Abstract
High-throughput experimentation (HTE) is an increasingly important tool in reaction discovery. While the hardware for running HTE in the chemical laboratory has evolved significantly in recent years, there remains a need for software solutions to navigate data-rich experiments. Here we have developed phactor™, a software that facilitates the performance and analysis of HTE in a chemical laboratory. phactor™ allows experimentalists to rapidly design arrays of chemical reactions or direct-to-biology experiments in 24, 96, 384, or 1,536 wellplates. Users can access online reagent data, such as a chemical inventory, to virtually populate wells with experiments and produce instructions to perform the reaction array manually, or with the assistance of a liquid handling robot. After completion of the reaction array, analytical results can be uploaded for facile evaluation, and to guide the next series of experiments. All chemical data, metadata, and results are stored in machine-readable formats that are readily translatable to various software. We also demonstrate the use of phactor™ in the discovery of several chemistries, including the identification of a low micromolar inhibitor of the SARS-CoV-2 main protease. Furthermore, phactor™ has been made available for free academic use in 24- and 96-well formats via an online interface.
Similar content being viewed by others
Introduction
Miniaturized high-throughput experimentation (HTE) has emerged as an accessible, reliable, economical, and environmentally friendly technique for the rapid discovery of new reactivities1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. Curated HTE data has proven to be increasingly valuable for predictive models15,16,17,18,19,20. While in experimental practice, the community has gravitated towards liquid handling techniques in glass shell microvials with tumble stir dowels, or in plastic 384 or 1536 wellplates1,4,5,6,7,8,9,10,11,12,13,14, a standard for HTE data handling has yet to be established. The organizational load required to perform a simple 24-well reaction array is generally manageable by repetitive notebook entries or with spreadsheets, yet managing multiple reaction arrays in a single day, or running ultraHTE in 1536 wellplates1, is challenging without information management software. Moreover, no readily available electronic lab notebook (ELN) can store HTE details in a tractable manner21,22 or provide a simple interface to extract data and results from multiple experiments simultaneously23,24. Contemporary HTE software provides HTE solutions but is only commercially available34,35. To continue developing HTE research and position data outputs for machine learning studies, detailed reaction data must be easily accessible for standardized rapid extraction and analysis25,26,27.
With these issues in mind, we developed the software phactor™ to streamline the collection of HTE reaction data. Our primary objective was to develop a robust yet generalizable HTE workflow solution that captures the nuances of chemical experimentation while reporting data in a standardized, machine-readable format. phactor™ minimizes the time and resources spent between experiment ideation and result interpretation. This enables creativity by freeing up time otherwise used thinking about experiment logistics, facilitates reaction discovery and optimization, and serves as a tool to bolster the amount of available reaction data reported in a standardized format. We have provided phactor™ as a free web service to the academic community, currently supporting 24- and 96-well formats, which can be accessed at https://phactor.cernaklab.com.
Results
phactor™ workflow overview
The workflow of a typical high-throughput experiment involves the design of the reaction array, preparation of reagent stock solutions, dosing of stock solutions according to the reaction array recipe (either by hand or with robotics), and evaluation of reaction outcome, followed by visualization and analysis of data and documentation of results. A standardized reaction template classifies substrates, reagents, and products (Fig. 1a). Interconnecting experimental results with online chemical inventories through this shared data format creates a closed-loop workflow for HTE-driven chemical research (Fig. 1b) and enables rapid reaction array design and analytics. While developing phactor™, we sought to maximize the automation of data movement and processing. Recognizing the rapidly accelerating chemical research software ecosystem28,29,30,31,32,33,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50, the philosophy behind phactor™’s data structure was to record experimental procedures and results in a machine-readable yet simple, robust, and abstractable format to naturally translate to other system languages (Supplementary Fig. 8). As such, the inputs and outputs of phactor™ can be procedurally generated or modified with basic Excel or Python knowledge to interface with any robot, analytical instrument, software, or custom chemical inventory containing metadata (e.g., the organophosphorous ligand platform Kraken51) such as reagent location, molecular weight, CAS number or SMILES string. Examples of interfacing phactor™ outputs with ORD20, XDL50, or EDBO+48 are shown in Supplementary Figs. 14–16.
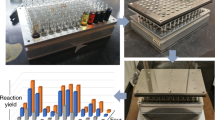
a Anatomy of a reaction as encoded by phactor™. b High-level software workflow of phactor™. Reaction arrays are designed from chemical inventories and reaction informatics. Resultant data is stored in delimited text (CSV) or in a relational database (SQLite3). phactor™ can convert results to Open Reaction Database (ORD)20 and Chemical Description Language (XDL)50 and is readily compatible with optimization programs such as EDBO+48 and LabMate.ML49. c Workflow of phactor™. Once the reaction array is designed, phactor™ provides human-readable or machine instructions to execute the dosing manually or robotically (UPLC ultra-performance liquid chromatography). d phactor™ supports custom volumes allowing for reaction arrays to be performed at any scale. At a minimum, the hardware needed to execute a reaction array is an autopipette and an array reactor block. e phactor™ facilitates the design and execution of ultraHTE in 1536 wellplates.
