supramolecular interactions (e.g., C−H···π or hydrogen bonding) make major contributions42,43,44,45,46,47,48. A handful of C2H6-selective MOFs have been recently discovered to show the preferential adsorption of both C2H2 and C2H6 over C2H4 via various supramolecular interactions49,50,51,52,53,54,55,56,57,58,59. However, the weak nature of supramolecular interactions commonly makes the C2H2 or C2H6 binding affinity not so sufficient, resulting in poor gas uptake or insufficient selectivity to preclude most of them from being highly selective (Fig. 1). For example, Azole-Th-1 and Zn(ad)(int) exhibit relatively high C2H6/C2H4 selectivity, whereas their C2H2 uptake and selectivity are quite low because of their insufficient C2H2 binding affinity49,54. UiO-67-(NH2)2 and CuTiF6-TPPY holds the benchmark C2H2 and C2H6 uptakes or selectivities, but limited by inadequate gas selectivities or uptakes53,59, respectively. Ideal adsorbents for one-step C2 separation should possess both high C2H2 and C2H6 adsorption capacities and selectivities over C2H4, which can maximize the productivity and purity of C2H4 purification. Besides separation performance, some other factors such as stability, economy feasibility, and synthesis scalability, also need to be considered for large-scale industrial applications. However, most of the reported C2H2/C2H6-selective MOFs suffer from the drawbacks of poor water stability, high cost, or low scalability, largely impeding their applications in industrial scenarios. Currently, there are no reports existing on kilogram-scale synthesis of MOFs relevant for one-step C2H2/C2H6/C2H4 separation. Developing ideal MOF adsorbents that fully merge high separation performance with high stability, economy feasibility and easy scalability of synthesis has never been achieved yet for this important one-step C2H4 purification.
To overcome the weakness nature of supramolecular interactions, an effective design strategy is to incorporate multiple supramolecular binding sites into MOFs with suitable pore sizes to enforce C2H2 and C2H6 binding affinity (Fig. 1), thus concurrently improving their uptake and selectivity over C2H4. Recent studies have shown that the incorporation of electronegative oxygen or nitrogen sites into MOFs can provide hydrogen-bonding interactions with acidic C2H2, and thus enhance C2H2 adsorption and separation capacities59,60,61,62,63. Further, such O/N binding sites may also enable the formation of more numbers of supramolecular interactions with C2H6 molecule than with C2H4, given that C2H6 has larger molecule size and more H atoms16, 64. With the above considerations in mind, we herein report a strategy of designing multiple supramolecular binding sites in microporous MOFs for highly efficient one-step separation of C2H4 from ternary C2 mixtures. We target this matter in a known and robust Al-based MOF, [Al(OH)PyDC]n (Al-PyDC, also called KMF-1 or MOF-313, H2PyDC = 2,5-pyrroledicarboxylate)65,66. This material consists of cis-corner-sharing octahedra AlO6 chains linked by N-containing aromatic ligands to form one-dimensional pore channels with a suitable size of 5.8 Å, wherein a large number of polar O sites and N-heterocyclic rings are densely arranged along pore channels to provide abundant supramolecular binding sites (Fig. 2). Al-PyDC thus exhibits one of the highest C2H2 and C2H6 uptakes (8.24 and 4.20 mmol g−1) and selectivities (4.3 and 1.9) over C2H4 at ambient conditions, outperforming most benchmark materials reported so far. Single-crystal X-ray diffraction (SCXRD) studies on gas-loaded Al-PyDC for all the C2 molecules visually unveiled that multiple O/N sites on channel-pore surfaces provide stronger multiple supramolecular interactions with both C2H2 and C2H6 over C2H4, accounting for both very high C2H2 and C2H6 uptakes and selectivities. Dynamic breakthrough experiments confirm its exceptional one-step C2H4 purification from 1/49.5/49.5 or 1/9/90 C2H2/C2H6/C2H4 mixtures at ambient conditions, affording the maximal polymer-grade C2H4 productivity of 0.66 or 1.61 mmol g−1. Most importantly, Al-PyDC was synthesized from two simple and commercially available reagents H2PyDC and AlCl3·6H2O using water as the sole solvent, and we showed that its synthesis can be easily scaled-up to multikilogram batches in a high yield of 92% at mild and water-based conditions. The production of Al-PyDC can be considered as a scalable green synthesis. The combined advantages of benchmark separation performance, high stability/recyclability, green synthesis and easy scalability make it as a benchmark material for industrial one-step C2H2/C2H6/C2H4 separation.
Results
Synthesis and structural characterization
The Al-PyDC samples were readily synthesized as microcrystalline powders by reaction of the simple dicarboxylate ligand (H2PyDC) and AlCl3·6H2O according to the previous literature with procedural optimization (see the Method for details)65. The crystal structures of the as-synthesized and activated Al-PyDC have been determined by Chang et al. based on the synchrotron powder diffraction data65. In spite of this, it is still highly important to obtain large enough single crystals of Al-PyDC for SCXRD analysis, which facilitates to not only check the accurate structure information but also determine gas-loaded crystal structures to visually identify the binding sites of C2 molecules. While it is very challenging to get large single crystals for Al-MOFs, we here successfully prepared large colorless square-block-shaped crystals for Al-PyDC through great endeavors to optimize the growth method (Supplementary Fig. 1). The SCXRD analysis revealed that hydrated Al-PyDC crystallizes in a non-centrosymmetric tetragonal space group (I41md) with unit cell parameters of a = b = 21.1895(3) Å and c = 10.6283(3) Å, which are consistent with the results obtained by synchrotron powder diffraction data. After removing all the guest molecules, the activated structure of Al-PyDC can transfer to a new tetragonal symmetry of I41/amd, as evidenced by the literature and our following gas-loaded crystal structures (Supplementary Table 4).
The crystal structure of the activated Al-PyDC is depicted in Fig. 2. Each octahedrally coordinated Al-centre is linked to four oxygen atoms from four PyDC linkers and two cis bridging OH− groups. The octahedral AlO6-polyhedras form one-dimensional (1D) four-fold helical chains via corner sharing, which are further interconnected with each other by the PyDC linkers to result in a 3D framework (Fig. 2a). As shown in Fig. 2b, Al-PyDC exhibits a 1D square-shaped pore channel with a pore window size of 5.8 Å in diameter along the c axis. This aperture size is larger than the kinetic diameter of all C2 molecules so as to favor the rapid diffusion of these gas molecules. Due to the full coordination of the Al-centre, there are no polar open metal sites (OMSs) existed in the framework of Al-PyDC. Most importantly, the surface of pore channels is surrounded by abundant naked oxygen atoms and N-containing aromatic rings originated from organic linkers and bridging OH− groups. Such nonpolar pore surface decorated by abundant O/N sites and aromatic rings may provide multiple supramolecular binding sites to preferentially interact with C2H6 than with C2H4 molecule (Fig. 2c). Further, recent studies on Al-MOFs have shown that the densely distributed oxygen atoms around the channels can provide high-density H-bonding interactions with C2H2 molecule, leading to high C2H2 uptakes and selectivities61,62. Therefore, we reasoned that such multiple supramolecular binding sites within OMS-free Al-PyDC may show the great potential to not only concurrently reinforce C2H2 and C2H6 binding affinity for high gas uptakes, but also provide more preferential adsorption of C2H2 and C2H6 over C2H4 for high selectivities.
