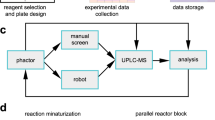
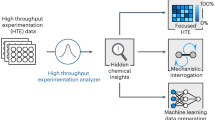
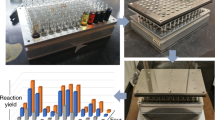
Abstract
Miniaturization is a tactic employed in many technologies to accelerate discovery through parallelized data collection. The miniaturization of chemical synthesis to the limits of chemoanalytical and bioanalytical detection could accelerate drug discovery by increasing the amount of experimental data collected per milligram of material consumed. Here we demonstrate the miniaturization of popular reactions used in drug discovery such as reductive amination, N-alkylation, N-Boc (tert-butyloxycarbonyl) deprotection and Suzuki coupling for utilization in 1.2 µl reaction droplets. Reaction methods were evolved to perform in high-boiling solvents at room temperature, enabling the diversification of precious starting materials, such as the complex natural product staurosporine.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The supporting information contains general information, experimental procedures, supplementary tables and images, and compound data and spectra. All raw high-throughput data can be accessed at https://github.com/cernaklab/medchem-reaction-miniaturization/tree/main.
References
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Faller, B., Ottaviani, G., Ertl, P., Berellini, G. & Collis, A. Evolution of the physicochemical properties of marketed drugs: can history foretell the future? Drug Discov. Today 16, 976–984 (2011).
Leeson, P. D. & Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov. 6, 881–890 (2007).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Mahjour, B., Shen, Y., Liu, W. & Cernak, T. A map of the amine–carboxylic acid coupling system. Nature 580, 71–75 (2020).
Shen, Y. et al. Automation and computer-assisted planning for chemical synthesis. Nat. Rev. Methods Prim. 1, 23 (2021).
Shim, E. et al. Predicting reaction conditions from limited data through active transfer learning. Chem. Sci. 13, 6655–6668 (2022).
Mahjour, B., Shen, Y. & Cernak, T. Ultrahigh-throughput experimentation for information-rich chemical synthesis. Acc. Chem. Res. 54, 2337–2346 (2021).
Moore, G. M. Cramming more components onto integrated circuits. Electronics 38, 114 (1965).
Krska, S. W., DiRocco, D. A., Dreher, S. D. & Shevlin, M. The evolution of chemical high-throughput experimentation to address challenging problems in pharmaceutical synthesis. Acc. Chem. Res. 50, 2976–2985 (2017).
Wong, H. & Cernak, T. Reaction miniaturization in eco-friendly solvents. Curr. Opin. Green Sustain. Chem. 11, 91–98 (2018).
Cernak, T. et al. Microscale high-throughput experimentation as an enabling technology in drug discovery: application in the discovery of (piperidinyl)pyridinyl-1H-benzimidazole diacylglycerol acyltransferase 1 inhibitors. J. Med. Chem. 60, 3594–3605 (2017).
Uehling, M. R., King, R. P., Krska, S. W., Cernak, T. & Buchwald, S. L. Organic chemistry: pharmaceutical diversification via palladium oxidative addition complexes. Science 363, 405–408 (2019).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Lin, S. et al. Mapping the dark space of chemical reactions with extended nanomole synthesis and MALDI-TOF MS. Science 361, eaar6236 (2018).
Santanilla, A. B. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 49–53 (2015).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Boström, J., Brown, D. G., Young, R. J. & Keserü, G. M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 17, 709–727 (2018).
Dombrowski, A. W., Aguirre, A. L., Shrestha, A., Sarris, K. A. & Wang, Y. The chosen few: parallel library reaction methodologies for drug discovery. J. Org. Chem. 87, 1880–1897 (2022).
Cooper, T. W. J., Campbell, I. B. & MacDonald, S. J. F. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew. Chem. Int. Ed. 49, 8082–8091 (2010).
Yu, Y. et al. Accelerating the discovery of DGAT1 inhibitors through the application of parallel medicinal chemistry (PMC). Bioorganic Med. Chem. Lett. 29, 1380–1385 (2019).
Richardson, P. & Abdiaj, I. in Flow Chemistry in Drug Discovery (eds Alcazar, J. et al.) 421–479 (Springer, 2021).
Gioiello, A., Piccinno, A., Lozza, A. M. & Cerra, B. The medicinal chemistry in the era of machines and automation: recent advances in continuous flow technology. J. Med. Chem. 63, 6624–6647 (2020).
Bogdan, A. R. & Organ, M. G. in Flow Chemistry for the Synthesis of Heterocycles (eds Sharma, U. K. & Van der Eycken, E. V.) 319–341 (Springer, 2018).
Perera, D. et al. A platform for automated nanomole-scale reaction screening and micromole-scale synthesis in flow. Science 359, 429–434 (2018).
Koppmann, R. Volatile Organic Compounds in the Atmosphere (Blackwell Publishing Ltd, 2007).
Alder, C. M. et al. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 18, 3879–3890 (2016).
Tu, N. P. et al. High-throughput reaction screening with nanomoles of solid reagents coated on glass beads. Angew. Chem. Int. Ed. 58, 7987–7991 (2019).
Hendershot, D. C. & Sarafin, A. Safe chemical reaction scale up. Chem. Health Saf. 12, 29–35 (2005).
Logsdon, D. L. et al. High-throughput screening of reductive amination reactions using desorption electrospray ionization mass spectrometry. Org. Process Res. Dev. 24, 1647–1657 (2020).