The event workflow of a typical phactor™ experiment is shown in Fig. 1c. The user selects desired reagents from the inventory for automatic field population or enters specific reagent entries manually, such as for a custom substrate. Once all relevant chemicals are selected, the reaction array layout is designed automatically or manually, as the user prefers. Reagent distribution instructions are generated to be executed either manually or by an interfacing liquid handling robot. Last-minute changes in the face of unforeseen circumstances during reaction setups, such as poor chemical solubility, chemical instability, or the need to premix reagents before dosing can be made at any time. Stock solutions are prepared in vials or wellplates and distributed to their respective locations on the reaction wellplate. Once the reactions are complete, they are quenched and analysed. Any data with a well-location map can be uploaded. This allows both data on reaction performance (e.g., UPLC-MS conversion) and biological assay results (e.g., bioactivity data) to be viewed in concert.
The workflows for executing HTE can vary depending on available equipment and desired throughput of the experiment. phactor™ incorporates these parameters into its user interface to ensure a consistent workflow experience agnostic of hardware capabilities (Fig. 1d). Examples herein demonstrate phactor™’s use with manual dosing as well as integration with the Opentrons OT-2 liquid handling robot for experiments of 384-well throughput or less, and the SPT Labtech mosquito® robot for 1536-well ultraHTE (Fig. 1e). Regardless of instrumentation or throughput, all results are stored in the same format, facilitating analysis of results across multiple experiments. Reaction discovery and library synthesis campaigns utilizing standard 24-, 96-, 384- and 1536-well experiments are described in detail52,53,54,55,56,57.
Experimental analysis
Phactor™ facilitates the discovery of new reactivity. Our lab is broadly interested in amine-acid coupling reactions52,53,55,56,57 and particularly amine-acid C–C coupling reactions55,57. Diverse chemistries discovered with the aid of phactor™ are shown in Fig. 2. Figure 2a shows the discovery of a deaminative aryl esterification53. In the reaction array design, an amine, activated as its diazonium salt (1), a carboxylic acid (2), one of three transition metal catalysts, with one of four ligands, in the presence or absence of a silver nitrate additive was to be dosed to each reaction well in acetonitrile, then stirred at 60 °C for 18 h. phactor™ automatically designed the reagent distribution recipe by splitting the plate into a simple four-row and six-column multiplexed array. At completion, a solution containing one molar equivalent of caffeine was added to each well as an internal standard. An aliquot of each reaction was transferred to a plastic wellplate, then diluted with acetonitrile for UPLC–MS analysis of the desired ester product (3). UPLC–MS output files were analysed by the commercial software Virscidian Analytical Studio, which provided a CSV file containing peak integration values for each of the 24 chromatographic traces. This file was fed into phactor™ to record the experimental outcome and produce the heatmap shown in Fig. 2a. Analysis on phactor™ indicated an 18.5% assay yield when using 30 mol% CuI, pyridine, and AgNO3, and these specific conditions were triaged for further study.
The reaction array design and results are shown here as displayed on phactor™. Colour bars adjacent to compound numbers correspond to the colour bars in the reaction array design grid generated by phactor™. Product/internal standard ratios are calculated using the observed UV-derived peak area, while assay yields account for differences in product absorptivity by calibrating to authentic samples of products. a Preliminary esterification hit leading to publication53. b Optimized oxidative indolization conditions towards the synthesis of umifenovir54. c Allylation catalyst/ligand concentration ratio and base reaction array analysed by conversion and selectivity.
In the example of Fig. 2b, we optimized the penultimate step in our synthesis of umifenovir54, an oxidative indolization reaction between 4 and 5 to produce 6. Inspired by the conditions of Glorius58, a reaction array was performed using copper catalysts and ligand/additive combinations. Four copper sources at 20 mol%: cuprous iodide, cuprous bromide, tetrakis(acetonitrile) copper(I) triflate, or cupric acetate, were distributed into the four rows while combinations of magnesium sulfate (0.0 equiv or 1.0 equiv) with 2-(1H-tetrazol-1-yl)acetic acid (L1), or 2,6-dimethylanilino(oxo)acetic acid (L2) at 40 mol% were distributed into the columns as DMSO solutions, with 3.0 equivalents of caesium carbonate added to every well as a suspension in DMSO. The reactions were manually arrayed in a glovebox, sealed, and stirred at 55 °C for 18 h. Well B3 (copper bromide with L1 and no magnesium sulfate) was found to perform best, and a 0.10 mmol scale-up reaction produced desired indole 6 in 66% isolated yield.
