Abstract
Varicose veins affect one-third of Western society, with a significant subset of patients developing venous ulceration, costing $14.9 billion annually in the USA. Current management consists of either compression stockings, or surgical ablation for more advanced disease. Most varicose veins patients report a positive family history, and heritability is ~17%. We describe the largest two-stage genome-wide association study of varicose veins in 401,656 individuals from UK Biobank, and replication in 408,969 individuals from 23andMe (total 135,514 cases and 675,111 controls). Forty-nine signals at 46 susceptibility loci were discovered. We map 237 genes to these loci, several of which are biologically plausible and tractable to therapeutic targeting. Pathway analysis identified enrichment in extracellular matrix biology, inflammation, (lymph)angiogenesis, vascular smooth muscle cell migration, and apoptosis. Using a polygenic risk score (PRS) derived in an independent cohort, we demonstrate its predictive utility and correlation with varicose veins surgery.
Similar content being viewed by others
Introduction
Varicose veins (VVs) are a very common manifestation of chronic venous disease, affecting over 30% of the population in Western countries1. In the USA, chronic venous disease affects over 11 million males and 22 million females aged 40–80 years2, meaning it is twice as prevalent as coronary heart disease3. Chronic venous insufficiency leads to serious complications in 10% of cases, including lipodermatosclerosis, venous ulceration and rarely amputation. Despite best care, 25–50% of venous leg ulcers remain unhealed after six months of treatment4. Ongoing management of venous leg ulcers costs around $14.9 billion annually and 4.5 million work days per year are lost to venous-related illness in the USA5,6. Despite this, at present no medical treatments exist for VVs. For symptomatic patients, endovenous ablation is the first-line treatment approach7. However, recurrence following surgery is 20%, with no difference in recurrence compared to conventional open surgery8.
VVs are thought to develop from a combination of valvular insufficiency, venous wall alterations, and haemodynamic changes that precipitate venous reflux, stasis and hypertension of the venous network, causing varicosities9,10. Risk factors for VVs include older age, female sex, pregnancy, a positive family history, obesity, tall height and previous deep-vein thrombosis (DVT)3,11,12. Many patients with VVs report a positive family history13, and among offspring with one affected parent the familial standardised incidence ratio is 2.3914, with a heritability of 17%15, suggesting a genetic component to aetiology. Two recent genome-wide association studies (GWAS) of VVs have been described. Ellinghaus et al.16 tested for associations in 323 cases and 4619 controls, with suggestive associations examined in an independent cohort totalling 1946 cases and 3146 controls. They reported two associations, mapped to EFEMP1 and KCNH8. Fukaya et al.17 used UK Biobank to identify a further 30 putative associations associated with VVs. However, their cases were defined only by the International Classification of Diseases (ICD) diagnostic codes, meaning that thousands of cases defined by operation codes were misclassified as controls18. Moreover, the genetic associations discovered were not replicated in an independent cohort.
To advance substantially our understanding of the aetiology and genetic architecture of VVs, we performed the largest genome-wide association study (GWAS) of surgically confirmed VVs. We use data from participants from the UK Biobank (n = 401,656) and confirm top associations in a large independent cohort of research participants with self-reported VVs from 23andMe (n = 408,969). We examine patterns of expression of genes in clinically relevant biologic pathways and prioritise targets for therapeutic development. Furthermore, we derive a polygenic risk score (PRS) for VVs in an independent cohort (FinnGen), and demonstrate its predictive utility in UK Biobank.
Results
The overall analytic workflow is shown in Fig. 1.
A discovery GWAS was performed in the UK Biobank cohort, with the top independent lead variants tested within the 23andMe replication cohort. Forty-nine independent variants at 46 loci met the Bonferroni-corrected threshold in the replication cohort, and were interrogated further in subsequent analyses.
Association analysis
Genome-wide testing of the UK Biobank discovery cohort (22,473 cases and 379,183 controls) yielded genome-wide significant associations (P < 5 × 10−8) at 108 risk loci (Supplementary Data 1). Conditional regression yielded a further seven independent signals at six of the 108 loci associated significantly with VVs. As expected, the large discovery sample led to the λGC showing inflation (1.25) (Supplementary Fig. 1). The linkage equilibrium score regression (LDSC) intercept (1.06), and attenuation ratio of 0.13 is consistent with polygenicity19. We estimated the total SNP heritability (h2g) for VVs in UK Biobank to be 8.04% (S.E. = 0.17%) using a variance components method, and 5.03% (S.E. = 0.30%) using LDSC19. The estimated heritability in the 23andMe dataset (113,041 cases and 295,928 controls) was 5.40% (S.E. = 0.30%).
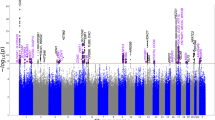
We tested the top 116 signals at the 108 risk loci in the independent 23andMe dataset. Of the 116 associated variants, 106 passed QC and were available in the 23andMe summary statistics. Forty-nine of 116 variants demonstrated significant association at a conservative Bonferroni-corrected threshold of P < 4.72 × 10−4. Thus, we identified 49 significant associations at 46 independent risk loci (Fig. 2; regional association plots for all 49 signals can be found in Supplementary Fig. 2). Allelic effects were concordant across both cohorts at all 49 replicated variants, with minimal evidence of heterogeneity between the two GWAS at all loci (Q-statistic > 0.05). Eighteen loci were previously reported (Supplementary Data 2). Sixty-seven variants at 67 loci did not replicate (Supplementary Data 3); however, there was high overall concordance in the effect sizes of the 106 SNPs between the two datasets (Pearson’s r2 = 0.84, P < 1 × 10−16; Supplementary Fig. 3A).
Manhattan plot showing genome-wide P-values plotted against position on each of the autosomes. The dark blue, light blue and green dots refer to the discovery UK Biobank Cohort, with the red dots corresponding to the 49 variants from the 23andMe cohort at each replicated locus (shown in Supplementary Data 2). The dark blue peaks correspond to the 46 loci that replicated in the 23andMe cohort at a Bonferroni-corrected threshold of P < 4.72 × 10−4. Candidate genes at each locus are named above each signal, with previously unreported genetic loci in blue, and previously described loci in black.
