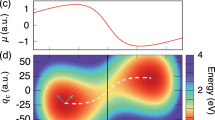
Abstract
Selectively exciting target molecules to high vibrational states is inefficient in the liquid phase, which restricts the use of IR pumping to catalyze ground-state chemical reactions. Here, we demonstrate that this inefficiency can sometimes be solved by confining the liquid to an optical cavity under vibrational strong coupling conditions. For a liquid solution of 13CO2 solute in a 12CO2 solvent, cavity molecular dynamics simulations show that exciting a polariton (hybrid light-matter state) of the solvent with an intense laser pulse, under suitable resonant conditions, may lead to a very strong (>3 quanta) and ultrafast (<1 ps) excitation of the solute, even though the solvent ends up being barely excited. By contrast, outside a cavity the same input pulse fluence can excite the solute by only half a vibrational quantum and the selectivity of excitation is low. Our finding is robust under different cavity volumes, which may lead to observable cavity enhancement on IR photochemical reactions in Fabry–Pérot cavities.
Similar content being viewed by others
Introduction
Controlling (electronic) ground-state chemical reaction rates with an external infrared (IR) laser is a long-standing goal of IR photochemistry. Although it is possible to trigger chemical reactions by exciting the reactants to high vibrational excited states via ladder climbing1, such IR-controlled chemical reactions are rarely efficient in the liquid phase because of competing relaxation processes that transfer vibrational energy to other degrees of freedom, either internal modes of the molecule or external modes of surrounding molecules (leading to a build-up of heat in the environment)2. Up to now, there have been only a few observations of selective IR photochemistry (i.e. reactions where an IR pump promotes selective reactions beyond a simple heating effect)3,4,5. That being said, if there were a general mechanism for targeting some molecules (e.g., reactants or solute molecules) and achieving highly vibrationally excited states on a timescale shorter than the lifetime of vibrational energy relaxation, one could imagine using IR light to help catalyze reactions of interest.
During the past decade, a novel means to control molecular properties has emerged through the formation of strong light–matter coupling between a vacuum electromagnetic field and a molecular (electronic or vibrational) transition of a large ensemble of molecules6,7,8,9. Under this collective strong light–matter coupling regime, the formed hybrid light–matter states, known as molecular polaritons, can suppress ultraviolet–visible (UV–vis) photochemical reactions10, alter ground-state reaction rates11,12,13, promote electronic conductivity14, and facilitate energy transfer between different molecular species15,16,17,18,19,20, etc. For example, under vibrational strong coupling (VSC)21,22 between an optical cavity mode and an equal liquid mixture of W(12CO)6 and W(13CO)6, Xiang et al. 20 have experimentally demonstrated that, by pumping the upper vibrational polariton (UP) with an external IR laser, intermolecular vibrational energy transfer between the two molecular species can be accelerated to a lifetime of tens of ps due to the intrinsically delocalized nature of polaritons.
Apart from the acceleration of energy transfer due to polariton delocalization, the other intriguing consequence of VSC is the enhancement of molecular nonlinear absorption7,23,24,25. Due to the anharmonicity of molecular vibrations, it is possible to tune a cavity in such a way that twice the lower polariton (LP) energy roughly matches the molecular 0 → 2 vibrational transition; in such a case, pumping the LP can facilitate molecular nonlinear absorption and promote the creation of highly vibrationally excited molecules7,24.
Here, we will numerically demonstrate that by combining the above two unique features of VSC—polariton delocalization and molecular anharmonicity—it is possible to selectively excite a set of molecules, i.e., a small concentration of solute molecules, to highly vibrationally excited states and leave the vast majority of solvent molecules barely excited via strongly exciting the solvent LP. We emphasize that this finding is of crucial importance, as it lays the foundations for using IR polaritonic chemistry to promote chemical reaction rates. Moreover, because the solvent LP is essentially composed of only solvent modes plus the cavity mode (with a negligible contribution from solute molecules), the observation of energy accumulation in the solute molecules must be regarded as vibrational energy transfer from solvent to solute, which is entropically unfavorable outside the cavity. The difference between our finding and conventional multiphoton molecular nonlinear absorption is also discussed in Supplementary Figs. 2, 3, and 8.
While many theoretical approaches26,27,28,29,30,31,32,33 have been developed recently in order to describe molecular polaritons, our numerical approach is classical cavity molecular dynamics (CavMD) simulation24,28,34, a new and promising tool for propagating coupled photon–nuclear dynamics in the condensed phase, though with the limitations of using a classical force field to describe realistic molecules; see Fig. 1 for the simulation setup and the section “Methods” (and also Supplementary Methods) for more details.
Nsub molecules are confined in a cavity formed by a pair of parallel metallic mirrors. These molecules are coupled to a single cavity mode (with two polarization directions) and are simulated in a periodic simulation cell. Our classical CavMD simulations show that, after a strong excitation of the solvent lower polariton (LP) with an IR laser pulse, the input energy can be selectively transferred to the highly excited dark states of the solute molecules, leaving the vast majority of the solvent molecules barely excited. The left cartoon demonstrates the quantum analog of our classical finding; see Supplementary Note 8 for details.