In Fig. 2c, the allylation of furanone 7 or furan 8 with reagents 9 or 10 was investigated. For each combination of nucleophile and electrophile, one of three ratios of Pd2dba3 to (S,S)-DACH-phenyl Trost ligand L3 was added, followed by the addition or omission of potassium carbonate as a base. Each reaction was run in toluene for 24 h at room temperature, quenched, and then analysed by UPLC–MS for conversion and selectivity. Multiplexed pie charts generated by phactor™ revealed that the conditions of well D3, with a 2:1 palladium catalyst to ligand loading and no base, generated the desired γ-regioisomer with the greatest selectivity, along with α-allylation and its olefin isomer when 8 was used.
An organocatalyzed asymmetric Mannich reaction is detailed in Supplementary Fig. 17. Aldehyde 13, p-anisidine (14), and ketone 15 are used to form desired product 16 via a solvent and catalyst reaction array that was analysed by TLC, which revealed the formation of undesired product 17.
With phactor™, HTE becomes an exercise in workflow execution, with automation of the organizational aspect of the experiment. This allows chemists to focus on the design and analysis of the reaction array, rather than workflow details (Fig. 3). Figure 3a–c displays three examples of 24-well experiments. Figure 3a shows an amide coupling reaction array performed in preparation for an ultra-high throughput direct-to-biology assay (vide infra), with the aim of producing inhibitors of the SARS-CoV-2 main protease (MPro)59. A screen of three anilines, two coupling agents HATU or DCC/HOBt, and a carboxylic acid, with or without base, produced all desired products. Amide 18 was produced in high conversion using HATU, DMAP, and DIPEA. Figure 3b and c detail the optimization of a recently published deaminative decarboxylative sp2–sp3 C–C coupling from an acid-activated as an N-acyl-glutarimide and an amine activated as a Katritzky salt55. Reaction array 3b screened 24 ligands in NMP under nickel catalysis to generate product 19, which was subsequently found with improved yield when using a bipyridine ligand and phthalimide as additives (Fig. 3c).
a–i Chemistries discovered via reaction arrays designed with phactor™. All input and output files used to produce reaction arrays (a–f) are provided via an online repository in addition to all compiled HTE results in a machine-readable format. Reaction schemes can be found in the “Selected screening examples” section of the Supplementary Information.
Figure 3d–f contains 96-well experiments designed with phactor™. Figure 3d and e show the results from a recently reported deaminative-decarboxylative sp3–sp3 C–C coupling55 that generated products 20 and 21. Three catalysts, four additives, four ligands, and two reductants were tested in Fig. 3d, and it was found that 20 was afforded at 47% product/internal standard with NiCl2•glyme as the catalyst, 4,4’-di-tert-butyl-2,2-bipyridine as ligand, zinc as the reductant, and lithium perchlorate as an additive. After additional optimization, the reaction array in Fig. 3e was run to test the reaction’s scope. Product 21 was afforded with optimal conditions of NiBr2•glyme, 4,4’-bis-trifluoromethyl-2,2’-bipyridine, and manganese in 1:1 dioxane:acetonitrile. Figure 3f shows the results of a 12-ligand—eight metal catalyst reaction array further developing the analogous sp2–sp3 decarboxylative deaminative C–C coupling, optimizing the yield of model product 2257.
Figure 3g–i show several ultraHTE reaction arrays. Figure 3g and h are reaction arrays produced in the development of our aryl amine esterification reaction53. Both reaction arrays were substrate-scope experiments, producing ester compounds such as 23 and 24. In Fig. 3g, lutidine and Cu(MeCN)4BF4 in benzonitrile showed good scope. Replacing lutidine with collidine expanded the reaction scope and reproducibility across quadruplicate measurements as shown in Fig. 3h. Finally, Fig. 3i contains the UPLC–MS results of a direct-to-biology reaction array towards the creation of SARS-CoV-2 MPro inhibitors, generating amides such as 25. Files needed for all 24- and 96-well experiments shown in Fig. 3 are provided as examples to learn the phactor™ workflow. The layouts for several reaction arrays are displayed in the Provided Examples section of the Supplementary Information.