In silico annotation
To interrogate and annotate SNPs at our susceptibility loci, we used FUMA (Functional Mapping and Annotation of GWAS)20. FUMA identified 5315 genome-wide significant candidate SNPs from our discovery cohort associated with VVs at 45 of the 46 replicated loci (Supplementary Fig. 4; Supplementary Data 4). Around 2% of candidate SNPs (n = 103) were exonic, of which 56 were non-synonymous (52 missense, two stop-gain, one splice site variant, and one frameshift variant). Of the non-synonymous variants, four missense variants were predicted to affect protein structure or function, and were in moderate linkage disequilibrium (LD) (r2 ≥ 0.22 and Dʹ ≥ 0.75) with the index SNP at three loci—12q13.12 (index SNP: rs7308356), 16q24.3 (index SNP: rs2002833) and 17q24.3 (index SNP: rs9895127) (Supplementary Data 5). This includes rs7184427 (A/G) (P = 9.10 × 10−40, OR = 1.19, r2index = 0.22, Dʹindex = 0.75), which causes a predicted deleterious p.Val250Ala substitution within PIEZO1 (SIFT21 score: 0). PIEZO1 encodes a mechanically active ion channel involved in the detection of vascular shear stress, and was previously associated with VVs and lymphoedema17,22.
Of the 4294 intronic and intergenic variants identified by FUMA (Supplementary Fig. 4), 3735 (87.0%) resided in open chromatin regions (Supplementary Data 4), and 163 demonstrated evidence of functionality with CADD (Combined Annotation-Dependent Depletion23) score ≥12.37, the threshold suggested for deleterious variants (Supplementary Data 6). Using RegulomeDB (RDB24) to investigate their regulatory functions, 17 had a RDB score of at least 2b (likely to affect binding) and eight had an RDB score of at least 1f (likely to affect binding and linked to expression of a gene target).
To narrow down the list of probable functional variants, we performed functionally informed fine-mapping25 for each replicated locus. Eighteen SNPs were identified with posterior probability >95% (Supplementary Table 1), including two likely functional variants in PIEZO1 (rs112070238 and rs8053350), affecting the 5ʹUTR and promoter regions, respectively.
Gene mapping
Positional mapping in FUMA SNP2GENE highlighted 204 genes based on genomic position at 38 loci (Supplementary Data 7)20. eQTL mapping, based on GTEx v8 tibial artery tissue26, mapped a total of 80 genes. Genome-wide, gene-based association analysis implemented in MAGMA v1.0727 identified 248 protein-coding genes significantly associated with VVs (P < 2.67 × 10−6); 117 were within our replicated loci (Supplementary Data 8; Supplementary Fig. 5).
In the summary-based Mendelian randomisation (SMR) analysis28, testing was performed for 4946 probes with a cis-eQTL at P < 5 × 10−8; a threshold of significance was set at PSMR < 1.01 × 10−5 (0.05/4,946). Forty-four genes passed the set significance threshold (PSMR < 1.01 × 10−5). To exclude SMR associations due to linkage, we performed HEIDI analysis across 44 significant genes −25 passed the HEIDI test (PHEIDI ≥ 1.12 × 10−3; Supplementary Data 9), 12 of which were within our VVs susceptibility loci, highlighting an association with VVs through pleiotropy rather than LD and co-localisation.
In summary, 237 unique genes were mapped by at least one mapping approach to 39 of the 46 susceptibility loci. Significant overlap between the mapping strategies was seen, with the majority of genes (54.9%, n = 130) being prioritised by two or more mapping approaches. Thirty-six genes were prioritised by three mapping approaches, and six genes (ATF1, AP1M1, DNAH10OS, FBLN7, LBH, WDR92) were prioritised by all four approaches.
Gene set, tissue-specific and pathway enrichment
To delineate gene sets and enriched pathways where the 237 prioritised genes converged, gene-set analysis was conducted in MAGMA v1.0727. Following MAGMA gene-set enrichment analysis, four Gene Ontology (GO) gene sets were significantly over-represented in our data: cardiovascular development (P = 1.56 × 10−8, n = 666); tube morphogenesis (P = 9.35 × 10−8, n = 778); blood vessel morphogenesis (P = 9.39 × 10−7, n = 555); and tube development (P = 1.68 × 10−6, n = 956) (Supplementary Table 2). Further, tissue-specific gene property analysis demonstrated significant gene expression in all three vascular tissue types present in GTEx 54 tissue types: coronary artery (P = 6.23 × 10−7, 2nd most enriched), tibial artery (P = 1.05 × 10−6, 3rd most enriched) and aorta (P = 3.92 × 10−5, 8th most enriched; Supplementary Fig. 6). MAGMA analysis of GTEx 30 general tissue types also demonstrated blood vessels to be a highly enriched tissue (P = 3.8 × 10−4, 3rd most enriched). Using eXploring Genomic Relations (XGR) analysis29, six canonical pathways were significantly enriched. This included enrichment for genes in pathways related to extracellular matrix biology, the VEGF and VEGFR signalling network, and intracellular Ca2+ signalling in the T-cell receptor (TCR) Pathway (Table 1).
Polygenic risk score analyses
In order to derive a polygenic risk score (PRS) without overfitting to the UKB cohort, we utilised VV summary statistics from the FinnGen cohort (17,027 cases, 190,028 controls), which were highly concordant with the UK Biobank GWAS (Pearson’s r2 = 0.95, P < 1 × 10−16; Supplementary Fig. 3B). Using multivariable logistic regression, we found that the FinnGen-derived PRS was an independent predictor for varicose vein case/control status (Fig. 3 and Supplementary Table 3). Individuals in the top PRS decile had markedly increased odds of VV diagnosis (OR 4.57, 95% CI 4.27–4.92, P < 1 × 10−300) compared to the bottom PRS decile. We hypothesised that VV cases who underwent surgery are phenotypically more severe and so would have a greater genetic liability than non-surgical VV cases. In a subgroup analysis of UK Biobank VV cases defined by self-reported diagnosis, ICD-10 or OPCS-4 code, history of surgery was associated with significantly increased PRS (0.407 vs. 0.330; P = 5.5 × 10−4), suggesting that the VV-PRS captures disease severity.