Gas adsorption measurements
The solvent-exchanged Al-PyDC sample was evacuated at room temperature for 12 h and then 393 K for 12 h until the outgas rate was 5 μmHg min−1, affording the fully activated material (Supplementary Fig. 2). The permanent porosity of the activated Al-PyDC was determined by nitrogen (N2) adsorption isotherms at 77 K. As shown in Fig. 3a, Al-PyDC takes up a 305 cm3 g−1 amount of N2 at 77 K and 1 bar, with a significant type I sorption behavior. The Brunauer−Emmett−Teller surface area and pore volume were calculated to be 1134 m2 g−1 and 0.472 cm3 g−1 (Supplementary Fig. 3), in good agreement with the values (1130 m2 g−1 and 0.473 cm3 g−1) reported in the literature65. The pore size distribution, determined by Non-Local Density Functional Theory (NLDFT) method based on 77 K N2 isotherms, shows a moderate pore size of 5.8 Å (Fig. 3a), which is consistent well with the value obtained from the crystal structure.
a N2 sorption isotherms of Al-PyDC at 77 K. Inset shows pore size distribution of Al-PyDC calculated based on NLDFT model. b Adsorption isotherms of Al-PyDC for C2H2, C2H6 and C2H4 at 296 K. c Predicted IAST selectivity curves for C2H2/C2H4 and C2H6/C2H4 mixtures at 296 K. d Comparison of C2H2 uptake and gas selectivities for Al-PyDC with the promising C2H2/C2H6-selective materials at ambient conditions. e Comparison of C2H6 uptake and gas selectivities for Al-PyDC with the indicated materials.
Pure-component adsorption isotherms of C2 hydrocarbons for Al-PyDC were measured at 273, 296, and 313 K up to 1 bar (Fig. 3b, Supplementary Figs. 4 and 5), respectively. Owing to the OMS-free and nonpolar pore surfaces, Al-PyDC exhibits an obviously preferential adsorption of C2H2 and C2H6 over C2H4 at 296 K (Fig. 3b). The C2 gas uptakes at 296 K and 1 bar follow the expected sequence of C2H2 (8.24 mmol g−1) > C2H6 (4.20 mmol g−1) > C2H4 (3.44 mmol g−1). This endows Al-PyDC with a promising capacity for efficient one-step separation of C2H4 from ternary mixtures. It is worthy to note that the C2H2 uptake (8.24 mmol g−1) at 1 bar and 296 K is the highest among all the C2H2/C2H6-selective MOFs reported so far (Fig. 3d and Supplementary Fig. 6), notably larger than that of most benchmark materials such as CuTiF6-TPPY (3.62 mmol g−1)53, TJT-100 (4.46 mmol g−1)55, NPU-1 (5.09 mmol g−1)56 and UiO-67-NH2 (5.90 mmol g−1)59. Further, the C2H6 uptake of Al-PyDC is also among the top-tier values for the relevant MOFs (Fig. 3e), surpassing that of Ag-PCM-102 (3.70 mmol g−1)52, CuTiF6-TPPY (2.82 mmol g−1)53, TJT-100 (3.75 mmol g−1)55 and comparable to Azole-Th-1 (4.47 mmol g−1)49 and NPU-1 (4.50 mmol g−1)56. Evidently, both the top-tier C2H2 and C2H6 uptakes make Al-PyDC as one of the best materials for ternary C2 separation. Further, we also investigated the time-dependent ads/desorption kinetics profiles of Al-PyDC at 296 K. As shown in Supplementary Fig. 7, Al-PyDC exhibits fast adsorption kinetics for all the C2 molecules and the adsorbed molecules can be completely removed under a high vacuum quickly, mainly attributed to its comparably large pore channels.
These adsorption discrepancies between C2 hydrocarbons can be partially explained by the experimental isosteric heat of adsorption (Qst), calculated by adsorption isotherms at different temperatures (Supplementary Figs. 9−11). As shown in Supplementary Fig. 12, the calculated Qst values at zero loading were determined to follow the order of C2H2 (35.3 kJ mol−1) > C2H6 (30.1 kJ mol−1) > C2H4 (27.8 kJ mol−1). Such higher Qst values for C2H2 and C2H6 compared with C2H4 confirm the stronger binding affinities of Al-PyDC with the former two gases, which is consistent well with that found in C2 gas uptakes. Compared to most reported C2H2/C2H6-selective materials, Al-PyDC also shows relatively higher Qst values for C2H2 and C2H6 (Supplementary Fig. 13), probably attributed to the multiple supramolecular binding sites observed in Al-PyDC.
Ideal Adsorbed Solution Theory (IAST) was utilized to estimate the adsorptive selectivity of Al-PyDC for both 1/99 C2H2/C2H4 and 50/50 C2H6/C2H4 mixtures. As shown in Fig. 3c, Al-PyDC exhibits both high C2H2/C2H4 selectivity of 4.3 and C2H6/C2H4 selectivity of 1.9 at 296 K and 1 bar. The C2H2/C2H4 selectivity is the highest among all of the reported C2H2/C2H6-selective MOFs except CuTiF6-TPPY53, significantly larger than that of some best-performing materials including Zn(ad)(int) (1.61)54, TJT-100 (1.8)55, and NPU-1 (1.4)56 and UiO-67-NH2 (2.1)59. Furthermore, the C2H6/C2H4 selectivity of Al-PyDC is also among the highest for the existing C2H2/C2H6-selective MOFs, outperforming Azole-Th-1 (1.46)49, TJT-100 (1.8)55, UiO-67-NH2 (1.7)59 and other benchmark materials. As shown in Figs. 3d, e, when we set the uptakes and selectivities as concurrent objectives for both C2H2/C2H4 and C2H6/C2H4 separations, Al-PyDC exhibits by far the best combination of very high uptakes and selectivities toward both separations, making it as the current benchmark for one-step separation of C2H4 from ternary C2 mixtures.