Discovery of a low micromolar inhibitor of SARS-CoV-2 MPro via direct-to-biology assay
An amide coupling experiment was planned based on an inventory of amines, largely anilines, and a tetrahydroquinoline carboxylic acid pharmacophore (26) found to be a potent inhibitor of MPro59. A preliminary 24-well amide coupling reaction array was performed to test the effectiveness of various amide coupling conditions for the acid and anilines (see Fig. 3a and Supplementary Fig. 21). A diluted aliquot from each well was subjected to a RapidFire™ MS binding assay to determine concentration-response curves60. Curves were found to correlate with yield and literature reported IC50 values were replicated utilizing this assay59. Following an optimized direct-to-biology ultraHTE workflow (Fig. 4a), amide coupling reactions were executed with the aim of making diverse amides, which were directly tested for activity against MPro in a single experiment. Conducting an experiment of this complexity using spreadsheet software would be challenging. However, with the use of phactor™, ultraHTE, and direct-to-biology experiments can be swiftly developed and assessed within a matter of minutes, especially if corresponding input or inventory files are in hand. Eight amide coupling conditions were tested in duplicate for each of the 80 amines, resulting in 1280 reactions (Fig. 4b). A key step in this workflow is the distribution of the 1536-well reaction plate into four 384-well analysis assay plates suitable for UPLC–MS or RapidFire™ analysis. As such, some of the wells in the 1536-well reaction plate were not utilized to account for the four control columns necessary in each of the four 384-well RapidFire™ assay plates to allow the calculation of Z prime for the assay (0.961)61. This distribution as well as the chemical and biological assay results are shown in Fig. 4c and d. Additional data analyses comparing chemical yield to biological response are shown in Supplementary Figs. 24 and 25. We note that reproducibility is a common concern in HTE and ultraHTE, and analyses of repeat experiments are provided in Supplementary Fig. 26. Both chemistry and biology assays are shown to be consistent, with 87% and 93% of data points having <10% error in the respective assays. From these analyses, three amides (27–29) were chosen for scale-up and IC50 determination, two of which (27 and 28) were previously unreported in the literature. Compound 28 was found to have an IC50 of 5.06 µM (Fig. 4e), competitive with the best-known MPro inhibitors in this series59. Notably, IC50 trends from pure compounds isolated on a larger reaction scale are well matched to the percent inhibition trends obtained on the nanomole scale.
a Event workflow for performing ultraHTE using phactor™ and a Mosquito robot. b Design of 1280 well amide coupling plate. 80 amines were selected to react with carboxylic acid 26. Eight conditions were run in duplicate for each amine. c Results of the amide coupling are shown as a product/internal standard integration ratio from a 2-min LCMS injection of each well. The Mosquito robot is utilized to split the size 1536 plate into four sizes 384 plates for LCMS and bioassay analysis. d Percent inhibition of SARS-CoV-2 MPro when treated with a sample of the reaction mixture from the corresponding well. The 1280 plate is visually recreated. e IC50 curves for three scaled-up compounds chosen from the reaction array. Compounds 27–29 display a range of assay and inhibitory responses.
Methods
General phactor™ workflow for general use and to recreate the chemistry reported in this and other manuscripts52,53,54,55,56,57
The workflow for phactor™ contains six stages: Settings, Factors, Chemicals, Analysis, and Report (Fig. 5). The first stage, Settings, simply begins the plate development process when provided an experiment name, reaction volume, and throughput (Supplementary Fig. 1a). The remaining stages can be optionally expedited with pre-generated workflow files that match a specified format.
The workflow files needed to reproduce the chemistry shown in Figs. 2a, b, and 3a–f are provided in an online repository. To replicate these studies, a 24- or 96-well reactor block, the corresponding amount of glass vials and stirbars, a hotplate with magnetic stirring, and autopipette will be required. The necessary solvents and reagents will also be required to the amounts as calculated on phactor™. Detailed specifications of hardware and chemical sources are provided in the “Experimental” section of the Supplementary Information. Having these files in hand provides an expedited utilization of phactor™—the user can directly drop any file that matches the chemical input specification directly into the Chemicals stage and click ‘Run’ to instantly get the procedural instructions for the experiments. Likewise dropping the provided corresponding analytical file into Analysis will display the reported results of the experiment.
Phactor™ can automatically distribute a list of chemicals into a multiplexed reaction array given that the chemicals are labelled as one of the reagent classes within the set {Electrophile, Nucleophile, Catalyst1, Catalyst2, Ligand1, Ligand2, Base/Acid, Reductant/Oxidant, Solvent1, Additive, or Other} and that the product of the amounts of each reagent class within the list is equal to or less than the wellplate size. For instance, a list containing six electrophiles, four nucleophiles, and a solvent can be automatically “plated” by phactor™. The desired plate design is specified in the Factors stage (Supplementary Fig. 1b).