Phenome-wide VV associations
To uncover associations between VV and other phenotypes, we first performed genetic correlation analysis using LD score regression19. Of the 176 traits from nine categories tested for genetic correlation, twelve traits from two categories (anthropometric and autoimmune) met our Bonferroni-corrected significance threshold (P < 5.56 × 10−3; Supplementary Table 4). All twelve significant traits were positively correlated with VVs (rg range: 9–21%). Eleven traits belonged to the anthropometric category and pertained to height and weight phenotypes, which are established risk factors for VVs3,11,12.
In the autoimmune trait category, we discovered systemic lupus erythematosus (SLE) to be correlated with VVs, sharing ~19% genetic overlap (P = 4.2 × 10−3, rg = 0.195). Of note, the C allele of variant rs17321999 at 2p23.1 (LBH), which is associated with an increased risk of SLE (P = 2.22 × 10−16, OR = 1.20)30, was also significantly associated with VVs in our discovery cohort (Pdisc = 3.20 × 10−14, OR = 1.09), and in high LD with lead SNP at 2p23.1 in the meta-analysis (rs9967884; Pmeta = 1.40 × 10−14, OR = 1.11; r2 = 0.87). We used the Open Targets Genetics platform31 to confirm the associations between VVs and SLE at the variant level, using a phenome-wide association study (PheWAS) approach (Supplementary Data 10); three of our 49 replicated lead variants (rs4849044, rs7773004, rs61863928) demonstrated an association with SLE.
As a complementary approach, we leveraged the FinnGen-derived VV-PRS to perform a phenome-wide polygenic risk score analysis using a set of curated time-to-event phenotypes (n = 694) in UK Biobank. Eighteen phenotypes met the phenome-wide significance threshold (P = 1 × 10−5) in the Cox regression (Supplementary Table 5). The most significantly associated phenotypes were “Phlebitis and thrombophlebitis”, “Unspecified monoarthritis”, “Obesity”, “Postphlebitic syndrome”, “Pulmonary embolism and infarction” and “Umbilical hernia”. To investigate whether these phenotypes are causally associated with VV, we performed bidirectional Mendelian randomisation using genetic instruments derived from our UK Biobank GWAS. Using the inverse-variance weighted (IVW) method as the primary analysis, we found evidence for raised body mass index being causal in VV, with no evidence for significant directional pleiotropy on MR-Egger sensitivity analysis (Supplementary Table 6). Two further suggestive causal associations were found: VVs (exposure phenotype) on thrombophlebitis risk and hernia (exposure phenotype) on VVs risk.
Drug-target enrichment analysis
Finally, we tested the amenability of our associated genes to drug targeting using the Open Targets Platform32. Of the 237 mapped genes, 200 genetic targets were identified32. Forty-two drug pathways reached nominal significance (P < 0.05), with the Butyrophilin family interactions pathway being most enriched (P = 2.6 × 10−7, six targets), followed by the Calcineurin-NFAT pathway (P = 9.4 × 10−4, two targets), and the Transcriptional regulation by RUNX1 pathway, which possessed the highest number of targets (P = 1.6 × 10−3, 14 targets; Supplementary Data 11). Tractability information for 105 gene targets was available, with 65 of 237 genes predicted to be tractable to antibody targeting to a high confidence, and 26 genes predicted to be tractable to small molecule targeting (Supplementary Data 12). Eight of the 237 genes overlapped with pharmacologically active targets with known pharmaceutical interactions (CDK10, COL27A1, GABBR1, KCNJ2, MAPK10, OPRL1, TNC and VEGFA) (Supplementary Data 13). Of note, VEGFA is a target for several antibody, protein and oligosaccharide agents, which are currently in phase 2, 3 and 4 clinical trials, including for several ocular vascular disorders.
Discussion
VVs cause significant morbidity and large healthcare costs. There is a compelling need for a deeper understanding of the biology of VVs in order to develop new therapeutic strategies. Our analysis represents the largest and most comprehensive GWAS to date of VVs, including 135,514 patients and 675,111 controls. We discovered 28 previously unreported risk loci (29 signals), and independently replicated 18 of 29 previously reported but non-replicated loci (20 of 32 known signals). Our in silico analyses demonstrated strong evidence of functional variants in VV-associated genomic regions. Furthermore, our pathway analyses establish a strong enrichment for genes expressed in the extracellular matrix, immune cell signalling and circulatory system development. We have also identified a previously unreported genetic correlation between VVs and systemic lupus erythematosus (SLE). Several prioritised genes demonstrate the potential for pharmacological targeting, and are currently under active investigation in other diseases. Lastly, we demonstrate the potential utility of a polygenic risk score (PRS) in VVs, demonstrating that genetic risk correlates with a more severe phenotype—a first step in facilitating personalised medicine approaches to management.
Biological drug targets
This study has identified several biological pathways as mediators of VV pathophysiology that may be of relevance to pharmacological targeting. These cluster broadly into five functional categories: extracellular matrix regulation, the immune response, angiogenesis and lymphangiogenesis, smooth muscle cell biology and apoptosis (Supplementary Data 14), and we will briefly examine the three functional categories that produced the most compelling evidence in this study.
Extracellular matrix regulation
VVs demonstrate an increased luminal diameter and intimal hypertrophy, features closely related to disruption of the extracellular matrix (ECM)33. Deposition of ECM in the perivascular space in VVs is also recognised—possibly a compensatory mechanism to reinforce an already weakened wall34. Venous dilatation and valve ring enlargement seen in VVs is thought to affect the ability of venous valves to co-apt, which in turn contributes to venous reflux and hypertension10. Indeed, there is a noted imbalance of ECM proteins in VVs, specifically collagen and elastin—with a preponderance of collagen compared to normal vein35. It is therefore possible that disruption in intrinsic connective tissue components of the vein wall or valves may contribute to VVs.