Binding-site determination
To visualize the locations of all C2 molecules and thus elucidate the origin of the observed higher C2H6 and C2H2 uptakes over C2H4, we carried out the in situ SCXRD experiments on gas-loaded Al-PyDC crystals. The SCXRD data were collected at 200 K and 1 bar. According to the C2H2-loaded SCXRD analysis, Al-PyDC was found to exhibit two types of binding sites (site-I and site-II) for the adsorbed C2H2 molecules (Fig. 4a). As shown in Fig. 4b, the adsorbed C2H2 molecule in site-I is mainly located at the corner of square-shaped pore channel. This C2H2 molecule exhibits multiple interactions with six carboxylate oxygen atoms through six C−H···O hydrogen bonds with the distances of 2.47–3.30 Å. In addition, C2H2 molecule also interacts with μ-OH site of the Al-octahedral chain through two supramolecular interactions (O−H···C = 1.98 Å), and with two C − H groups from surrounding pyrrole rings through weak supramolecular interactions (CC2H2···Hpyrrole = 2.40 Å). The C2H2 binding site-II was found to be located around the channel wall and in close proximity to the PyDC linker (Fig. 4c). The site-II acetylene molecule interacts with three carboxylate oxygen atoms through C−H···O hydrogen bonds (2.41–3.35 Å) and with one pyrrole ring to form the van der Waals (vdW) interaction (C−H···π = 3.18 Å). Further, the C2H2 molecule also binds with two N−H groups from two PyDC linkers through weak supramolecular interactions (N−H···CC2H2 = 2.32/2.88 Å). Such multiple supramolecular interactions in site-I and site-II were found to cooperatively interact with the adsorbed C2H2 molecules. Most importantly, as shown in Fig. 4d and Supplementary Fig. 14, due to the dense distribution of these two types of binding sites within the channels, each adsorbed C2H2 molecule in site-I interacts with two adjacent C2H2 molecules in site-II through four C−H···C−H interactions (3.78–4.40 Å). Such cooperative interactions between guest molecules enable the dense packing of C2H2 molecules inside the pore channels, resulting in the ultrahigh C2H2 uptake capacity of Al-PyDC. Full occupation of these adsorption sites corresponds to 10.1 mmol g−1 gas uptake, which is close to the saturated C2H2 uptake (11.1 mmol g−1) at 196 K (Supplementary Fig. 15). This also implies that about 81.6% of these C2H2 adsorption sites are occupied at 296 K and 1 bar.
a The SCXRD structure of C2H2-loaded Al-PyDC viewed along the c axis, indicating two types of C2H2 binding sites. b Illustration of C2H2 binding site-I, (c) C2H2 binding site-II, and (d) dense packing of C2H2 molecules within Al-PyDC. e The C2H6 binding site and (f) C2H4 binding site in gas-loaded Al-PyDC, determined by SCXRD analysis.
For C2H6 molecule, the adsorption location is approximately the same as that of C2H2 binding site-II. Due to its larger molecular size, C2H6 adopts an adsorption orientation different from that of site-II C2H2 molecule because of the steric restriction. The C2H6 orientation is perpendicular to the PyDC linker axis. As shown in Fig. 4e, multiple supramolecular interactions were observed between the adsorbed C2H6 molecule and the host framework. Each C2H6 molecule is H-bonded to twelve carboxylate O atoms around the pore through fourteen C−H···O interactions with the distances of 3.54–4.65 Å. In addition, C2H6 molecule also interacts with two pyrrole N atoms through three C−H···N interactions (3.80–4.18 Å) and with pyrrole ring on both sides through four C−H···π interactions (3.33–3.71 Å). In comparison, the adsorbed C2H4 molecules are located at the similar positions in the square-shaped channels. As depicted in Fig. 4f, each C2H4 molecule interacts with carboxylate O atoms to form only six C−H···O H-bonds with the distances of 3.83–4.08 Å, with two pyrrole N atoms through C–H···N interactions (3.21 and 3.42 Å) and with pyrrole ring on both sides through two C−H···π interactions (4.26 Å). Evidently, due to the more number of H atoms and larger molecular size of C2H6, the adsorbed C2H6 molecule exhibits much more number of weak supramolecular interactions with the framework, leading to the stronger binding affinity than C2H4 molecule. This can be further confirmed by the higher experimental binding energies of C2H6 (30.1 kJ mol−1) than C2H4 (27.8 kJ mol−1) observed in Al-PyDC. All of the above results can visually elucidate the adsorption and separation phenomenon on C2 gas mixtures.
Dynamic breakthrough studies
Dynamic breakthrough experiments were first conducted on a packed column filled with activated Al-PyDC to evaluate the separation performance for binary C2H6/C2H4 (50/50) and C2H2/C2H4 (1/99) mixtures under a gas flow of 1.25 mL min−1 at 296 K. As shown in Fig. 5a, owing to the ultrahigh C2H2 uptake and large C2H2/C2H4 selectivity, Al-PyDC exhibits a highly efficient separation capacity for 1/99 C2H2/C2H4 mixture, wherein C2H4 gas first eluted through the adsorption bed at 34 min, while C2H2 breakthrough did not occur until 92 min. During this time interval, pure C2H4 production (> 99.999%) from the outlet effluent for a given cycle was calculated to be 7.93 mmol g−1. This C2H4 productivity is even much higher than some top-performing materials for single C2H2/C2H4 separation, such as UTSA-100a (1.13 mmol g−1)11 and SIFSIX-3-Zn (1.94 mmol g−1)37. Further, the efficient separation of C2H4 from C2H6/C2H4 mixture can be also accomplished by Al-PyDC (Fig. 5b), with a high pure C2H4 productivity of 0.68 mmol g−1 at the outlet. This productivity is higher than some promising C2H6-selective materials such as Cu(Qc)2 (0.42 mmol g−1)42, MAF-49 (0.28 mmol g−1)65 and even comparable to the benchmark Fe2(O2)(dobdc)2 (0.79 mmol g−1)14.
Experimental column breakthrough curves of Al-PyDC for (a) C2H2/C2H4 (1/99), (b) C2H6/C2H4 (50/50) and (c) C2H2/C2H6/C2H4 (1/49.5/49.5) mixtures under ambient conditions. d Experimental column breakthrough curves of Al-PyDC for C2H2/C2H6/C2H4 (1/9/90) mixtures under ambient conditions. e Comparison of pure C2H4 productivity for Al-PyDC (the averaged value was obtained from five independent tests, and the error bar is the standard deviation) with the top-performing materials reported. f Twenty separation cycles of breakthrough experiments on Al-PyDC for C2H2/C2H6/C2H4 (1/9/90) mixture.