Once the factors have been input and saved, the user can add reagents to be used in the experiment in the Chemicals stage (Supplementary Fig. 1c). These reagents can be either input by hand via textbox, where the user personally reports the reagent name, molecular weight, reagent class label, and density or reagents can be added directly from an online inventory. All chemicals are associated with a colour, which can be changed by clicking on the chemical’s corresponding colour bar in the table. If factors were specified in the previous stage, a checklist is provided to ensure the requirements for the automatic distribution are met. Once all lines are green, hitting run will design the plate on the following Grid stage. A third alternative to populating reagents is to upload a CSV file containing reagent data in a specified format. As mentioned, example files corresponding to the experiments in this manuscript are provided in an online repository. In the case where the input reagent file contains a distribution of reagents that would perfectly fill out the reaction plate, the factors are automatically updated and thus factors need not be specified in the factors stage.
Following the Chemicals stage is the Grid stage, which is prepopulated if the automatic distribution feature was utilized. At this stage, an interactive grid allows the user to add or remove reagents to wells in bulk. Individual wells in the digital reaction array can be selected to view reaction details. In addition to the reaction array grid, a table containing the recipes for stock solutions, which indicate the mass of reagent and volume of solvent to be added to each source vial. The volume and locations of aliquots from each source vial are shown as well, to be executed via autopipette or liquid handling robot. Finally, a suite of buttons interfacing the designed reaction array with a variety of hardware and software is provided. On the provided version, MassLynx and Virscidian files are preformatted and provided with prepopulated metadata to facilitate the characterization of the reaction array.
When the reaction array is fully designed, the recipe can be downloaded. Stock solutions are dosed according to the generated procedure, and the reaction plate is moved to a reactor with the appropriate atmosphere, pressure, and temperature. After the designated reaction time has elapsed, the reactions are quenched, and aliquots of each reaction are transferred to an analytical plate containing internal standard in a UPLC–MS compatible solvent. Once the analytical plate has been characterized, the resultant file containing well locations and output results can be dropped into the Analysis stage of phactor™. Here an interactive triptych display allows the user to view several output results in the same view. Clicking on a well displays the input and outputs of the reaction, as well as an image of the product if specified.
The final stage is Report. An overall one-page summary of the results is shown here via visualizations of inputs and outputs. Additionally, statistics of the results are calculated, and the top-performing reagents are shown. Most importantly, here is where the user can download the compiled results of the entirety of the reaction array in a single CSV file. This file contains the inputs and metadata associated with each reaction of the reaction array, including reagent labels, SMILES, molarities, and solvents, as well as the output values associated with each reaction. This file can be trivially collated with other plate outputs to make supermassive reaction datasets.
Machine learning Python scripts for phactor™ output
In the provided repository an example Python notebook is provided to facilitate downstream machine learning analyses from the phactor™ output file. In this example notebook, tSNE plots are generated from collated from a deaminative-decarboxylative sp3–sp3 C–C coupling optimization campaign dataset (Fig. 6). Product SMILES are encoded into fingerprints using RDKit, and fingerprint matrices are fed into the tSNE algorithm to reduce the dimensionality of the dataset into two dimensions. These are subsequently plotted and coloured by the average output value of each product to provide a rapid visualization of the reaction manifold. Areas of reaction success and failure are then readily identified in a singular display. Notebooks for conversion to heatmaps and kernel density estimate plots are also provided. Sample notebooks converting phactor™ output to ORD, EDBO+, and XDL are also provided within the repository. All the provided scripts can be easily modified by changing the input file string to work with other datasets.
Standardized output files can be rapidly merged to create massive datasets. Shown is a tSNE (t-distributed stochastic neighbour embedding) of all products made in the decarboxylative–deaminative sp3–sp3 C–C coupling detailed in ref. 55, coloured by average product/internal standard.
In conclusion, we present the HTE ELN phactor™, which records all details of an experiment to allow for robust reproduction and accelerated discovery. phactor™ stores all experimental details in a machine-readable yet tractable and interpretable format with an SQL database and on the cloud to facilitate the use of downstream statistical analysis. As all reaction arrays are stored in a centralized database, bulk analyses of multiple reaction arrays can be performed. phactor™ provides an exposed API that can be used to develop interfaces to other robots, assays, and software. Examples of various integrations and code infrastructures are shown in the “Discussion” section of the Supplementary Information. Furthermore, we are currently developing additional integrations with AI and ML models to generate and evaluate reaction arrays62 and to discover additional amine acid couplings via automated mining of relevant literature63,64,65,66,67. We hope that phactor™’s ease of use provides increased accessibility to HTE and HTE data in the chemistry community. Registration-free and non-commercial use of phactor™ in 24- and 96-well formats is available through https://phactor.cernaklab.com/.
Inclusion and ethics
Local researchers were included throughout the entirety of the research process, and all roles and responsibilities were agreed to ahead of the research.