Our prioritised genes significantly overlapped with canonical pathways relating to ECM components, including Collagen Type XXVII Alpha 1 Chain (COL27A1) and EGF-containing Fibulin-like Extracellular Matrix Protein 1 (EFEMP1).
rs753085 (P = 2.17 × 10−11, OR = 1.07) is in an intron of COL27A1. COL27A1 is a fibrillar collagen in the extracellular matrices of several tissues, including blood vessels36. COL27A1 expression has been demonstrated to be reduced in VV samples37. Moreover, our drug-enrichment analysis demonstrated COL27A1 to be a pharmacologically active target, with pharmaceutical agents currently being investigated in several clinical trials (Supplementary Data 13), demonstrating its potential candidacy as a therapeutic target for VV prevention or treatment.
In our GWAS, we replicated the previously described association between rs3791679 and VVs (P = 1.59 × 10−13, OR = 1.08)16. rs3791679 resides in an enhancer region of EFEMP1, and is believed to be the causal variant at this locus38. EFEMP1 encodes the ECM glycoprotein fibulin-339, which plays a key role in maintaining the integrity of elastic tissues16, and is highly expressed in vein endothelial cells. Interestingly, the same A allele of rs3791679 is also associated with abdominal hernia susceptibility40; this pleiotropy may in part explain our Mendelian randomisation finding that placed hernias on the causal pathway for VVs.
Fibulin-3 has been found to antagonise vascular development by reducing the expression of the matrix metalloproteases, MMP2 and MMP3, and increasing expression of tissue inhibitors of metalloproteases (TIMPs) in endothelial cells41. The saphenofemoral junction in VVs demonstrates reduced expression of MMP2, and heightened expression of TIMP1 and MMP1 protein levels42. Alterations in expression of these enzymes may therefore result in weakness in the venous wall and predispose patients to VVs. Our drug-enrichment analysis found fibulin-3 to be tractable to antibody targeting to a high confidence; moreover, metformin has been demonstrated to downregulate fibulin-3 through downregulation of MMP243. Fibulin-3 therefore represents another potential pharmacological target for VVs.
The immune response
Chronic Inflammation in the venous wall has been proposed to play a role in VV aetiopathology33. When compared to normal veins, enhanced expression of inflammatory mediators has been observed10. VVs contain increased mast cells, monocytes and macrophages compared to normal veins10.
We defined five inflammation-associated risk loci in our GWAS. Of particular note is rs78216177 (Pmeta = 5.80 × 10−14, OR = 1.10), in an intron of DOCK8 that has roles in both innate and adaptive immune systems. Deletion of DOCK8 is strongly associated with Hyper-IgE syndrome, a type of primary immunodeficiency that affects multiple systems, including the vasculature44. Vascular abnormalities in hyper-IgE syndrome include aneurysmal changes and abnormalities in great vessels.
Notably, we also discovered a significant genetic overlap between VVs and SLE, an autoimmune disease, in the genetic correlation analyses, albeit not in the phenome-wide analysis of VV-polygenic risk score. The associations between VVs and SLE were confirmed at the variant level using a PheWAS approach, with three of 49 replicated lead variants (rs4849044, rs7773004, rs61863928) demonstrating an association with SLE. Furthermore, lead variant rs1471251 (P = 8.33 × 10−11, OR = 1.06) is a known eQTL of AFF1, which is associated with SLE45. Reinforcing this shared genetic basis, a VVs-associated variant at LBH, rs17321999 (Pdisc = 3.20 × 10−14, OR = 1.09), was also previously associated with SLE (P = 2.22 × 10−16, OR = 1.20)30.
XGR analysis demonstrated enrichment of “intracellular calcium signalling in the CD4 + T-cell receptor (TCR) pathway” (P = 1.9 × 10−3, Z = 3.5), specifically highlighting genes NFATC2 and PPP3R1, which are intimately involved in this pathway. rs3787184 (Pmeta = 2.51 × 10−36, OR = 1.16) was one of 18 SNPs identified as likely causal variants in our functionally informed fine-mapping (Supplementary Table 1), and resides in a promoter region of NFATC2. rs2861819 (Pmeta = 2.65 × 10−77, OR = 1.20) is in an intergenic region ~19 kb upstream of PPP3R1. PPP3R1 encodes calcium binding B (CnB), a subunit of calcineurin, a Ca2+influx-activated serine/threonine-specific phosphatase that interacts with NFAT transcription factors in the regulation of naive T-cell activation46. VVs are defined by clustering and infiltration of T lymphocytes34, which are predominantly distributed close to the venous valve agger47, a fibroelastic structure located at the base of venous valves where tunica media meets adventitia. Therefore, we hypothesise that aberrant PPP3R1 and NFATC2 expression could alter calcium signalling in T-cells, which may contribute to the valvular pathology seen in VVs. The Calcineurin-NFAT pathway was also the second-most enriched in our drug-target enrichment analysis, and may represent a novel therapeutic approach to VVs.
Angiogenesis and lymphangiogenesis
Disruption in normal angiogenic processes can lead to VVs48, potentially because of a failure to develop properly formed venous walls and valves, or to repair defects following vascular stress injury. MAGMA gene-set analysis revealed enrichment of several gene sets relating to tube formation and morphology, with the VEGF/VEGFR signalling network also being enriched in the XGR analysis.
rs11967262 at 6p21.1 (Pmeta = 1.45 × 10−19, OR = 1.09) lies in an intergenic region ~7 kb upstream of vascular endothelial growth factor A (VEGFA). VEGFA is a critical regulator of angiogenesis, and is fundamental to maintaining the integrity and functionality of the vessel wall49. VEGFA is a selective endothelial mitogen, binding to its receptor VEGFR2 to induce endothelial cell proliferation, migration and differentiation. VEGFA and VEGFR2 expression are significantly enhanced in the wall of VVs compared to normal veins, particularly in VVs complicated by thrombophlebitis50; interestingly, our Mendelian randomisation analysis found evidence for a causal role played by VVs in thrombophlebitis risk. Plasma levels of VEGFA have also been demonstrated to be significantly increased in patients with VVs51. VEGFA also causes vasodilatation, which reduces vessel tone, leading to stasis and the release of oxygen free radicals, which contributes to vein wall weakness52. Intriguingly, VEGFA also promotes inflammation via expression of intercellular and vascular cell adhesion molecules, linking angiogenesis to immune dysregulation53. Of note, anti-VEGFA agents are currently being investigated in several clinical trials for the treatment of retinal vein occlusion (Supplementary Data 13). The VEGF axis therefore represents a promising candidate for therapeutic targeting in the treatment of VVs.