Results
Polaritonic energy transfer in pure liquid
Before presenting the main finding of this manuscript—selective energy transfer to solute molecules—we investigate how exciting a polariton leads to a nonuniform energy redistribution in a pure liquid 12CO2 system (with Nsub = 216 molecules explicitly propagated in a simulation cell) at room temperature (300 K). In Fig. 2a, we plot the equilibrium IR spectrum outside a cavity (black line) or inside a cavity (red line), where the IR spectrum is calculated by evaluating the auto-correlation function of the total dipole moment for the molecular subsystem24,35. Inside the cavity, the C=O asymmetric stretch mode (peaked at ω0 = 2327 cm−1) is resonantly coupled to a cavity mode (at ωc = 2320 cm−1 corresponding to the vertical blue line) with an effective coupling strength \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u. In this collective VSC regime, a pair of polaritons [LP (ωLP = 2241 cm−1) and UP (ωUP = 2428 cm−1)] forms and these two peaks are separated by a Rabi splitting of 187 cm−1.
a Equilibrium IR spectrum for pure liquid 12CO2 outside a cavity (black line) or inside a cavity with coupling \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u. (red line); the vertical dashed blue line denotes the cavity mode at 2320 cm−1. b The corresponding nonequilibrium local IR spectrum for each molecule after a resonant excitation of the LP at 2241 cm−1 with a strong external pulse. The external pulse takes the form \({E}_{0}\cos (\omega t+\phi ){{{{{{{{\bf{e}}}}}}}}}_{x}\) (0.1 < t < 0.6 ps) with fluence 632 mJ/cm2; the corresponding pulse spectrum is also plotted as the yellow curve on the left panel. The nonequilibrium spectrum is calculated by evaluating the dipole autocorrelation function of each CO2 molecule over time interval 1–6 ps. The red arrow marks the frequency of the LP and also the external pulse, and the LP lifetime (0.2 ps, calculated from photonic energy dynamics24) is labeled at the bottom. c Equilibrium IR spectrum (red line) for 4.63% 13CO2 in a 12CO2 solution when \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u. The gray line denotes the 13CO2 IR spectrum outside the cavity; again, the black line denotes the pure 12CO2 IR spectrum outside the cavity. d The corresponding nonequilibrium local IR spectrum for each molecule plotted in a similar manner as (b). The responses of 12CO2 (left) and 13CO2 (right) are separated by the vertical dashed line. e–h The same plots as in (a–d) but with a larger effective light-matter coupling (\(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u.). Note that with an appropriate LP frequency, the LP is more likely to transfer energy to the 13CO2 solute molecules than to the 12CO2 solvent. See Supplementary Methods for simulation details, the definition of the CO2 force field, and the definition of the spectra signals.
We now excite the LP with a strong pulse of the form \({{{{{{{\bf{E}}}}}}}}(t)={E}_{0}\cos (\omega t){{{{{{{{\bf{e}}}}}}}}}_{x}\) (0.1 < t < 0.6 ps) with fluence 632 mJ/cm2 (E0 = 6 × 10−3 a.u.); see the yellow curve in Fig. 2a for the corresponding pulse spectrum. This fluence will be used below and throughout the present manuscript, and the pulse is assumed to interact only with the molecular subsystem. Note that, as shown in Supplementary Fig. 6, for the same amount of pumped energy, using a Gaussian pulse (instead of a square pulse) does not meaningfully alter the reported results; similarly, as shown in Supplementary Fig. 5, if we assume that the external pulse activates the cavity mode directly (rather than the molecular subsystem), the reported results are effectively unchanged.
In Fig. 2b, after the LP pumping, the resulting energy distribution among molecules is characterized by calculating the response of every 12CO2 molecule in the simulation cell after the LP excitation. The individual molecular response is calculated by evaluating the auto-correlation function of each single-molecule dipole moment (which we call the “local" IR spectrum)24 during a time window 1 < t < 6 ps (immediately after the LP excitation). Note that the response measured is different from the usual IR spectrum—which is calculated by evaluating the auto-correlation function of the total dipole moment of a molecular system (as in Fig. 2a) and reflects the dynamics of the molecular bright mode; the local IR spectrum measured here reflects the dynamics of individual molecules and can be expressed as a linear combination of molecular bright and dark modes. Because the total number of molecules explicitly accounted for in the simulation is large (with Nsub = 216), the local IR spectrum is dominated by the density of dark modes.
In Fig. 2b, every column of pixels represents the local IR spectrum of one molecule (in total Nsub = 216 molecules) during 1–6 ps and the color bar (from light yellow to deep red) denotes a logarithmic scaled spectroscopic intensity. Clearly, after the LP excitation (where the LP lifetime fitted from photonic energy dynamics24 is 0.2 ps), the polaritonic energy is nonuniformly transferred to different 12CO2 molecules (which are composed mostly of vibrational dark modes). While most molecules are only weakly excited and have a peak near 2320 cm−1 (the equilibrium IR peak; see also Fig. 2a), a dozen of molecules are strongly excited and show an intense, red-shifted peak near 2200 cm−1. Because we use an anharmonic force field24 to simulate carbon dioxide molecules, the presence of intense, red-shifted peaks implies that the LP energy is mostly transferred to a small fraction of 12CO2 molecules.