Data availability
All sample files and data generated in this study have been deposited in an online GitHub repository68. https://github.com/cernaklab/public-phactor-example-files.
Code availability
All relevant interfacing scripts generated in this study have been deposited in an online GitHub repository68. https://github.com/cernaklab/public-phactor-example-files.
References
Mahjour, B., Shen, Y. & Cernak, T. Ultrahigh-throughput experimentation for information-rich chemical synthesis. Acc. Chem. Res. 54, 2337–2346 (2021).
Shen, Y. et al. Automation and computer-assisted planning for chemical synthesis. Nat. Rev. Methods Prim. 1, 1–23 (2021).
Mennen, S. M. et al. The evolution of high-throughput experimentation in pharmaceutical development and perspectives on the future. Org. Process Res. Dev. 23, 1213–1242 (2019).
Wong, H. & Cernak, T. Reaction miniaturization in eco-friendly solvents. Curr. Opin. Green Sustain. Chem. 11, 91–98 (2018).
Uehling, M. R., King, R. P., Krska, S. W., Cernak, T. & Buchwald, S. L. Pharmaceutical diversification via palladium oxidative addition complexes. Science 363, 405 (2019).
Lin, S. et al. Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS. Science 361, eaar6236 (2018).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Cernak, T. et al. Microscale high-throughput experimentation as an enabling technology in drug discovery: application in the discovery of (piperidinyl)pyridinyl-1H-benzimidazole diacylglycerol acyltransferase 1 inhibitors. J. Med. Chem. 60, 3594–3605 (2017).
Kutchukian, P. S. et al. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 7, 2604–2613 (2016).
Buitrago Santanilla, A. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49 (2015).
Gesmundo, N. et al. Miniaturization of popular reactions from the medicinal chemists’ toolbox for ultrahigh-throughput experimentation. Nat. Synth. https://doi.org/10.1038/s44160-023-00351-1 (2023).
Krska, S. W., DiRocco, D. A., Dreher, S. D. & Shevlin, M. The evolution of chemical high-throughput experimentation to address challenging problems in pharmaceutical synthesis. Acc. Chem. Res. 50, 2976–2985 (2017).
Wagen, C. C., McMinn, S. E., Kwan, E. E. & Jacobsen, E. N. Screening for generality in asymmetric catalysis. Nature https://doi.org/10.1038/s41586-022-05263-2 (2022).
Shevlin, M. Practical high-throughput experimentation for chemists. ACS Med. Chem. Lett. 8, 601–607 (2017).
Zuranski, A. M., Martinez Alvarado, J. I., Shields, B. J. & Doyle, A. G. Predicting reaction yields via supervised learning. Acc. Chem. Res. 54, 1856–1865 (2021).
Schleinitz, J. et al. Machine learning yield prediction from NiCOlit, a small-size literature data set of nickel catalyzed C–O couplings. J. Am. Chem. Soc. 144, 14722–14730 (2022).
Stevens, J. M. et al. Advancing base metal catalysis through data science: insight and predictive models for Ni-catalyzed borylation through supervised machine learning. Organometallics 41, 1847–1864 (2022).
Lexa, K. W. et al. Application of machine learning and reaction optimization for the iterative improvement of enantioselectivity of cinchona-derived phase transfer catalysts. Org. Process Res. Dev. 26, 670–682 (2021).
Beker, W. et al. Machine learning may sometimes simply capture literature popularity trends: a case study of heterocyclic Suzuki–Miyaura coupling. J. Am. Chem. Soc. 144, 4819–4827 (2022).
Kearnes, S. M. et al. The open reaction database. J. Am. Chem. Soc. 143, 18820–18826 (2021).
Kanza, S. et al. Electronic lab notebooks: can they replace paper? J. Cheminform. 9, 1–15 (2017).
Higgins, S. G., Nogiwa-Valdez, A. A. & Stevens, M. M. Considerations for implementing electronic laboratory notebooks in an academic research environment. Nat. Protoc. 17, 179–189 (2022).
Tremouilhac, P. et al. Chemotion ELN: an open source electronic lab notebook for chemists in academia. J. Cheminform. 9, 54 (2017).
Barillari, C. et al. openBIS ELN-LIMS: an open-source database for academic laboratories. Bioinformatics 32, 638–640 (2016).
Cole, J. M. The chemistry of errors. Nat. Chem. 14, 973–975 (2022).
Christ, C. D., Zentgraf, M. & Kriegl, J. M. Mining electronic laboratory notebooks: analysis, retrosynthesis, and reaction based enumeration. J. Chem. Inf. Model. 52, 1745–1756 (2012).
Yano, J. et al. The case for data science in experimental chemistry: examples and recommendations. Nat. Rev. Chem. 6, 357–370 (2022).