Mahjour, B. et al. Rapid planning and analysis of high-throughput experiment arrays for reaction discovery. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2022-hcnng (2022).
Buitrago Santanilla, A., Christensen, M., Campeau, L. C., Davies, I. W. & Dreher, S. D. P2Et phosphazene: a mild, functional group tolerant base for soluble, room temperature Pd-catalyzed C-N, C-O, and C-C cross-coupling reactions. Org. Lett. 17, 3370–3373 (2015).
Bruno, N. C., Tudge, M. T. & Buchwald, S. L. Design and preparation of new palladium precatalysts for C-C and C-N cross-coupling reactions. Chem. Sci. 4, 916–920 (2013).
Thomas, A. A. & Denmark, S. E. Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: the missing link. Science 352, 329–332 (2016).
Kutchukian, P. S. et al. Chemistry informer libraries: a chemoinformatics enabled approach to evaluate and advance synthetic methods. Chem. Sci. 7, 2604–2613 (2016).
Dreher, S. D. & Krska, S. W. Chemistry informer libraries: conception, early experience, and role in the future of cheminformatics. Acc. Chem. Res. 54, 1586–1596 (2021).
Abdel-Magid, A. F. & Mehrman, S. J. A review on the use of sodium triacetoxyborohydride in the reductive amination of ketones and aldehydes. Org. Process Res. Dev. 10, 971–1031 (2006).
Abdel-Magid, A. F., Carson, K. G., Harris, B. D., Maryanoff, C. A. & Shah, R. D. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. Studies on direct and indirect reductive amination procedures. J. Org. Chem. 61, 3849–3862 (1996).
Afanasyev, O. I., Kuchuk, E., Usanov, D. L. & Chusov, D. Reductive amination in the synthesis of pharmaceuticals. Chem. Rev. 119, 11857–11911 (2019).
Pels, K. & Kodadek, T. Solid-phase synthesis of diverse peptide tertiary amides by reductive amination. ACS Comb. Sci. 17, 152–155 (2015).
Bhattacharyya, S. Titanium(IV) isopropoxide and sodium borohydride: a reagent of choice for reductive amination. Tetrahedron Lett. 35, 2401–2404 (1994).
Mattson, R. J., Pham, K. M., Leuck, D. J. & Cowen, K. A. An improved method for reductive alkylation of amines using titanium(IV) isopropoxide and sodium cyanoborohydride. J. Org. Chem. 55, 2552–2554 (1990).
Neidigh, K. A., Avery, M. A., Williamson, J. S. & Bhattacharyya, S. Facile preparation of N-methyl secondary amines by titanium(IV) isopropoxide-mediated reductive animation of carbonyl compounds. J. Chem. Soc., Perkin Trans. 1 16, 2527–2531 (1998).
Huang, X. et al. Discovery and hit-to-lead optimization of non-ATP competitive MK2 (MAPKAPK2) inhibitors. ACS Med. Chem. Lett. 2, 632–637 (2011).
Huang, X. et al. A three-step protocol for lead optimization: quick identification of key conformational features and functional groups in the SAR studies of non-ATP competitive MK2 (MAPKAPK2) inhibitors. Bioorg. Med. Chem. Lett. 22, 65–70 (2012).
Fiore, M., Forli, S. & Manetti, F. Targeting mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2, MK2): medicinal chemistry efforts to lead small molecule inhibitors to clinical trials. J. Med. Chem. 59, 3609–3634 (2016).
Weis, E., Johansson, M., Korsgren, P., Martín-Matute, B. & Johansson, M. J. Merging directed C-H activations with high-throughput experimentation: development of iridium-catalyzed C-H aminations applicable to late-stage functionalization. JACS Au 2, 906–916 (2022).
Acknowledgements
We thank S. Krska (Merck & Co., Inc.) for helpful discussions. This study was supported by the University of Michigan College of Pharmacy (start-up funds to T.C.), NIH (R01GM144471 to T.C.), the American Chemical Society MEDI Division (predoctoral fellowship to B.M.) and the National Defense Medical Center of Taiwan (fellowship to Y.-T.K.).
Author information
Authors and Affiliations
Contributions
N.G., K.D., Y.-T.K., R.Z. and T.C. performed experiments. R.F. and S.D. assisted in preliminary experiments. B.S. and J.S. performed analysis of products. J.L.D. and B.M. and performed data analysis. N.G., J.L.D., B.M. and T.C. wrote the paper. All authors reviewed the data. T.C. supervised the work.
Corresponding author
Ethics declarations
Competing interests
The majority of experimental work was performed at Merck & Co., Inc., Rahway, NJ, USA. The Cernak Lab at University of Michigan has received research funding or in-kind donations from MilliporeSigma, Relay Therapeutics, Janssen Therapeutics, Entos, Inc., SPT Labtech and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. T.C. is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, holds equity in Scorpion Therapeutics, and is a cofounder and equity holder of Entos, Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Magnus Johansson, Steven Mennen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Peter Seavill, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details, Sections 1–8 and Supplementary Figs. 1–7.
Supplementary Data 1
Excel file containing all raw high-throughput experimentation data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gesmundo, N., Dykstra, K., Douthwaite, J.L. et al. Miniaturization of popular reactions from the medicinal chemists’ toolbox for ultrahigh-throughput experimentation. Nat. Synth 2, 1082–1091 (2023). https://doi.org/10.1038/s44160-023-00351-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00351-1
This article is cited by
-
Rapid planning and analysis of high-throughput experiment arrays for reaction discovery
Nature Communications (2023)