Next, we examined the separation performance of Al-PyDC for actual C2H2/C2H6/C2H4 ternary mixtures at the same conditions. Figure 5c reveals that complete separation of both C2H6 and C2H2 from 3-component C2H2/C2H6/C2H4 (1/49.5/49.5) mixture can be fulfilled by Al-PyDC. It was found that C2H4 was first eluted at 33 min to yield a pure gas with an undetectable amount of C2H6 and C2H2 (the detection limit of the instrument is 0.01%). After that, C2H6 gas secondly passed through the adsorption bed at 46 min, and the adsorbent retained C2H2 lastly until 92 min. During the time interval between C2H4 and C2H6/C2H2 breakthrough, pure C2H4 productivity of Al-PyDC from the outlet effluent for a given cycle was calculated up to 0.66 mmol g−1. This C2H4 productivity is even higher than the previously benchmark UiO-67-(NH2)2 (0.55 mmol g−1)59. Given the fact that the C2 fraction obtained from cracked gas sometimes contains a small portion of C2H6 (ca. 6–10%), we further evaluated its separation capacity on a ternary C2H2/C2H6/C2H4 (1/9/90) mixture at the same conditions. As shown in Fig. 5d, the much later breakthrough times of both C2H2 and C2H6 (100 and 43 min) than that of C2H4 (29 min) reveal that simultaneous removal of C2H2 and C2H6 can be fulfilled by Al-PyDC. The productivity of pure C2H4 (over 99.9%) from the outlet effluent was calculated up to 1.61 mmol g−1 (Fig. 5e), which is the highest among the reported best-performing C2H2/C2H6-selective materials including Azole-Th-1 (1.34 mmol g−1, 1/9/90)49, Zn(ad)(int) (1.43 mmol g−1, 1/10/89)54 and TJT-100 (0.69 mmol g−1, 0.5/0.5/99)55. The highly efficient separation capacity can be still retained when the flow rate of the mixed gases accelerated to 5.0 and 10.0 mL min−1 (Supplementary Fig. 17).
For practical applications, the adsorbent should possess good recyclability and structural stability. To test the recyclability of Al-PyDC, we firstly carried out cycling experiments on Al-PyDC for single-component C2H2 or C2H6 adsorption at 1 bar and 296 K, followed by desorption under vacuum at room temperature. As shown in Supplementary Fig. 18, the experimental cycling results indicate that there was no noticeable loss in C2H2 and C2H6 adsorption capacities for Al-PyDC over 30 cycles. Next, breakthrough separation experiments on both C2H2/C2H6/C2H4 (1/49.5/49.5) and C2H2/C2H6/C2H4 (1/9/90) mixtures were cycled numerous times to further assess the recyclability of Al-PyDC (Fig. 5f and Supplementary Figs. 19−21), wherein the sample was fully regenerated between cycles under sweeping He gas at 373 K. The cycling results show that there was no obvious loss in the separation capacity for Al-PyDC over 20 cycles, confirming its good recyclability for this separation. Given that the actual feed streams typically contain small amount of acidic gases (e.g., H2S),67 the breakthrough experiments for 1/9/90 C2H2/C2H6/C2H4 mixture with 1000 ppm H2S were performed on Al-PyDC to investigate the effect of acidic H2S gas on separation performance. As shown in Supplementary Fig. 22, the separation performance of Al-PyDC remains almost unchanged to afford the comparable C2H4 productivity of 1.57 mmol g−1, and the separation capacity can be preserved over six continuous cycles under the presence of acid gases. These results have demonstrated that Al-PyDC can be recycled for repeated separation cycles without any loss of performance even under the presence of acid gases.
Material stability and scale-up synthesis
Besides separation performance, material stability and scale-up synthesis are two most important concerns for industrial applications. We first examined the chemical stability of Al-PyDC after treatment under different conditions, monitored by powder X-ray diffraction (PXRD) and gas adsorption measurements. As shown in Fig. 6a, after immersion in water, boiling water, and aqueous solutions of pH 1 and 12 for 3 days, the PXRD studies revealed that the framework of Al-PyDC can retain its structural integrity without any phase change and loss of crystallinity observed. Such ultrahigh chemical stability was also confirmed by N2 adsorption isotherms at 77 K and C2H2/C2H6 uptake capacities at 296 K after different treatment, wherein all the gas adsorption amounts show no obvious decrease compared to those of the pristine sample (Supplementary Fig. 23). In addition, scanning electron microscope (SEM) and optical images of Al-PyDC crystals showed that there are no obvious changes observed in their morphology and surface after the treatment with water and pH solutions (Fig. 6b and Supplementary Fig. 24). The variable temperature PXRD patterns indicate that Al-PyDC is thermally stable up to 350 °C (Supplementary Fig. 25). Therefore, this material shows one of the best chemical and thermal stabilities among the reported MOFs (Supplementary Table 8), even comparable to some representative stable MOFs including MIL-101(Cr), BUT-12 and PCN-250.68,69,70 Considering that the actual working environment would contain trace amount of acid gases in feed streams67, we further assess the stability of Al-PyDC under acid gas circumstances. We first carried out the repeated adsorption/desorption cycling experiments for C2H6 gas containing 1000 ppm acidic H2S gas, in which the sample was regenerated by N2 sweeping at 373 K. As shown in Fig. 6c, the C2H6 adsorption capacity can be maintained with no noticeable loss after 50 cycles in the presence of acidic H2S. Further, the breakthrough cycling experiments on C2H2/C2H6/C2H4 mixtures containing 1000 ppm H2S further confirmed that the separation capacity of Al-PyDC can be preserved without any loss of performance over six cycles under the presence of acid gases (Supplementary Fig. 22). The PXRD pattern and 77 K N2 sorption isotherms after repeated breakthrough cycles indicate that Al-PyDC can maintain its structural integrity (Supplementary Fig. 26). These results confirm its high structural stability under practical circumstances.
a PXRD patterns and C2H2 adsorption isotherms and (b) SEM images of Al-PyDC samples after treatment with different conditions. c Gas adsorption on Al-PyDC over 50 consecutive adsorption-desorption cycles for C2H6 gas containing 1000 ppm H2S at 296 K, in which the sample was regenerated by N2 sweeping at 373 K. d The kilogram-scale synthesis of Al-PyDC by a green and facile method. e PXRD patterns and C2H2 adsorption isotherms of Al-PyDC samples obtained from various-scale synthesis. f The comprehensive comparison of separation performance, stability, economic feasibility and scalability for Al-PyDC and other benchmark materials.