Roch, L. M. et al. ChemOS: an orchestration software to democratize autonomous discovery. PLoS ONE 15, e0229862 (2020).
Christensen, M. et al. Data-science driven autonomous process optimization. Commun. Chem. 4, 1–12 (2021).
Häse, F. et al. Olympus: a benchmarking framework for noisy optimization and experiment planning. Mach. Learn.: Sci. Technol. 2, 035021 (2021).
Shiri, P. et al. Automated solubility screening platform using computer vision. Iscience 24, 102176 (2021).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Daponte, J. A., Guo, Y., Ruck, R. T. & Hein, J. E. Using an automated monitoring platform for investigations of biphasic reactions. ACS Catal. 9, 11484–11491 (2019).
ACD/Labs. Katalyst D2D. https://www.acdlabs.com/products/spectrus-platform/katalyst-d2d/ (2023).
Unchained Labs. LEA. https://www.unchainedlabs.com/unchained-labs-makes-automation-workflows-cinch-releases-lea-9/ (2023).
Lu, J., Paci, I. & Leitch, D. C. A broadly applicable quantitative relative reactivity model for nucleophilic aromatic substitution (SN Ar) using simple descriptors. Chem. Sci. 13, 12681–12695 (2022).
Lu, J., Donnecke, S., Paci, I. & Leitch, D. C. A reactivity model for oxidative addition to palladium enables quantitative predictions for catalytic cross-coupling reactions. Chem. Sci. 13, 3477–3488 (2022).
Allen, C. L., Leitch, D. C., Anson, M. S. & Zajac, M. A. The power and accessibility of high-throughput methods for catalysis research. Nat. Catal. 2, 2–4 (2019).
Angello, N. H. et al. Closed-loop optimization of general reaction conditions for heteroaryl Suzuki–Miyaura coupling. Science 378, 399–405 (2022).
Molga, K. et al. A computer algorithm to discover iterative sequences of organic reactions. Nat. Synth. 1, 49–58 (2022).
Molga, K., Szymkuc, S. & Grzybowski, B. A. Chemist ex machina: advanced synthesis planning by computers. Acc. Chem. Res. 54, 1094–1106 (2021).
Mikulak-Klucznik, B. et al. Computational planning of the synthesis of complex natural products. Nature 588, 83–88 (2020).
Badowski, T., Gajewska, E. P., Molga, K. & Grzybowski, B. A. Synergy between expert and machine‐learning approaches allows for improved retrosynthetic planning. Angew. Chem. Int. Ed. 59, 725–730 (2020).
Gajewska, E. P. et al. Algorithmic discovery of tactical combinations for advanced organic syntheses. Chem 6, 280–293 (2020).
Jaworski, W. et al. Automatic mapping of atoms across both simple and complex chemical reactions. Nat. Commun. 10, 1434 (2019).
Grzybowski, B. A. et al. Chematica: a story of computer code that started to think like a chemist. Chem 4, 390–398 (2018).
Gallegos, L. C., Luchini, G., St. John, P. C., Kim, S. & Paton, R. S. Importance of engineered and learned molecular representations in predicting organic reactivity, selectivity, and chemical properties. Acc. Chem. Res. 54, 827–836 (2021).
Torres, J. A. G. et al. A multi-objective active learning platform and web app for reaction optimization. J. Am. Chem. Soc. 144, 19999–20007 (2022).
Reker, D., Hoyt, E. A., Bernardes, G. J. L. & Rodrigues, T. Adaptive optimization of chemical reactions with minimal experimental information. Cell Rep. Phys. Sci. 1, 100247 (2020).
Hammer, A. J. S., Leonov, A. I., Bell, N. L. & Cronin, L. Chemputation and the standardization of chemical informatics. JACS Au 1, 1572–1587 (2021).
Gensch, T. et al. A comprehensive discovery platform for organophosphorus ligands for catalysis. J. Am. Chem. Soc. 144, 1205–1217 (2022).
Mahjour, B., Shen, Y., Liu, W. & Cernak, T. A map of the amine–carboxylic acid coupling system. Nature 580, 71–75 (2020).
Shen, Y., Mahjour, B. & Cernak, T. Development of copper-catalyzed deaminative esterification using high-throughput experimentation. Commun. Chem. 5, 1–8 (2022).
Lin, Y. et al. Reinforcing the supply chain of umifenovir and other antiviral drugs with retrosynthetic software. Nat. Commun. 12, 7327 (2021).
Zhang, Z. & Cernak, T. The formal cross-coupling of amines and carboxylic acids to form sp3–sp3 carbon–carbon bonds. Angew. Chem. Int. Ed. 60, 27293–27298 (2021).