The nonuniform polaritonic energy redistribution in Fig. 2b stems from polariton-enhanced molecular nonlinear absorption, a mechanism which has been shown experimentally (via two-dimensional IR spectroscopy)23, analytically25, and numerically (via CavMD)24. Quantum-mechanically speaking, when twice the LP energy roughly matches the 0 → 2 vibrational transition of molecules, the LP can serve as a “virtual state" to enhance molecular nonlinear absorption of light and directly create highly vibrational excited molecules within a sub-ps timescale. In the present case (see Fig. 2a), the LP frequency sits between the fundamental 12C = O asymmetric stretch (ω0 = 2327 cm−1) and the 1 → 2 vibrational transition (which is roughly 2200 cm−1). Hence, polariton-enhanced molecular nonlinear absorption can occur once the LP is strongly excited. Moreover, since molecules have different orientations and different local electrostatic environments (which leads to different instantaneous vibrational frequencies), only a small subset of molecules can interact strongly with the LP through the nonlinear channel (which requires an exact frequency match), thus leading to a vast difference in energy redistribution among molecules. In the end, some solvent molecules become highly excited and others do not. This quantum-mechanical interpretation of polariton-enhanced molecular nonlinear absorption is easy to understand. A classical analog can be pictured as well. Once the LP is excited and starts to dephase, due to the inhomogeneous local molecular environment, some molecules receive more polaritonic energy than others. Due to molecular anharmonicity, these high-energy molecules oscillate with red-shifted, closer-to-LP frequencies, leading to an even stronger interaction between these high-energy molecules and the LP. Such “self-catalyzing" can, under strong LP excitation, eventually lead to some molecules being strongly and nonlinearly excited. While keeping in mind that our calculations are in fact classical, below we will use the quantum description because we feel the latter is more standard in the literature.
Polaritonic energy transfer to solute molecules
Let us now show that the mechanism above can be utilized to selectively transfer the energy from an IR laser pulse to a few target molecules in a liquid system. For the sake of simplicity, let us choose these target molecules to be a few 13CO2 molecules and investigate how to achieve selective energy transfer to these 13CO2 solute molecules (which are dissolved in a liquid 12CO2 solution).
For a solution of 4.63% (10/216) 13CO2 molecules dissolved in liquid 12CO2 (in total Nsub = 216 molecules are included in the simulation cell), the red line in Fig. 2c plots the equilibrium IR spectrum inside the same cavity as in Fig. 2a, b (with ωc = 2320 cm−1 and \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u.). Because the concentration of the 13CO2 solute is small, inside the cavity, the positions of the polaritons are largely unchanged compared with the case of pure liquid 12CO2 (in Fig. 2a). Note that the equilibrium IR spectrum for pure liquid 13CO2 system outside a cavity is also plotted as the gray line in Fig. 2c, where the 13C=O asymmetric stretch peaks at 2262 cm−1.
For this liquid mixture, after a strong excitation of the LP (again with a pulse fluence of 632 mJ/cm2), Fig. 2d plots the transient local IR spectra for every 12CO2 and 13CO2 molecule. Here, the 12CO2 are plotted on the left and the 13CO2 molecules are plotted on the right-hand side of the vertical dashed line. Figure 2d shows that there is now a competition between exciting 12CO2 and 13CO2 molecules, as molecules of both isotopes exhibit intense, red-shifted peaks.
This conclusion is illustrated quantitatively in Fig. 3a, where we plot the dynamics of the average C=O bond potential energy (minus the thermal energy kBT) per 12CO2 (gray line) or 13CO2 (red line) molecule after the strong LP excitation. Due to the LP excitation [where the time window of the pulse (0.1 < t < 0.6 ps) is represented with a yellow region], the vibrational energy of the 13CO2 solute molecules can reach roughly four times that of the 12CO2 solvent molecules and each solute molecule can absorb roughly a vibrational quantum (~2 × 103 cm−1) of the input energy. Note that Fig. 3a is drawn with a logarithmic scale; see the black arrow which denotes the energy difference. By contrast, outside the cavity, when the 13C = O asymmetric stretch of the liquid mixture is resonantly excited by the same pulse (Fig. 3c), the 13CO2 vibrational energy reaches roughly twice the energy of the 12CO2 solvent molecules, and the energy absorption of each solute molecule is roughly half a vibrational quantum (~103 cm−1). Our finding indicates that the cavity environment can meaningfully improve the selectivity and also the absolute value of solute excitation by a factor of two (though a factor of two is perhaps not a huge effect).
Three conditions are compared: (a) \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u. after exciting the LP at 2241 cm−1 (the same as Fig. 2d); (b) \(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u. after exciting the LP at 2177 cm−1 (the same as Fig. 2h); and (c) outside the cavity after exciting the 13C=O asymmetric stretch at 2262 cm−1. In each subplot, the 12CO2 solvent is drawn with a gray line and the 4.63% 13CO2 solute is drawn with a red line. The y-axis is plotted in a logarithmic scale, and the yellow region denotes the time window (0.1 < t < 0.6 ps) during which the external pulse is applied. Note that although the nonequilibrium local IR spectrum in Fig. 2 was calculated using a single trajectory, 40 trajectories are averaged here to obtain an ensemble-averaged result; see Supplementary Methods for simulation details.
Improving the selectivity of energy transfer to solute molecules by tuning the Rabi splitting
In order to improve the selectivity of energy transfer from the LP, we need to both (i) suppress the energy transfer to the 12CO2 solvent molecules and also (ii) enhance the energy transfer to the 13CO2 solute molecules. As mentioned above, the nonuniform polaritonic energy transfer stems from polariton-enhanced molecular nonlinear absorption, a mechanism that requires twice the LP frequency to roughly match the 0 → 2 vibrational transition. In other words, by changing the LP frequency relative to the vibration of one molecular species, we can enhance or suppress polaritonic energy transfer to that molecular species according to a frequency match or mismatch.