With respect to large-scale practical applications, it is crucial that the scale-up synthesis of MOFs should be viable from a technical and economic point of view. The cost analysis for various MOF syntheses indicates that the raw material costs are often prohibitively high, especially for organic linkers or using organic solvents as reaction solutions. Further, synthetic conditions can also have an important influence on the economics. For instance, the necessary use of high-temperature or high-pressure apparatus for MOF syntheses would be not only costly but also bring high expenses on safety precautions. Al-PyDC exhibits none of these disadvantageous conditions. The reported literature showed that Al-PyDC can be prepared from simple and commercially available reagents of H2PyDC and Al2(SO4)3·18H2O in water solvent at a temperature of 120 °C65. However, the synthetic temperature (120 °C) is still much higher than the water boiling point, which is detrimental to the scale-up synthesis. To reduce the synthetic temperature, we here optimized the reaction conditions by changing the base equivalents and aluminum salt (see the Method for details). Our experiments revealed that Al-PyDC can be readily synthesized from H2PyDC and AlCl3·6H2O in water solvent at a much lower temperature (85 °C). These mild and water-based conditions can be considered as a scalable green synthesis, which are particularly advantageous from safety and environmental aspects. As shown in Fig. 6d and Supplementary Fig. 27, the kilogram-scale production of Al-PyDC sample was easily performed by using a reflux-based synthesis method in a 30 L reaction vessel. This protocol provided more than 1.0 kg of as-synthesized Al-PyDC per reaction batch in a high yield of 92% within 9 h, resulting in an exceptional space-time yield (STY) over 126 kg m−3 day−1. For a comparison, the STYs of zeolites are commonly in the range of 50–150 kg m−3 day−112. The Al-PyDC products synthesized at this large-scale exhibit similar crystallinity and gas uptake capacities compared to the material produced at a small scale, as verified by PXRD analysis and gas adsorption isotherms (Fig. 6e and Supplementary Fig. 29). Further, dynamic breakthrough experiments on activated Al-PyDC sample synthesized in this low temperature procedure showed the same separation capacity for ternary C2 hydrocarbon mixtures (Supplementary Fig. 29), without any loss in C2H4 productivity.
A perfect adsorbent for industrial one-step C2H4 purification from ternary mixtures should meet the following criteria: (1) large C2H2 and C2H6 adsorption capacities; (2) high C2H2/C2H4 and C2H6/C2H4 selectivities; (3) viable chemical/thermal stability; (4) economic feasibility; (5) easy scalability of production. As shown in Fig. 6f, we comprehensively compare all the above criteria between Al-PyDC and other benchmark materials. We have shown that Al-PyDC can meet all of these criteria. In comparison, other benchmark MOFs have better reported properties in one or more of the above-mentioned criteria, but not in all of them. For instance, several benchmark MOFs (e.g., Azole-Th-1, NPU-1, and UiO-67-NH2) show highly efficient separation performance for one-step C2H4 purification; however, they suffer from either the use of toxic organic solvents (e.g., dimethylformamide) and harsh synthesis conditions, or contain expensive and complicated organic linkers (Supplementary Table 8 and Supplementary Fig. 30). These drawbacks lead to an extremely high difficulty on the scale-up production of these materials, making most of them unfavorable for large-scale industrial applications. By far, there are no reports existed on kilogram-scale synthesis of MOFs relevant for one-step C2H2/C2H6/C2H4 separation. With Al-PyDC, we demonstrated that a high-yielding (>90%) and scalable (~1.05 kg) synthesis from simple and commercially available reagents was achieved in water solution and with a high STY value, making the cost of Al-PyDC synthesis more affordable. In terms of separation performance, Al-PyDC exhibits one of the highest C2H2 and C2H6 uptakes and selectivities over C2H4 at ambient conditions, providing a maximum pure C2H4 productivity for ternary mixtures. Overall, the combined superiorities of green synthesis method, benchmark separation performance, high stability, economic feasibility and easy scalability of production make this material as a promising adsorbent to address the challenges for industrial one-step C2H4 purification from ternary mixtures.
Discussion
In summary, we have proposed and demonstrated a scalable and robust Al-MOF (Al-PyDC) with multiple supramolecular binding sites for highly efficient one-step C2H4 purification from ternary C2 mixtures. The gas-loaded SCXRD studies of Al-PyDC visually identified that the low-polarity pore surfaces with abundant O/N sites provide stronger multiple supramolecular interactions with C2H2 and C2H6 over C2H4, thus simultaneously optimizing the C2H2 and C2H6 adsorption uptakes and selectivities. This material thereby achieves the top-tier C2H2 and C2H6 uptakes (8.24 and 4.20 mmol g−1) and selectivities (4.3 and 1.9) over C2H4 at ambient conditions, outperforming most of the benchmark materials reported to date. The breakthrough experiments affirmed that Al-PyDC can simultaneously separate C2H2 and C2H6 from ternary C2H2/C2H6/C2H4 mixtures, affording the record high polymer-grade C2H4 productivity of 1.61 mmol g−1. Most remarkably, Al-PyDC is highly water/pH stable and can be easily produced at kilogram-scale using a green synthesis method. The comprehensive features of high separation performance, notable stability/recyclability, economic feasibility and easy scalability of synthesis make this material as the current benchmark for industrial one-step C2H4 purification applications. This work also provides an effective approach of designing multiple supramolecular binding sites in microporous MOFs to concurrently enforce C2H2 and C2H6 binding affinity for boosting this important gas separation.
Methods
Materials
Aluminum chloride hexahydrate (AlCl3·6H2O, CAS: 231-208-1, purity 99.9%) was purchased from Aladdin, 1H-Pyrrole-2,5-dicarboxylic acid (H2PyDC, CAS: 937-27-9, purity 97%) was purchased from Bidepharm (China). N2 (99.999%), C2H4 (99.9%), C2H6 (99.99%), C2H2 (99.6%), He (99.999%) and mixed gases of C2H2/C2H4 (1/99, v/v), C2H6/C2H4 (50/50), C2H2/C2H6/C2H4 (1/49.5/49.5) and C2H2/C2H6/C2H4 (1/9/90) were purchased from JinGong Company (China). All chemicals were used without further purification.
Synthesis of Al-PyDC powder
The Al-PyDC powder sample was synthesized based on the method reported in the previous literature with modification65. H2PyDC (310 mg, 2 mmol) and NaOH (240 mg, 6 mmol) were dissolved in H2O (6 mL) by sonication for 10 min to obtain clear solution. Afterwards, aqueous AlCl3 solution (1 M, 2 mL) was added. The reaction mixture was then kept at 85 °C under refluxed condition for 9 h. The resulting white precipitate of Al-PyDC was separated by using filtration and washed with H2O and ethanol.