McGrath, A., Zhang, R., Shafiq, K. & Cernak, T. Repurposing amine and carboxylic acid building blocks with an automatable esterification reaction. Chem. Commun. 59, 1026–1029 (2023).
Douthwaite, J. L. et al. The formal cross-coupling of amines and carboxylic acids to form sp3–sp2 carbon–carbon bonds. J. Am. Chem. Soc. 145, 10930–10937 (2023).
Wang, C., Rakshit, S. & Glorius, F. Palladium-catalyzed intermolecular decarboxylative coupling of 2-phenylbenzoic acids with alkynes via C−H and C−C bond activation. J. Am. Chem. Soc. 132, 14006–14008 (2010).
Rossetti, G. G. et al. Non-covalent SARS-CoV-2 Mpro inhibitors developed from in silico screen hits. Sci. Rep. 12, 2505 (2022).
Malla, T. R. et al. Mass spectrometry reveals potential of β-lactams as SARS-CoV-2 M pro inhibitors. Chem. Commun. 57, 1430–1433 (2021).
Zhang, J.-H., Chung, T. D. Y. & Oldenburg, K. R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4, 67–73 (1999).
Mahjour, B., Hoffstadt, J. & Cernak, T. Designing chemical reaction arrays using phactor and ChatGPT. Preprint at Chem Rxiv https://doi.org/10.26434/chemrxiv-2023-2tfdv (2023).
Lu, P., Sanchez, C., Cornella, J. & Larrosa, I. Silver-catalyzed protodecarboxylation of heteroaromatic carboxylic acids. Org. Lett. 11, 5710–5713 (2009).
Gooßen, L. J., Linder, C., Rodríguez, N., Lange, P. P. & Fromm, A. Silver-catalysed protodecarboxylation of carboxylic acids. Chem. Commun. 7173–7175 https://doi.org/10.1039/B912509D (2009).
Dow, N. W. et al. Decarboxylative borylation and cross-coupling of (hetero) aryl acids enabled by copper charge transfer catalysis. J. Am. Chem. Soc. 144, 6163–6172 (2022).
Xu, P., López-Rojas, P. & Ritter, T. Radical decarboxylative carbometalation of benzoic acids: a solution to aromatic decarboxylative fluorination. J. Am. Chem. Soc. 143, 5349–5354 (2021).
Witzel, S. et al. Excitation of aryl cations as the key to catalyst-free radical arylations. Cell Rep. Phys. Sci. 2, 100325 (2021).
Mahjour, B. et al. Rapid planning and analysis of high-throughput experiment arrays for reaction discovery. GitHub https://doi.org/10.5281/zenodo.7951534 (2023).
Acknowledgements
O.G.M. and A.T. thank Michigan Drug Discovery for supporting the MPro inhibition screening assay (MDD-22105S to A.T.). We would like to thank the Center for Structural Biology and the Proteomics and Peptide Synthesis core at the University of Michigan for supplying the MPro enzyme and the substrate peptide for the MPro inhibition assay, respectively. We would like to thank the Center for Chemical Genomics at the University of Michigan for providing access to different instruments. We gratefully acknowledge funding from MilliporeSigma (Y.L.), Relay Therapeutics (J.D.), Janssen Therapeutics (A.M.), the National Science Foundation (CHE-2236215) (T.C.) an ACS MEDI Predoctoral Fellowship (B.M.), as well as generous gifts of liquid handling automation from SPT Labtech and Merck & Co., Inc. Initial studies were funded by startup funds from the University of Michigan College of Pharmacy. We gratefully acknowledge funding from Schmidt Futures.
Author information
Authors and Affiliations
Contributions
B.M. wrote and deployed the software and developed, performed, and analysed chemical and biological experiments. B.M., Y.S., Y.L., and R.Zhao. performed chemical experiments. O.G.M. and A.T. performed the inhibition assay. B.M. and T.C. wrote the manuscript with input from all authors. T.C. conceived and supervised the project. R.Z, Y.S, A.M., Y.L., Z.Z, J.L.D., and T.C. provided feedback on software development. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The Cernak Lab has received research funding or in-kind donations from MilliporeSigma, Relay Therapeutics, Janssen Therapeutics, SPT Labtech, and Merck & Co., Inc. T.C. holds equity in Scorpion Therapeutics and is a cofounder of and equity holder in Entos, Inc. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahjour, B., Zhang, R., Shen, Y. et al. Rapid planning and analysis of high-throughput experiment arrays for reaction discovery. Nat Commun 14, 3924 (2023). https://doi.org/10.1038/s41467-023-39531-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-39531-0
This article is cited by
-
Exploring the combinatorial explosion of amine–acid reaction space via graph editing
Communications Chemistry (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.