Let us first focus on the side of the 12CO2 molecules and investigate how to suppress the energy transfer to the 12CO2 molecules with an increased Rabi splitting. For a pure liquid 12CO2 system, when the effective light–matter coupling strength is increased from \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u. to \(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u., the red line in Fig. 2e plots the equilibrium IR spectrum inside the cavity with ωc = 2320 cm−1. For the larger light–matter coupling (\(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u.), the Rabi splitting increases by a factor of two and the LP frequency red-shifts from 2241 cm −1 (in Fig. 2a–d) to 2177 cm−1. Now, as shown in Fig. 2f, after resonant excitation of the LP with a strong laser pulse, polaritonic energy transfer to 12CO2 molecules is largely suppressed compared with Fig. 2b. The suppression of polaritonic energy transfer is consistent with a longer LP lifetime (2.9 ps) relative to that in Fig. 2b (0.2 ps); this longer LP lifetime can be observed in Fig. 2f by visualizing the near-uniform intensity among all molecules at the LP frequency (positioned by the red arrow) over the time scale 1–6 ps, which corresponds to a long-lived molecular bright state.
Second, consider the case where the liquid mixture of 4.63% 13CO2 in a 12CO2 solution experiences a larger light–matter coupling (with \(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u.). Because the concentration of the 13CO2 solute molecules is small, the equilibrium IR spectrum inside the cavity (Fig. 2g) remains unchanged compared with the case of pure liquid 12CO2 (Fig. 2e). After resonantly exciting the LP with a strong laser pulse, as shown in Fig. 2h, we find that the polaritonic energy mostly transfers to the 13CO2 molecules (on the right-hand side of the vertical dashed white line). Quantitatively speaking, Fig. 3b plots the corresponding dynamics of the average C=O bond potential energy for the solute and solvent molecules. Here, within the time window of the laser pulse (t < 1 ps), the solute molecules can be excited to a state of roughly three vibrational quanta (recall that the frequency of the C=O asymmetric stretch is rough ~2 × 103 cm−1), which is six times of the outside-cavity solute energy absorption (Fig. 3c). As far as selectivity is considered, within t < 5 ps, the solute molecules absorb an energy 56 times larger than the solvent molecules (see the black arrow), a difference which is one order of magnitude larger than the case of outside the cavity (Fig. 3c). In short, although we found above that, when \(\widetilde{\varepsilon }=2\times 1{0}^{-4}\) a.u., there is a competition between the polaritonic energy transfer to 12CO2 and 13CO2 molecules, we now find that when \(\widetilde{\varepsilon }=4\times 1{0}^{-4}\) a.u., 13CO2 clearly wins.
The high selectivity of this polaritonic energy transfer to the solute molecules can be explained as follows. First, since the LP frequency is unchanged compared with the pure liquid 12CO2 system in Fig. 2e, polariton-enhanced molecular nonlinear absorption for the 12CO2 solvent molecules remains greatly suppressed. Second, for the larger Rabi splitting, the vibrational frequency difference between the LP and 13C=O asymmetric stretch (see red and gray lines in Fig. 2g) is suitable to allow for polariton-enhanced molecular nonlinear absorption of the solute molecules. This statement is consistent with the shortened LP lifetime in Fig. 2h (0.6 ps) as compared with τLP = 2.9 ps in Fig. 2f. Altogether, these two facts lead to a high selectivity in energy transfer. A summary of the quantum picture of our finding—a selective polariton energy transfer to the second excited solute dark states when twice the solvent LP frequency roughly matches the solute 0 → 2 transition—is plotted in cartoon form on the left-hand side of Fig. 1; see also Supplementary Note 8 for details.
Dependence on system size and solute concentration
One important issue we must address is the dependence of polaritonic energy transfer on molecular system size. This study is necessary because usual VSC setups in experiments (e.g., Fabry–Pérot microcavities) contain a macroscopic number of molecules, while we have only simulated a molecular system of Nsub = 216 coupled to the cavity. Therefore, we must check how our conclusions vis a vis VSC effects depend on molecular system size.
Following the approach from ref. 24, in Fig. 3b, we enlarge the molecular system by simultaneously increasing both the number of 13CO2 and 12CO2 molecules (maintaining a constant proportion) and we decrease the light–matter coupling strength (\(\widetilde{\varepsilon }\)); these two effects are chosen in a balanced manner (\(\widetilde{\varepsilon }\propto 1/\sqrt{{N}_{{{{{\mbox{sub}}}}}}}\)) so as to keep the molecular density and Rabi splitting the same as in Fig. 2g, h. For systems with different sizes, after the same strong excitation of the LP, Fig. 4 plots the dynamics of the average C=O bond potential energy for the 13CO2 and 12CO2 molecules. Here, the top and bottom lines denote the dynamics of 13CO2 and 12CO2 molecules, respectively. Lines with different colors (from black to orange) denote molecular systems of different sizes (from Nsub = 216 to Nsub = 216 × 16). Note that after the pulse, the 13CO2 molecules are hot and start to cool down, while the weakly excited 12CO2 molecules start to heat up. Figure 4 also clearly shows that, although the later-time vibrational relaxation and energy transfer dynamics (t > 5 ps) can depend on the size of the molecular system34, we do observe a consistent selective polaritonic energy transfer behavior at early times (t < 5 ps) for all molecular system sizes. Therefore, we tentatively conclude that our numerical findings here should be observable in cavities with different volumes, including Fabry–Pérot microcavities (that are usually used to study collective VSC) and plasmonic cavities, an emerging platform for studying VSC36,37.