Crystallization of Al-PyDC
Single crystals were obtained by fully dissolving H2PyDC (39 mg, 0.25 mmol) in aqueous NaOH solution (0.05 M, 5 mL). Afterwards, aqueous AlCl3 solution (0.05 M, 5 mL) was slowly added. The resulting solution was incubated for 48 h in a pre-heated oven at 100 °C.
Scalable synthesis of Al-PyDC
H2PyDC (0.9 kg, 5.7 mol) and NaOH (0.684 kg, 17.1 mol) were dissolved in deionized water (17 L) at room temperature with stirring for 30 min to obtain clear solution. Afterwards, AlCl3·6H2O (1.374 kg, 5.7 mol) was dissolved in 3 L deionized water and transferred to a glass material-feeding funnel. The AlCl3 aqueous solution was added at a rate of 3 L per hour to the reaction vessel, resulting in the formation of white precipitate. The solution was then heated and kept at 85 °C for 9 h under reflux condition with the spinner rotating at 150 rpm. After that, the solid product was collected in a 20 L filtration funnel and thoroughly washed with deionized water and ethanol, then vacuum dried at 393 K overnight to obtain ~1.05 kg product with a yield of 92%.
Sample characterization
The SEM images were obtained from a Hitachi S4800 field-emission scanning electron microscopy (FE-SEM). The microscopy images were captured on an Olympus IX 71 inverted fluorescent microscope. The PXRD patterns in the 2θ = 2–45° range were measured on an X’Pert PRO diffractometer at room temperature using a Cu-Kα (λ = 1.54184 Å) radiation source. Thermogravimetric analysis (TGA) was measured on a TA SDT-650 instrument and the sample was heated under N2 flow (50 mL min−1) with a heating rate of 5 K min−1.
Gas sorption measurements
Before the test, the as-synthesized sample was solvent-exchanged with high-purity methanol over eight times within 3 days to the complete removal of guest solvent molecules from the framework. The solvent-exchanged sample was degassed for 12 h at room temperature and then for another 12 h at 393 K until the outgas rate was 5 μmHg min−1. Gas sorption isotherms of C2H2, C2H6, and C2H4 were obtained from a Micromeritics ASAP 2020 instrument, and the adsorption tube was kept at a constant temperature of 273 K, 296 K and 313 K by using a Julabo water bath. N2 sorption isotherms were recorded by using a Micromeritics ASAP 2460 instrument at 77 K under liquid N2 bath. Kinetic and equilibrium adsorption measurements were carried out using an Intelligent Gravimetric Analyzer (IGA001, Hiden, UK) under diverse test conditions.
Single-crystal X-ray diffraction
SCXRD data were collected at 170 K for Al-PyDC-hydrated, and at 200 K for C2H2@Al-PyDC, C2H6@Al-PyDC and C2H4@Al-PyDC on an Agilent Supernova CCD diffractometer equipped with graphite-monochromatic enhanced Cu-Kα radiation (λ = 1.54184 Å). A single crystal of solvent-exchanged Al-PyDC was selected and placed into a capillary glass tube with inner diameter of 0.1 mm. This crystal was activated at 393 K for 4 h, and the capillary glass tube was filled by pure C2H2, C2H6 or C2H4 gas up to 1 bar and then sealed to obtain C2H2-loaded, C2H6-loaded or C2H4-loaded Al-PyDC crystal. The datasets were corrected by empirical absorption correction using spherical harmonics, implemented in the SCALE3 ABSPACK scaling algorithm. The structure was solved by direct methods and refined by full matrix least-squares methods with the SHELX-97 program package. During crystal structure analysis for C2H6@Al-PyDC, we found that guest C2H6 molecules exhibited highly positional disorder within the channels. By atomic identification and refinement, we determined that the asymmetric unit of each C2H6 molecule within the channel includes four carbon atoms (C5, C6, C8, C9) and eight hydrogen atoms (H5A, H5C, H6A, H6C, H8A, H8C, H9A, H9C). Among them, four carbon atoms and four hydrogen atoms (H5A, H6A, H8A, H9A) are located on a mirror plane (−x, y, z), while two carbon atoms (C6 and C8) are located on a mirror plane (x, 1/2−y, z) and a 2-fold rotation axis (1/2−x, y, 1/2−z). The asymmetric unit of C2H6 is generated by these symmetry operations, resulting in four completely disordered C2H6 molecules at each site, with an occupancy of 25%. All the crystal data are summarized in Supplementary Table 4, and ORTEP style illustrations of all structures are provided in Supplementary Figs. 31–34.
Breakthrough experiments
The breakthrough curves were obtained from a dynamic gas breakthrough apparatus equipped with stainless steel column (Φ 4 × 120 mm). The weight of sample packed in the column was 0.34 g. The mixed gas flows of (1) C2H2/C2H4 (1/99, v/v), (2) C2H6/C2H4 (50/50), (3) C2H2/C2H6/C2H4 (1/49.5/49.5) or (4) C2H2/C2H6/C2H4 (1/9/90) were introduced into breakthrough apparatus with the rate of 1.25, 5 and 10 mL min−1 at 296 K and 1 bar, respectively. The outlet gas was monitored using a gas chromatography (GC-2014C, SHIMADZU) equipped with thermal conductivity detector (TCD, detection limit 0.1 ppm). The concentration of the outlet gas was calibrated by detecting the standard gas mixture. After each breakthrough experiment, the sample can be regenerated under a purging He gas with a flow of 10 mL min−1 at 373 K for 1 h.
Cycling experiments
The cycling experiments for single-component C2H2 or C2H6 were performed on a Micromeritics ASAP 2020 surface area analyzer. The activated sample was exposed to C2H2 or C2H6 gas at 1 bar and 296 K until saturation, followed by desorption under vacuum at room temperature to 0.001 bar. The cycling experiments for C2H6 with 1000 ppm H2S were measured by using DSC/TGA Discovery SDT 650. After being fully activated at 393 K under N2 flow for 2 h, the adsorbent was exposed to C2H6 gas containing 1000 ppm H2S (100 mL min−1) at 296 K for 60 min, followed by regeneration at 373 K under N2 flow (200 mL min−1) for 30 min. Cycling breakthrough experiments for C2H2/C2H6/C2H4 mixture were performed using a dynamic gas breakthrough apparatus equipped with stainless steel column. The weight of sample packed in the column was 0.418 g. The mixed gas flows of (1) C2H2/C2H6/C2H4 (1/49.5/49.5), (2) C2H2/C2H6/C2H4 (1/9/90) or (3) C2H2/C2H6/C2H4 (1/9/90) with 1000 ppm H2S were introduced into breakthrough apparatus with the rate of 1.25 mL min−1 at 296 K and 1 bar. After each breakthrough experiment, the sample can be fully regenerated under a purging He gas with a flow of 10 mL min−1 at 373 K for 30 min.