Positional and MAGMA mapping highlighted genes at four loci relating to lymphangiogenesis. Indeed, the lymphatic system develops from veins, and its function is intimately related with the venous circulation, draining extracellular fluid back into circulation. It is therefore feasible that similar genetic defects may result in either lymphoedema, varicose veins or a combination of the two conditions. PROX1 is a master inducer gene necessary for the development of lymphatic vasculature54, and PROX1knock-out mice are deficient of lymphatic vasculature55. During developmental lymphangiogenesis, PROX1 has been shown to be necessary for the formation of lymphovenous valves56,57, suggesting it may predispose to VV development by causing defects in venous valves. rs340875, (Pmeta = 4.22 × 10−20, OR = 1.09) is ~2 kb upstream of PROX1, and we also fine-mapped two probable functional variants at this locus (Supplementary Table 1).
Furthermore, PROX1 is co-expressed and functions alongside the transcription factor FOXC2 in lymphatic valve-forming cells at the earliest stage of lymphatic development58. Mutations in FOXC2 cause hereditary lymphoedema-distichiasis—a disease characterised by VVs and peripheral lymphoedema—highlighting genetic overlap between the two disorders59. A previous analysis also suggested FOXC2 to be implicated in the development of varicose veins in the general population60, but we did not find evidence of association between varicose veins and FOXC2 in our study. In fact, the only gene identified in this study known to be associated with a Mendelian disease that results in VVs is PIEZO1, which also predisposes to both VVs and lymphoedema (Supplementary Table 7).
Polygenic risk score
Over two million people in the USA have advanced chronic venous disease2, and around 500,000 per year undergo invasive surgical procedures. Our polygenic risk score (PRS) analysis of operated vs unoperated VVs cases represents an important proof-of-principle, demonstrating the feasibility of enhanced prognostication in enabling the subset of VVs patients that will require surgical intervention to be identified. Preventive strategies in high-risk individuals could include prophylactic compression stocking use, or early ablation procedures to mitigate the risk of venous ulceration. The benefits of early endovenous ablation in improving healing of venous leg ulcers has been demonstrated61. Future research may also enable the identification of those at high risk of recurrence following surgery, a significant problem in current management of VVs patients.
Strengths and limitations
Certain limitations of this study must be acknowledged. While the discovery GWAS in UK Biobank used a combination of hospital diagnostic codes, operation codes and self-report codes, VV cases in 23andMe were identified based on self-report alone, meaning that the phenotyping for the replication GWAS was necessarily less stringent. Moreover, rather than undertake a formal meta-analysis between the discovery and replication GWAS, we independently tested the association for the 116 independent lead SNPs that were genome-wide significant in the UK Biobank discovery GWAS. Thus, sub-threshold signals in the discovery GWAS that may have reached genome-wide significance in the replication GWAS, or under meta-analysis, were not identified. Furthermore, not having access to the full summary statistics for the replication GWAS also meant that our in silico analyses were performed on the summary statistics from the discovery GWAS alone.
Finally, we acknowledge that out of the many VVs-associated genes identified by a multitude of gene-prioritisation and mapping methods, only a small minority have robust evidence linking them to VVs through a truly causal variant. Moreover, our eQTL-based gene mapping and summary-based Mendelian randomisation analyses were limited by our lack of access to eQTL data for venous tissue. We therefore had to employ GTEx tibial artery tissue data as an imperfect surrogate, reasoning that it is the anatomically, histologically, and embryologically closest tissue to lower limb veins within the GTEx dataset. Functional validation will be required in diseased venous tissue to confidently associate these candidate susceptibility variants and genes to VVs.
Several strengths of the paper mitigate these limitations. We have performed the largest GWAS of VVs to date by a considerable margin, with 135,514 cases and 675,111 controls. We have stringently controlled our false-positive rate by reporting only the loci that were genome-wide significant in the discovery GWAS and that subsequently replicated, so we can be confident in the veracity of these 49 signals. This is reflected in the plethora of biologically plausible genes, gene clusters and biological pathways that were associated with these loci. Furthermore, by including surgical codes for phenotyping in the UK Biobank discovery GWAS, we were able to identify a considerably greater number of cases than a previous GWAS that also used the UK Biobank resource but relied on ICD diagnostic codes alone (22,473 vs. 9577 cases)17,18. As a general principle of case ascertainment, we believe there is much to be gained by seeking out individuals who have undergone surgery for a disease: given the inevitable risk of complications, surgery is generally reserved for those at the more phenotypically severe end of the spectrum who have failed non-surgical treatment. Finally, the predictive capabilities of a VVs polygenic risk score derived in an independent cohort not only underscores the validity of the VVs-associated polygenic signals uncovered in our discovery GWAS, but also opens the door to the future use of genetic risk-stratification in improving prognostication and guiding decision-making in the management of VVs patients.
In summary, we have described the largest GWAS to date of VVs, a highly prevalent disease with a substantial health and socioeconomic cost. We discovered 49 variants at 46 loci that predispose to VVs. We have identified pathways and genes involved in extracellular matrix regulation, inflammation, vascular and lymphatic development, smooth muscle cell activity and apoptosis, all of which are biologically plausible contributors to the pathobiology of VVs, and provide excellent candidates for further investigation of venous biology. Several genes appear tractable to pharmacological targeting (notably VEGFA, COL27A1, EFEMP1, PPP3R1 and NFATC2), and may represent viable therapeutic targets in the future management of VV patients. Importantly, our polygenic risk score represents a first step towards better prognostication in patients with VVs.