The Rabi splitting and molecular number density have been fixed. Black lines correspond to the data in Fig. 3b (Nsub = 216), and the lines from dark red to orange represent increasing molecular systems where Nsub is increased by a factor of N (N = 2, 4, 8, 16). In all cases, we excite the LP and measure the amount of potential energy in the C=O bonds. Note that the selective polaritonic energy transfer to the 13CO2 solute molecules is fairly robust against molecular system size, indicating that the current finding may hold for collective VSC with large cavity volumes but under the same Rabi splitting, i.e., smaller \(\widetilde{\varepsilon }\); see text below and also Supplementary Fig. 1 for an explanation of the system size dependence.
Note that the robustness of the early-time (<1 ps) polaritonic decay dynamics against the system size can be rationalized by, e.g., Fermi’s golden rule calculations. Although the interaction between the polariton and each dark mode scales with 1/N (where N denotes the total molecular number) via intermolecular interactions, since there are N−1 dark modes that allow polaritonic energy transfer (or dephasing), when Fermi’s golden rule is used to describe the polaritonic relaxation dynamics, the 1/N and N−1 factors cancel with each other, leading to consistent early-time dynamics during polaritonic relaxation versus the system size. This argument explains why the early time dynamics (<1 ps) in Fig. 4 do not depend sensitively on N. That being said, for the later-time (>5 ps) dynamics, which involve energy transfer and vibrational relaxation, the same argument shows that the presence of a cavity will play a role and lead to N-dependent dynamics. After all, consider the following energy transfer channel that we might expect to be important after we have excited a set of 13CO2 molecules: 13CO2 → polaritons → 12CO2. Since there are only two (not N) polaritons, we do not expect this pathway to play an important role once the system size is very large, leading to slower vibrational relaxation and energy transfer dynamics in later times; see ref. 34 for a detailed study.
Finally, in Fig. 5, we plot the dynamics of the averaged C=O bond potential energy per 13CO2 solute or 12CO2 solvent molecule as a function of time for different system sizes—but now with a fixed number (10) of solute molecules. In other words, we increase only the number of 12CO2 solvent molecules (so that the solute concentration is decreased). For all curves, as above, the total Rabi splitting is kept constant by decreasing the effective coupling strength \(\widetilde{\varepsilon }\), which corresponds physically to placing the system in a larger volume cavity.
This plot is the same as Fig. 4 except for that the 13CO2 solute molecular number is fixed as 10 when the system size enlarges. Note that, when the system size is increased (or when the solute concentration is decreased), both the solvent and solute molecules are more heavily pumped under the same IR excitation of the LP. See text for details of this counter-intuitive feature.
Perhaps counter-intuitively, Fig. 5 shows that the C=O bond energy of both the solvent and solute molecules increases as the number of solvent molecules increases. These findings can be explained as follows. On the one hand, as for the solute molecules, when the number of solvent molecules increases, the total transition dipole of the solvent bright mode also increases, such that the total amount of energy absorbed through the polariton grows when the same incident pulse is applied—and each solute molecule will recover more energy from the polariton through the nonlinear channel (since the energy absorbed by the polariton divided by the solute number grows). On the other hand, when the concentration of the solute decreases (under larger system sizes), the pumped solvent polariton cannot efficiently transfer energy to its favorite acceptor (the solute), such that the polariton transfers more energy to the solvent molecules. Finally, with regards to selectivity (i.e., energy of solute divided by energy of solvent), these two effects clearly work against each other. Empirically, we find that the selectivity reaches a maximum value of 61 times (see the vertical brown arrow) and the solute excitation can exceed 104 cm−1 (4–5 quanta) when we double the system size (i.e. we add slightly more than two times the number of solvent molecules so that the solute concentration becomes 2.32%, the brown lines). Very interestingly, in Supplementary Fig. 7 we show that the selectivity also goes through a maximum value as a function of the pulse fluence, confirming our assertion that energy transfer to the 13CO2 species is dominated by a nonlinear transition: at low intensities, this mechanism is inefficient, while at high intensities the 13CO2 species energy absorption becomes saturated.
The findings above cannot be achieved outside a cavity. For example, on the one hand, when the solvent bright mode is pumped outside a cavity, since the excited bright mode dephases on a much faster timescale than the polaritons38, and because there are no resonance conditions between the solvent bright mode and the solute, energy transfer from the solvent to solute is not efficient and occurs on a much longer timescale than 1 ps. On the other hand, when the solute vibration is directly excited outside the cavity (see Fig. 3c), the energy absorbed by the system is much smaller than the inside-cavity case (where energy absorption is dominated by the solvent polariton).
Discussion
To summarize, we have numerically demonstrated that energy from a strong IR laser pulse can be selectively transferred to solute molecules by pumping the LP of the solvent molecules. This selective energy transfer behavior requires the LP to have an appropriate frequency to selectively enhance molecular nonlinear absorption for the solute (but not solvent) molecules and can be optimized by changing the solute concentration. Consequently, the transient vibrational energy difference between the solute and the solvent molecules can exceed the free-space case by more than one order of magnitude. Since our CavMD simulations have shown some key agreements with VSC experiments (including the signature of polariton-enhanced molecular nonlinear absorption), and because the current finding of selective energy transfer to the solute molecules is robust against the cavity volume, we believe that this finding is also amenable to experimental verification. For example, although the molecule-resolved nonequilibrium local IR spectrum (see Fig. 2) cannot be directly observed experimentally, for Fabry–Pérot microcavities, pump-probe or 2D-IR spectroscopy should be able to verify the main predictions of this paper. For example, once a small fraction of solute molecules is added, the observation of a strong reduction of the solvent LP lifetime when twice the solvent LP frequency roughly matches the solute 0 → 2 transition would provide one indirect piece of evidence supporting our mechanism. Moreover, for certain experiments, after pumping the solvent LP, the observation of bond dissociation events would serve as a direct piece of evidence confirming that solute molecules have been highly excited.