Data availability
Crystallographic data for the structures in reported this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2242152−2242155. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All the other relevant data that support the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon request.
References
Sholl, D. S. & Lively, R. P. Seven chemical separations to change the world. Nature 532, 435–437 (2016).
Ren, T., Patel, M. & Blok, K. Olefins from conventional and heavy feedstocks: energy use in steam cracking and alternative processes. Energy 31, 425–451 (2006).
Sadrameli, S. M. Thermal/catalytic cracking of liquid hydrocarbons for the production of olefins: a state-of-the-art review II: catalytic cracking review. Fuel 173, 285–297 (2016).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal−organic frameworks. Science 341, 1230444 (2013).
Adil, K. et al. Gas/vapour separation using ultra-microporous metal−organic frameworks: insights into the structure/separation relationship. Chem. Soc. Rev. 46, 3402–3430 (2017).
Zhao, X., Wang, Y., Li, D.-S., Bu, X. & Feng, P. Metal−organic frameworks for separation. Adv. Mater. 30, 1705189 (2018).
Han, X., Yang, S. & Schröder, M. Porous metal−organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 3, 108–118 (2019).
Wang, H., Liu, Y. & Li, J. Designer metal−organic frameworks for size-exclusion-based hydrocarbon separations: progress and challenges. Adv. Mater. 32, 2002603 (2020).
Chen, Z., Kirlikovali, K. O., Li, P. & Farha, O. K. Reticular chemistry for highly porous metal−organic frameworks: the chemistry and applications. Acc. Chem. Res. 55, 579–591 (2022).
Yang, S. et al. Supramolecular binding and separation of hydrocarbons within a functionalized porous metal−organic framework. Nat. Chem. 7, 121–129 (2015).
Hu, T.-L. et al. Microporous metal-organic framework with dual functionalities for highly efficient removal of acetylene from ethylene/acetylene mixtures. Nat. Commun. 6, 7328 (2015).
Li, J.-B. et al. A scalable metal-organic framework as a durable physisorbent for carbon dioxide capture. Science 374, 1464–1469 (2021).
Bonneau, M. et al. Tunable acetylene sorption by flexible catenated metal−organic frameworks. Nat. Chem. 14, 816–822 (2022).
Li, L. et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362, 443–446 (2018).
Li, J. et al. Metal-organic framework containing planar metal-binding sites: efficiently and cost-effectively enhancing the kinetic separation of C2H2/C2H4. J. Am. Chem. Soc. 141, 3807–3811 (2019).
Zeng, H. et al. Cage-interconnected metal−organic framework with tailored apertures for efficient C2H6/C2H4 separation under humid conditions. J. Am. Chem. Soc. 141, 20390–20396 (2019).
Zhang, Z. et al. Efficient trapping of trace acetylene from ethylene in an ultramicroporous metal−organic framework: synergistic effect of high-density open metal and electronegative sites. Angew. Chem. Int. Ed. 59, 18927–18932 (2020).
Shen, J. et al. Simultaneous interlayer and intralayer space control in two-dimensional metal−organic frameworks for acetylene/ethylene separation. Nat. Commun. 11, 6259 (2020).
Geng, S. et al. Scalable room-temperature synthesis of highly robust ethane-selective metal−organic frameworks for efficient ethylene purification. J. Am. Chem. Soc. 143, 8654–8660 (2021).
Baldwin, L. A., Crowe, J. W., Pyles, D. A. & McGrier, P. L. Metalation of a mesoporous three-dimensional covalent organic framework. J. Am. Chem. Soc. 138, 15134–15137 (2016).
Bereciartua, P. J. et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017).
Chai, Y. et al. Control of zeolite pore interior for chemoselective alkyne/olefin separations. Science 368, 1002–1006 (2020).
Su, K., Wang, W., Du, S., Ji, C. & Yuan, D. Efficient ethylene purification by a robust ethane-trapping porous organic cage. Nat. Commun. 12, 3703 (2021).
Cadiau, A., Adil, K., Bhatt, P. M., Belmabkhout, Y. & Eddaoudi, M. A metal-organic framework−based splitter for separating propylene from propane. Science 353, 137–140 (2016).
Bae, Y.-S. et al. High propene/propane selectivity in isostructural metal−organic frameworks with high densities of open metal site. Angew. Chem. Int. Ed. 51, 1857–1860 (2012).
He, T. et al. Trace removal of benzene vapour using double-walled metal-dipyrazolate frameworks. Nat. Mater. 21, 689–695 (2022).
Zeng, H. et al. Orthogonal-array dynamic molecular sieving of propylene/propane mixtures. Nature 595, 542–548 (2021).
Li, L. et al. Discrimination of xylene isomers in a stacked coordination polymer. Science 377, 335–339 (2022).
Zhang, X.-W., Zhou, D.-D. & Zhang, J.-P. Tuning the gating energy barrier of metal-organic framework for molecular sieving. Chem 7, 1006–1019 (2021).
Wang, G.-D. et al. Boosting ethane/ethylene separation by MOFs through the amino-functionalization of pores. Angew. Chem. Int. Ed. 61, e202213015 (2022).
Li, J.-R., Kuppler, R. J. & Zhou, H.-C. Selective gas adsorption and separation in metal−organic frameworks. Chem. Soc. Rev. 38, 1477–1504 (2009).
Chen, K.-J. et al. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture. Science 366, 241–246 (2019).
Li, B. et al. Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 136, 8654–8660 (2014).
Jiang, Y. et al. Benchmark single-step ethylene purification from ternary mixtures by a customized fluorinated anion-embedded MOF. Nat. Commun. 14, 401 (2023).
Mohamed, M. H. et al. Designing open metal sites in metal−organic frameworks for paraffin/olefin separations. J. Am. Chem. Soc. 141, 13003–13007 (2019).
Bloch, E. D. et al. Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 335, 1606–1610 (2012).
Cui, X. et al. Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016).
Li, B. et al. An ideal molecular sieve for acetylene removal from ethylene with record selectivity and productivity. Adv. Mater. 29, 1704210 (2017).
Luna-Triguero, A. et al. Acetylene storage and separation using metal-organic frameworks with open metal sites. ACS Appl. Mater. Interfaces 11, 31499–31507 (2019).
Verma, G., Ren, J., Kumar, S. & Ma, S. New paradigms in porous framework materials for acetylene storage and separation. Eur. J. Inorg. Chem. 2021, 4498–4507 (2021).
Niu, Z. et al. A MOF-based ultra-strong acetylene nano-trap for highly efficient C2H2/CO2 separation. Angew. Chem. Int. Ed. 60, 5283–5288 (2021).