Methods
Ethical approval and consent
UK Biobank obtained ethical approval from the North West Multi-Centre Research Ethics Committee (MREC) (11/NW/0382) to collect and disseminate data and samples from participants (for more details: ukbiobank.ac.uk/ethics). This study was conducted under UK Biobank study ID 22572. All participants provided informed consent for their genotype data to be used for this research. The consent procedures for UK Biobank are provided elsewhere (for more details: www.ukbiobank.ac.uk/). All 23andMe participants were obtained from the customer base of 23andMe, Inc. Genotyping of participants was performed by the 23andMe Personal Genome Service. All 23andMe research participants provided informed consent for their genotype data to be utilised for research purposes under a protocol approved by the external AAHRPP-accredited IRB, Ethical & Independent Review Services. Further details regarding the consent processes of 23andMe can be found elsewhere (23andme.com/en-gb/about/consent).
Population and phenotype definition
The UK Biobank is a population-level resource comprising a prospective cohort of approximately 500,000 participants, recruited at age 40–69 years, at 22 centres across England, Scotland and Wales, between 2006 and 201062. Participants underwent whole-genome genotyping and data linkage with their medical records was performed to permit deep phenotyping63. The characteristics of the full UK Biobank cohort are described in detail elsewhere64. In the discovery analysis, VV cases were identified from the UK Biobank data showcase (ukbiobank.ac.uk) using the following diagnostic, operative and self-report codes (Supplementary Table 8):
-
1.
Primary and/or secondary ICD-10 codes for varicose veins (I83)
-
2.
Primary and/or secondary OPCS code for varicose vein surgery: ((L84-L88)
-
3.
Self-reported operation code for varicose vein surgery (1479)
-
4.
Self-reported non-cancer illness code for varicose veins (1494)
In summary, 27,165 individuals from the UK Biobank cohort possessed at least one of the above codes and were classified as VVs cases. Following quality control (QC), we identified 22,473 VV cases and the remaining 379,183 individuals were defined as controls.
In the 23andMe replication cohort, participants answered the question “Do you have varicose veins on your legs? (Yes/Not Sure/No)”. VV cases were identified if they answered “Yes”, while controls were those who answered “No”. In all, 113,041 VV cases and 295,928 controls were included in the final replication analysis.
Genotyping
UK Biobank participants were genotyped on UK BiLEVE (49,950 participants) and UK Biobank Axiom arrays (438,427 participants), with ~95% shared content. In all, 805,426 directly genotyped variants from 488,377 participants were available prior to QC. The 23andMe replication cohort was genotyped on one of four custom arrays (v1/v2, v3, v4, v5). Illumina HumanHap550+ BeadChip was used for v1/v2 (1680 cases, 4882 controls) and the Illumina OmniExpress+ BeadChip was used for v3 (21,342 cases, 56,448 controls). For v4 a fully customised array (58,883 cases, 148,637 controls) was used, and for v5, the Illumina Infinium Global Screening Array was used (31,136 cases, 85,961 controls). Successive arrays contained significant overlap with all previous arrays.
Quality control
Quality control (QC) for the UK Biobank discovery cohort was conducted using PLINK v1.965 (https://zzz.bwh.harvard.edu/plink/) and R v3.3.1, as previously described (Supplementary Fig. 7)66. Briefly, all SNPs with a call rate <90% were removed. Following which, sample-level QC was conducted—individuals were excluded if: (i) they demonstrated heterozygosity >3 S.D. from the mean (calculated using UK Biobank’s PCA-adjusted heterozygosity values, Data Field 20004); (ii) there was disparity between genetically inferred sex (Data Field 22001) and self-reported sex (Data Field 31) or individuals with aneuploidy of sex chromosomes (Data Field 22019); and (iii) had a call rate <98%. Further, we removed all individuals who were not of white British ancestry (based on PCA and self-reported ethnicity (Data Field 22006).
To confirm individuals were of white British-ancestry, we combined our UK Biobank data with individuals identified as GBR from the 1000 Genomes Project (www.1000genomes.org), and using FlashPCA v2.0 (github.com/gabraham/flashpca/) we identified agreement between the two datasets. Following this analysis, 86,693 individuals were excluded from our discovery GWAS. Using a linear mixed model implemented in BOLT-LMM67 allowed us to include and account for related individuals. Next, conducting SNP-level QC, we excluded 230,562 SNPs based on: (i) a call rate <98%, (ii) Hardy–Weinberg Equilibrium (HWE) P < 1 × 10−4, (iii) MAF < 1%. We further excluded six individuals who were visual outliers when autosomal heterozygosity was plotted against call rate. Our post-QC discovery GWAS, therefore, consisted of 401,667 individuals and 547,011 genotyped SNPs (Supplementary Fig. 7). Following QC, our final discovery GWAS consisted of 401,667 individuals of white British ancestry and 547,011 genotyped single-nucleotide polymorphisms (SNPs).
For the 23andMe replication analysis, samples were restricted to individuals from European ancestry determined through an analysis of local ancestry68. A maximal set of unrelated individuals was chosen for each analysis using a segmental identity-by-descent (IBD) estimation algorithm, defining related individuals if they shared more than 700 cM IBD, including regions where the two individuals share either one or both genomic segments IBD. Cases were preferentially retained in the analysis. Variant QC was applied independently to genotyped and imputed GWAS results. The SNPs failing QC were flagged based on multiple criteria, such as HWE P-value, call rate, imputation R-square and test statistics of batch effects.
Imputation
The phasing and imputation of UK Biobank has been previously described63. Briefly, using SHAPEIT369 (jmarchini.org/shapeit3/), autosome phasing was conducted, with a reference panel based on Phase 3 1000 Genomes Project data70. Imputation was performed using a combined HRC reference panel (www.haplotype-reference-consortium.org/) and a merged UK10000/1000 Genomes Phase 3 Panel71. This resulted in an imputation file consisting of 92,693,895 autosomal SNPs, short indels and large structural variants63. In 23andMe, out-of-sample modified versions of the Beagle graph-based haplotype phasing algorithm72 and Eagle2 v2.373 algorithm were used to phase samples. We used a 23andMe internally developed tool, which implements the Beagle graph-based haplotype phasing algorithm, modified to separate the haplotype graph construction and phasing steps. We imputed samples against a single unified imputation reference panel combining the 1000 Genomes Phase 3 haplotypes70 with the UK10K imputation reference panel71 using Minimac374.