In principle, our finding might be one route to IR laser-controlled chemistry on an electronic ground-state surface, which remains difficult to achieve in the liquid phase. In order to selectively excite the (presumably chemically active) solute molecules in an efficient way, it would be best if the solvent molecules were to have a relatively simple vibrational structure so that the dephasing channel from the solvent polariton to the solvent dark modes can be largely forbidden. For the solute molecules, however, a complicated vibrational density of states might actually enhance the energy transfer from the solvent polariton. One must wonder if one potential application of the present manuscript would be isotope separation, where two similar molecules can be distinguished by a vibrational degree of freedom. Finally, for the cases where the vibrational relaxation is so strong that the transient excitation of the solute molecules would not facilitate IR photochemistry, the current mechanism may also allow us to achieve selective heating of the local geometry, e.g., by putting the solute molecules inside a protein, perhaps one can control a selective denaturation of proteins without heating up the whole liquid sample. Overall, there are clearly many future possibilities for using collective VSC to modify molecular properties.
Methods
The CavMD approach is formulated in the dipole gauge and includes the self-dipole term in the light–matter Hamiltonian, thus preserving gauge invariance39,40,41. The motivation for using classical CavMD for simulating nonequilibrium vibrational dynamics inside the cavity is twofold. First, for molecular vibrational relaxation and energy transfer dynamics in the condensed phase and outside the cavity, classical (nonequilibrium) molecular dynamics simulations have been shown to often agree well with experiments42,43,44,45,46; moreover, cavity photons are harmonic oscillators, whose dynamics can usually be captured well by classical dynamics45. By combining the classical motion of cavity photons and molecules, CavMD is expected to capture the essential physics in many VSC experiments. Second, CavMD has already correctly captured some key features of both equilibrium and nonequilibrium VSC experiments23,38,47,48, including (i) the asymmetry of a Rabi splitting28,47, (ii) polaritonic relaxation to vibrational dark modes on a timescale of ps or sub-ps24,38,48, (iii) polariton-enhanced molecular nonlinear absorption24—a phenomenon whose signature is a delayed population gain in the first excited state of vibrational dark modes after strongly exciting the LP7,23, and (iv) dark-mode relaxation dynamics for systems with large cavity volumes34,38. Altogether, classical CavMD appears to be a suitable choice between accuracy and affordability for simulating nonequilibrium vibrational dynamics under VSC.
The fundamental equations underlying CavMD and all simulation details for exciting a polariton in a liquid carbon dioxide system (including the force field) can be found in refs. 24,28; see also Supplementary Methods for a comprehensive explanation. In short, as shown in Fig. 1, we imagine Nsub molecules in a periodic simulation cell coupled to a single cavity mode (with two polarization directions). The effective coupling strength between each molecule and the cavity mode is denoted as \(\widetilde{\varepsilon }\), which is defined as
Here, \(\varepsilon =\sqrt{{m}_{{\rm {c}}}{\omega }_{{\rm {c}}}^{2}/{{\Omega }}{\epsilon }_{0}}\) denotes the true light–matter coupling strength between each molecule and the cavity mode, where ωc, Ω and ϵ0 denote the cavity mode frequency, the effective cavity volume, and the vacuum permittivity, respectively, and mc = 1 a.u. denotes the auxiliary mass for the cavity photons (see Supplementary Methods for details). Ncell denotes the number of periodic simulation cells, the value of which can be determined by fitting the experimentally observed Rabi splitting. As discussed in Supplementary Methods, when CavMD is used to simulate VSC in Fabry–Pérot microcavities, we must always check that our results on a small subsystem are not spurious. We check for this possibility by running a protocol that enlarges the molecular system size (while fixing the Rabi splitting and molecular density)—the results in the asymptotic limit of a very large Nsub correspond to the true experimental result. See Fig. 4 for such a system size enlarging process.
For the simulations reported here, by using an explicit force field defined in Supplementary Methods, we include vibrational relaxation and dephasing for the molecular subsystem. We have assumed that the molecular force field remains the same inside versus outside the cavity, i.e., both intramolecular and intermolecular interactions are unchanged under VSC. For nonpolar molecules, although a recent first-principle calculation shows that intermolecular interactions can be modified by several cm−1 when a few molecules are confined in a cavity49, we anticipate that, compared with thermal fluctuations at 300 K, such a small modification can be neglected when the molecular number is large. We disregard cavity loss in the manuscript, which should usually be reasonable for Fabry–Pérot microcavities. Note that the main finding of this manuscript—selective polaritonic energy transfer to solute molecules—occurs mostly within 1 ps after the laser pumping, while a typical cavity mode lifetime for a Fabry–Pérot microcavity is ~5 ps23. In Supplementary Note 2 we have verified that a cavity lifetime larger than 1 ps would not meaningfully alter the results corresponding to Fabry–Pérot microcavities (i.e., when Nsub is large). The code for reproducing this work, which is implemented based on the I–PI interference50 and LAMMPS51, is open-source and available at Github52.