Lin, R.-B. et al. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal−organic frameworks. J. Am. Chem. Soc. 140, 12940–12946 (2018).
Yang, H. et al. Pore-space-partition-enabled exceptional ethane uptake and ethane-selective ethane−ethylene separation. J. Am. Chem. Soc. 142, 2222–2227 (2020).
Qazvini, O. T., Babarao, R., Shi, Z.-L., Zhang, Y.-B. & Telfer, S. G. A robust ethane-trapping metal−organic framework with a high capacity for ethylene purification. J. Am. Chem. Soc. 141, 5014–5020 (2019).
Yang, L. et al. Adsorption in reversed order of C2 hydrocarbons on an ultramicroporous fluorinated metal-organic framework. Angew. Chem. Int. Ed. 61, e202204046 (2022).
Di, Z. et al. A metal-organic framework with nonpolar pore surfaces for the one-step acquisition of C2H4 from a C2H4 and C2H6 mixture. Angew. Chem. Int. Ed. 61, e202210343 (2022).
Pires, J. et al. Enhancement of ethane selectivity in ethane−ethylene mixtures by perfluoro groups in Zr-based metal-organic frameworks. ACS Appl. Mater. Interfaces 11, 27410–27421 (2019).
Gücüyener, C., van den Bergh, J., Gascon, J. & Kapteijn, F. Ethane/ethene separation turned on its head: selective ethane adsorption on the metal−organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 132, 17704–17706 (2010).
Xu, Z. et al. A robust Th-azole framework for highly efficient purification of C2H4 from a C2H4/C2H2/C2H6 mixture. Nat. Commun. 11, 3163 (2020).
Wang, Y. et al. One-step ethylene purification from an acetylene/ethylene/ethane ternary mixture by cyclopentadiene cobalt-functionalized metal−organic frameworks. Angew. Chem. Int. Ed. 60, 11350–11358 (2021).
Wang, Y. et al. Guest-molecule-induced self-adaptive pore engineering facilitates purification of ethylene from ternary mixture. Chem 8, 3263–3274 (2022).
Sikma, R. E. et al. Low-valent metal ions as MOF pillars: a new route toward stable and multifunctional MOFs. J. Am. Chem. Soc. 143, 13710–13720 (2021).
Zhang, P. et al. Synergistic binding sites in a hybrid ultramicroporous material for one-step ethylene purification from ternary C2 hydrocarbon mixtures. Sci. Adv. 8, eabn9231 (2022).
Ding, Q. et al. One-step ethylene purification from ternary mixtures in a metal−organic framework with customized pore chemistry and shape. Angew. Chem. Int. Ed. 61, e202208134 (2022).
Hao, H.-G. et al. Simultaneous trapping of C2H2 and C2H6 from a ternary mixture of C2H2/C2H4/C2H6 in a robust metal−organic framework for the purification of C2H4. Angew. Chem. Int. Ed. 57, 16067–16071 (2018).
Zhu, B. et al. Pore engineering for one-step ethylene purification from a three-component hydrocarbon mixture. J. Am. Chem. Soc. 143, 1485–1492 (2021).
Wang, G.-D. et al. One-step C2H4 purification from ternary C2H6/C2H4/C2H2 mixtures by a robust metal-organic framework with customized pore environment. Angew. Chem. Int. Ed. 61, e202205427 (2022).
Cao, J.-W. et al. One-step ethylene production from a four-component gas mixture by a single physisorbent. Nat. Commun. 12, 6507 (2021).
Gu, X.-W. et al. Immobilization of Lewis basic sites into a stable ethane-selective MOF enabling one-step separation of ethylene from a ternary mixture. J. Am. Chem. Soc. 144, 2614–2623 (2022).
Gong, W. et al. Efficient C2H2/CO2 separation in ultramicroporous metal−organic frameworks with record C2H2 storage density. J. Am. Chem. Soc. 143, 14869–14876 (2021).
Ye, Y. et al. Metal−organic framework based hydrogen-bonding nanotrap for efficient acetylene storage and separation. J. Am. Chem. Soc. 144, 1681–1689 (2022).
Pei, J. et al. Dense packing of acetylene in a stable and low-cost metal−organic framework for efficient C2H2/CO2 separation. Angew. Chem. Int. Ed. 60, 25068–25074 (2021).
Moreau, F. et al. Unravelling exceptional acetylene and carbon dioxide adsorption within a tetra-amide functionalized metal-organic framework. Nat. Commun. 8, 14085 (2017).
Liao, P.-Q., Zhang, W.-X., Zhang, J.-P. & Chen, X.-M. Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 6, 8697 (2015).
Cho, K. H. et al. Rational design of a robust aluminum metal-organic framework for multi-purpose water-sorption-driven heat allocations. Nat. Commun. 11, 5112 (2020).
Yaghi, O. M., Hanikel, N. & Lyu, H. Multivariate and other metal−organic frameworks, and uses thereof. WO WO2020/112899A1, June 4 (2020).
Fakhroleslam, M. & Sadrameli, S. M. Thermal/catalytic cracking of hydrocarbons for the production of olefins; a state-of-the-art review III: process modeling and simulation. Fuel 252, 553–566 (2019).
Yuan, S. et al. Stable metal−organic frameworks: design, synthesis, and applications. Adv. Mater. 30, 1704303 (2018).
Wang, K. et al. Construction and application of base-stable MOFs: a critical review. Chem. Soc. Rev. 51, 6417–6441 (2022).
Wang, B. et al. Highly stable Zr(IV)-based metal−organic frameworks for the detection and removal of antibiotics and organic explosives in water. J. Am. Chem. Soc. 138, 6204–6216 (2016).
Acknowledgements
This research was supported by the National Science Foundation of China (52073251, B.L. and U22A20251, G.Q.), the Zhejiang Provincial Natural Science Foundation of China (No. LR22E030003, B.L.), and the Science Technology Department of Zhejiang Province (2022C01225, G.Q.).
Author information
Authors and Affiliations
Contributions
E.W. and X.-W.G. synthesized and characterized the MOF samples, and measured the adsorption isotherms. E.W., X.Z., H.W., and W.Z. collected and analyzed the single-crystal X-ray diffraction data. E.W., X.-W.G., and D.L. measured and analyzed the breakthrough data. B.L. and G.Q. directed and supervised the project. B.L. wrote the paper, and all authors contributed to revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Gaurav Verma and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, E., Gu, XW., Liu, D. et al. Incorporation of multiple supramolecular binding sites into a robust MOF for benchmark one-step ethylene purification. Nat Commun 14, 6146 (2023). https://doi.org/10.1038/s41467-023-41692-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41692-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.