Association analysis
In the UK Biobank, we performed GWAS using a linear mixed non-infinitesimal model implemented in BOLT-LMM v2.367 across 547,011 genotyped SNPs (minor allele frequency (MAF) ≥ 0.01) and 8,397,536 imputed SNPs (MAF ≥ 0.01 and INFO score ≥0.90), adjusting for genotyping platform and genetic sex. The reference genetic map used was hg19 and linkage disequilibrium scores were generated from European-ancestry individuals taken from the BOLT-LMM package. The genome-wide significance threshold in this analysis was set at P < 5 × 10−8. Conditional regression analysis was performed at the top signal at each of 109 associated loci in BOLT-LMM67, excluding the MHC region due to the high density of genes and high linkage of variants.
In 23andMe, summary statistics were generated via logistic regression assuming an additive model for allelic effects. Association analysis was performed adjusting for age, sex, the first five principal components, and genotyping platform. The top independent variants from the discovery GWAS were tested for their association with VVs in the 23andMe cohort. Of the 116 genome-wide significant variants in the discovery GWAS, 106 were present in, and passed QC within the replication cohort. The Bonferroni-corrected significance threshold for replication was set at P < 4.72 × 10−4 (0.05/106). Data for all top independent variants that were available in both UK Biobank and 23andMe and that met the SNP QC within 23andMe were meta-analysed using a fixed-effects meta-analysis performed in GWAMA v2.2.275.
Functional annotation of SNPs
To annotate SNPs at our susceptibility loci, SNP2GENE was performed in Functional Mapping and Annotation of GWAS (FUMA) v1.3.320 (https://fuma.ctglab.nl/) using summary statistics from the UK Biobank cohort and default settings. FUMA defined risk loci borders by using all independent genome-wide significant SNPs (r2 < 0.6), and identified all SNPs that were in linkage disequilibrium (LD) with one of these candidate SNPs. Using ANNOVAR (https://annovar.openbioinformatics.org/en/latest/), candidate SNPs within the replicated loci were annotated on the basis of genomic location. Exonic SNPs were investigated further using gnomAD and Ensembl genome browsers (www.ensembl.org/index.html) to identify non-synonymous missense variants (see URLs). All candidate SNPs were annotated with Combined Annotation-Dependent Depletion (CADD)23 (cadd.gs.washington.edu/), RegulomeDB24 (www.regulomedb.org/), and 15-core chromatin states (Roadmap ChromHMM model76) to predict any regulatory or transcription effects from chromatin states at each SNP.
Fine-mapping
For each locus that was replicated in the 23andMe summary statistics (n = 45), functionally informed fine-mapping was performed using Polyfun-SuSiE v0.11.9225 using a 2MB window around each window SNP with the maximum number of causal SNPs set as 5 and a posterior probability threshold of 95%. The LD reference was derived from 1000 Genomes project European population. Fine-mapping was implemented using the echolocatoR package v0.2.2 for R77.
Candidate gene mapping
Four gene mapping approaches—positional mapping, eQTL mapping, MAGMA gene mapping (www.ctg.cncr.nl/software/magma), and summary-based Mendelian randomisation (SMR)—were used to map putative genes at the replicated loci. For FUMA positional mapping, all genome-wide significant SNPs at each locus were mapped to genes within a positional window of 10 Kb20. eQTL gene mapping was used to map genes based on having at least one genome-wide significant cis-eQTL in GTEx v8 tibial artery tissue20. Using MAGMA v.1.0727, we conducted a genome-wide, gene-based association study, testing 17,966 protein-coding genes. The significance threshold was set at P < 2.78 × 10−6 (0.05/17966).
To identify gene expression levels associated with VVs due to pleiotropy, SMR and HEIDI analyses were performed using SMR v0.71028. Summary statistics from the UK Biobank discovery GWAS were used, alongside eQTL data for GTEx V7 tibial artery (www.gtexportal.org/home/). The top-associated eQTL for each gene was used as an instrumental variable to examine association with VVs. A Bonferroni-corrected significance for all SMR probes was set at PSMR < 1.01 × 10−5 (0.05/4946 probes). Subsequently, a HEIDI test was conducted across all SMR-significant probes to examine for heterogeneity in SMR estimates (significance set at P < 0.05/ number of PSMR-significant probes).
Gene set, tissue-specific and pathway enrichment analysis
Gene-set analysis was implemented in MAGMA v1.0727 across 15,496 gene sets derived from MSigDB v8.078 with a significance threshold of P < 3.23 × 10−6 (0.05/15496) (Supplementary Data 15). Tissue-specific gene property analysis was also performed in MAGMA v1.07 to determine the expression of the protein-coding genes in VV-related tissue types in GTEx v8.026.
Pathway analysis was performed in eXploring Genomic Relations (XGR v1.1.3)29 (https://cran.r-project.org/web/packages/XGR/index.html). XGR ontology enrichment analysis was performed across all mapped genes, in “canonical pathways” with the following settings: hypergeometric distribution testing, any number of genes annotated, any overlap with input genes, and an adjusted FDR < 0.05.
SNP-based heritability, genetic correlation and PheWAS
The SNP-based heritability for the UK Biobank cohort was computed using BOLT-REML v2.3, a variance components method79, and LD score (LDSC) regression using LDSC v1.0.119. LDSC was also used to calculate the LDSC intercept, mean chi-squared test and attenuation score, and SNP-based heritability (h2g) in the 23andMe cohort80. This approach calculates heritability for a specific phenotype by regression of a SNP’s association statistic onto its LDSC80. LDSC regression was also used to calculate the genetic correlation between VVs and 176 preselected traits, across nine trait categories: metabolites, glycaemic traits, autoimmune diseases, anthropometric traits, smoking behaviour, lipids, cardiometabolic traits, reproductive traits and haematological traits, from publicly available GWAS data within LD Hub (https://ldsc.broadinstitute.org/)80. Traits were selected based on putative epidemiological associations with VVs from the literature. We confirmed associations between VVs and the correlated traits at the variant level by employing a PheWAS approach. Each of 49 replicated variants was searched in Open Targets Genetics31 (https://genetics.opentargets.org/, accessed 25/02/2022), and we collated all phenotypes with P-value < 0.005 in the databases available on the platform (UK Biobank, FinnGen, and GWAS Catalog). All phenotypes associated with the 49 variants are shown in Supplementary Data 10.