Data availability
All the data shown here, including the script files to generate the raw molecular dynamics trajectories and the data for plotting, are available at https://github.com/TaoELi/cavity-md-ipi52.
Code availability
The code for reproducing this paper, including a tutorial for installation and simulations, is available at https://github.com/TaoELi/cavity-md-ipi52.
References
Maas, D., Duncan, D., Vrijen, R., van der Zande, W. & Noordam, L. Vibrational ladder climbing in NO by (sub)picosecond frequency-chirped infrared laser pulses. Chem. Phys. Lett. 290, 75 (1998).
Owrutsky, J. C., Raftery, D. & Hochstrasser, R. M. Vibrational relaxation dynamics in solutions. Annu. Rev. Phys. Chem. 45, 519 (1994).
Delor, M. et al. Toward control of electron transfer in donor-acceptor molecules by bond-specific infrared excitation. Science 346, 1492 (2014).
Stensitzki, T. et al. Acceleration of a ground-state reaction by selective femtosecond-infrared-laser-pulse excitation. Nat. Chem. 10, 126 (2018).
Heyne, K. & Kühn, O. Infrared laser excitation controlled reaction acceleration in the electronic ground state. J. Am. Chem. Soc. 141, 11730 (2019).
Herrera, F. & Owrutsky, J. Molecular polaritons for controlling chemistry with quantum optics. J. Chem. Phys. 152, 100902 (2020).
Xiang, B. & Xiong, W. Molecular vibrational polariton: Its dynamics and potentials in novel chemistry and quantum technology. J. Chem. Phys. 155, 050901 (2021).
Garcia-Vidal, F. J., Ciuti, C. & Ebbesen, T. W. Manipulating matter by strong coupling to vacuum fields. Science 373, eabd0336 (2021).
Li, T. E., Cui, B., Subotnik, J. E. & Nitzan, A. Molecular polaritonics: chemical dynamics under strong light–matter coupling. Annu. Rev. Phys. Chem. 73, https://doi.org/10.1146/annurev-physchem-090519-042621 (2022).
Hutchison, J. A., Schwartz, T., Genet, C., Devaux, E. & Ebbesen, T. W. Modifying chemical landscapes by coupling to vacuum fields. Angew. Chem. Int. Ed. 124, 1624 (2012).
Thomas, A. et al. Ground-state chemical reactivity under vibrational coupling to the vacuum electromagnetic field. Angew. Chem. Int. Ed. 55, 11462 (2016).
Thomas, A. et al. Tilting a ground-state reactivity landscape by vibrational strong coupling. Science 363, 615 (2019).
Imperatore, M. V., Asbury, J. B. & Giebink, N. C. Reproducibility of cavity-enhanced chemical reaction rates in the vibrational strong coupling regime. J. Chem. Phys. 154, 191103 (2021).
Orgiu, E. et al. Conductivity in organic semiconductors hybridized with the vacuum field. Nat. Mater. 14, 1123 (2015).
Coles, D. M. et al. Polariton-mediated energy transfer between organic dyes in a strongly coupled optical microcavity. Nat. Mater. 13, 712 (2014).
Zhong, X. et al. Energy transfer between spatially separated entangled molecules. Angew. Chem. Int. Ed. 56, 9034 (2017).
Sáez-Blázquez, R., Feist, J., Fernández-Domínguez, A. I. & García-Vidal, F. J. Organic polaritons enable local vibrations to drive long-range energy transfer. Phys. Rev. B 97, 241407 (2018).
Du, M. et al. Theory for polariton-assisted remote energy transfer. Chem. Sci. 9, 6659 (2018).
Georgiou, K., Jayaprakash, R., Othonos, A. & Lidzey, D. G. Ultralong range polariton assisted energy transfer in organic microcavities. Angew. Chem. Int. Ed. 60, 16661 (2021).
Xiang, B. et al. Intermolecular vibrational energy transfer enabled by microcavity strong light–matter coupling. Science 368, 665 (2020).
Shalabney, A. et al. Coherent coupling of molecular resonators with a microcavity mode. Nat. Commun. 6, 5981 (2015).
Long, J. P. & Simpkins, B. S. Coherent coupling between a molecular vibration and Fabry–Perot optical cavity to give hybridized states in the strong coupling limit. ACS Photonics 2, 130 (2015).
Xiang, B. et al. State-selective polariton to dark state relaxation dynamics. J. Phys. Chem. A 123, 5918 (2019).
Li, T. E., Nitzan, A. & Subotnik, J. E. Cavity molecular dynamics simulations of vibrational polariton-enhanced molecular nonlinear absorption. J. Chem. Phys. 154, 094124 (2021).
Ribeiro, R. F., Campos-Gonzalez-Angulo, J. A., Giebink, N. C., Xiong, W. & Yuen-Zhou, J. Enhanced optical nonlinearities under strong light–matter coupling. Phys. Rev. A. 103, 063111 (2021)
Campos-Gonzalez-Angulo, J. A. & Yuen-Zhou, J. Polaritonic normal modes in transition state theory. J. Chem. Phys. 152, 161101 (2020).
Li, X., Mandal, A. & Huo, P. Cavity frequency-dependent theory for vibrational polariton chemistry. Nat. Commun. 12, 1315 (2021).
Li, T. E., Subotnik, J. E. & Nitzan, A. Cavity molecular dynamics simulations of liquid water under vibrational ultrastrong coupling. Proc. Natl. Acad. Sci. USA 117, 18324 (2020).
Sidler, D., Schäfer, C., Ruggenthaler, M. & Rubio, A. Polaritonic chemistry: collective strong coupling implies strong local modification of chemical properties. J. Phys. Chem. Lett. 12, 508 (2021).
Flick, J., Ruggenthaler, M., Appel, H. & Rubio, A. Atoms and molecules in cavities, from weak to strong coupling in quantum-electrodynamics (QED) chemistry. Proc. Natl. Acad. Sci. USA 114, 3026 (2017).
Haugland, T. S., Ronca, E., Kjønstad, E. F., Rubio, A. & Koch, H. Coupled cluster theory for molecular polaritons: changing ground and excited states. Phys. Rev. X 10, 041043 (2020).
Triana, J. F., Hernández, F. J. & Herrera, F. The shape of the electric dipole function determines the sub-picosecond dynamics of anharmonic vibrational polaritons. J. Chem. Phys. 152, 234111 (2020).
Luk, H. L., Feist, J., Toppari, J. J. & Groenhof, G. Multiscale molecular dynamics simulations of polaritonic chemistry. J. Chem. Theory Comput. 13, 4324 (2017).
Li, T. E., Nitzan, A. & Subotnik, J. E. Collective vibrational strong coupling effects on molecular vibrational relaxation and energy transfer: numerical insights via cavity molecular dynamics simulations. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202103920 (2021).
Gaigeot, M.-P. & Sprik, M. Ab initio molecular dynamics computation of the infrared spectrum of aqueous uracil. J. Phys. Chem. B 107, 10344 (2003).
Brawley, Z. T., Storm, S. D., Contreras Mora, D. A., Pelton, M. & Sheldon, M. Angle-independent plasmonic substrates for multi-mode vibrational strong coupling with molecular thin films. J. Chem. Phys. 154, 104305 (2021).
Yoo, D. et al. Ultrastrong plasmon–phonon coupling via epsilon-near-zero nanocavities. Nat. Photonics 15, 125 (2021).
Grafton, A. B. et al. Excited-state vibration–polariton transitions and dynamics in nitroprusside. Nat. Commun. 12, 214 (2021).
Schäfer, C., Ruggenthaler, M., Rokaj, V. & Rubio, A. Relevance of the quadratic diamagnetic and self-polarization terms in cavity quantum electrodynamics. ACS Photonics 7, 975 (2020).
Taylor, M. A. D., Mandal, A., Zhou, W. & Huo, P. Resolution of gauge ambiguities in molecular cavity quantum electrodynamics. Phys. Rev. Lett. 125, 123602 (2020).
Stokes, A. & Nazir, A. Implications of gauge-freedom for nonrelativistic quantum electrodynamics. Preprint at arXiv https://arxiv.org/abs/2009.10662 (2020).
Heidelbach, C., Fedchenia, I. I., Schwarzer, D. & Schroeder, J. Molecular-dynamics simulation of collisional energy transfer from vibrationally highly excited azulene in compressed CO2. J. Chem. Phys. 108, 10152 (1998).
Kabadi, V. N. & Rice, B. M. Molecular dynamics simulations of normal mode vibrational energy transfer in liquid nitromethane. J. Phys. Chem. A 108, 532 (2004).
Kandratsenka, A., Schroeder, J., Schwarzer, D. & Vikhrenko, V. S. Nonequilibrium molecular dynamics simulations of vibrational energy relaxation of HOD in D2O. J. Chem. Phys. 130, 174507 (2009).
Miller, W. H. A classical/semiclassical theory for the interaction of infrared radiation with molecular systems. J. Chem. Phys. 69, 2188 (1978).
Orel, A. E. & Miller, W. H. Classical model for laser induced nonadiabatic collision processes. J. Chem. Phys. 73, 241 (1980).
Vergauwe, R. M. A. et al. Modification of enzyme activity by vibrational strong coupling of water. Angew. Chem. Int. Ed. 58, 15324 (2019).
Xiang, B. et al. Two-dimensional infrared spectroscopy of vibrational polaritons. Proc. Natl. Acad. Sci. USA 115, 4845 (2018).
Haugland, T. S., Schäfer, C., Ronca, E., Rubio, A. & Koch, H. Intermolecular interactions in optical cavities: An ab initio QED study. J. Chem. Phys. 154, 094113 (2021).
Kapil, V. et al. i-PI 2.0: a universal force engine for advanced molecular simulations. Comput. Phys. Commun. 236, 214 (2019).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1 (1995).
Li, T. E. Cavity molecular dynamics simulations tool sets (v0.1). Zenodo https://doi.org/10.5281/zenodo.6629538 (2022).
Acknowledgements
This material is based upon work supported by the U.S. National Science Foundation under Grant No. CHE1953701 (A.N.); and US Department of Energy, Office of Science, Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division under Award No. DE-SC0019397 (J.E.S.).
Author information
Authors and Affiliations
Contributions
T.E.L. conceived the project, performed simulations and plotted the figures, T.E.L., A.N., and J.E.S. discussed the results and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Jacopo Fregoni, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, T.E., Nitzan, A. & Subotnik, J.E. Energy-efficient pathway for selectively exciting solute molecules to high vibrational states via solvent vibration-polariton pumping. Nat Commun 13, 4203 (2022). https://doi.org/10.1038/s41467-022-31703-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-31703-8
This article is cited by
-
Theoretical formulation of chemical equilibrium under vibrational strong coupling
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.