Polygenic risk score
The varicose veins polygenic risk score (VV-PRS) was derived in FinnGen (https://www.finngen.fi/en), a public-private partnership project combining genotype data from Finnish biobanks and digital health record data from Finnish health registries. Genotyping, phenotyping, and quality control of the FinnGen cohort has been reported previously81, and varicose veins phenotype definition is available at https://risteys.finngen.fi/phenocode/I9_VARICVE.
Summary statistics for varicose vein ICD-10 diagnosis (I9_VARICVE) in FinnGen were downloaded from https://console.cloud.google.com/storage/browser/finngen-public-data-r5/summary_stats/. There were 17,027 VVs cases and 190,028 controls. Summary statistics were lifted over from GRCh38 to GRCh37 using the “default_lift_data” function in the gnomAD package v0.4.0 for Hail v0.2.77 (https://hail.is/). Non-autosomal SNPs were removed and SNP-allele pairs were matched with the provided UK Biobank-derived LD reference comprising high-quality HapMap3 variants using the “snp_match” function in the bigsnpr v1.9.11 package for R. SNPs with an absolute MAF difference >0.1 between FinnGen and UKB were excluded (n = 36,656), leaving a total of 1,004,944 SNPs. VVs polygenic risk score was derived using LDpred282 (automatic model), following instructions in the package vignette. Model convergence was visually confirmed by inspection of the chain plot. The PLINK2 linear scoring function (–score) was used to calculate the per-sample score for unrelated European participants in UK Biobank (n = 381,544).
To validate the predictive utility of this score, we performed logistic regression of varicose vein case/control status (defined as above) against PRS decile, adjusted for year of birth, sex, genotyping array, recruitment centre, body mass index (at recruitment) and PC1-10.
For phenome-wide VV-PRS analysis, Phecode83-level time-to-event data was extracted from UKB phenotype data as described previously84, and varicose vein-specific phenotypes were removed. For each phenotype, Cox regression was performed for phenotype against Z-scored PRS adjusted for year of birth, sex, genotyping array, recruitment centre and PC1-10.
Bidirectional two-sample Mendelian randomisation analyses
Genetic instruments were extracted from summary statistics by thresholding at a significance level of 5 × 10−8 and clumping according to r2 < 0.01 using a 1000 Genomes European population reference. Varicose vein instruments were extracted from the summary statistics generated above, while other instruments were extracted from the IEU OpenGWAS project85 (https://gwas.mrcieu.ac.uk/). Exposure and outcome data were harmonised using the “harmonise_data” function in the TwoSampleMR v0.5.6 for R (https://mrcieu.github.io/TwoSampleMR/). The primary analysis performed was the inverse-variance weighted (IVW) method, assuming that all instrumental variables are valid. As sensitivity analyses, we additionally performed MR-Egger regression to identify available of significant directional pleiotropy. All analyses were implemented in the TwoSampleMR package for R.
Drug-target enrichment analysis
The prioritised genes at our replicated loci were queried on the Open Targets Platform32, assessing whether encoded proteins were tractable to small molecule or antibody targeting, or drug targets in any phase of clinical trial. Genes were also analysed for enriched drug pathways, with a nominal P < 0.05 threshold of significance.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Discovery GWAS summary statistics from UK Biobank have been deposited in the Oxford University Research Archive and are available for download at: https://doi.org/10.5287/bodleian:8J26woZQg. Full UK Biobank data can be accessed by direct application to UK Biobank. Genotype data for 23andMe research participants have not been deposited in public repositories, as consent for this was not obtained in the study protocol. Summary statistics can be accessed from 23andMe by qualified researchers who enter into agreement with 23andMe that protects subjects’ confidentiality. Investigators wishing to collaborate with 23andMe can email dataset-request@23andme.com or apply via the 23andMe research website (www.23andme.com/en-gb/research).
References
Evans, C. J., Fowkes, F. G. R., Ruckley, C. V. & Lee, A. J. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J. Epidemiol. Commun. Health 53, 149–153 (1999).
Gloviczki, P. et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 53, 2S–48S (2011).
Brand, F. N., Dannenberg, A. L., Abbott, R. D. & Kannel, W. B. The epidemiology of varicose veins: The Framingham Study. Am. J. Prev. Med. 4, 96–101 (1988).
Singer, A. J., Tassiopoulos, A. & Kirsner, R. S. Evaluation and management of lower-extremity ulcers. N. Engl. J. Med 377, 1559–1567 (2017).
Rice, J. B. et al. Burden of venous leg ulcers in the United States. J. Med. Econ 17, 347–356 (2014).
Stanley, J. C. et al. Vascular surgery in the United States: Workforce issues: report of the society for vascular surgery and the international society for cardiovascular surgery, North American Chapter, Committee on Workforce Issues. J. Vasc. Surg. 23, 172–181 (1996).
Marsden, G., Perry, M., Kelley, K. & Davies, A. H. Diagnosis and management of varicose veins in the legs: summary of NICE guidance. BMJ (Online) 347, f4279 (2013).
O’Donnell, T. F., Balk, E. M., Dermody, M., Tangney, E. & Iafrati, M. D. Recurrence of varicose veins after endovenous ablation of the great saphenous vein in randomized trials. J. Vasc. Surg. Venous Lymphat. Disord. 4, 97–105 (2016).
Raffetto, J. D. & Khalil, R. A. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology 23, 85–98 (2008).
Lim, C. S. & Davies, A. H. Pathogenesis of primary varicose veins. Br. J. Surg. 96, 1231–1242 (2009).
Lee, A. J., Evans, C. J., Allan, P. L., Ruckley, C. V. & Fowkes, F. G. R. Lifestyle factors and the risk of varicose veins: Edinburg Vein Study. J. Clin. Epidemiol. 56, 171–179 (2003).
Sisto, T. et al. Prevalence and risk factors of varicose veins in lower extremities: Mini-Finland health survey. Eur. J. Surgery Acta Chir. 161, 405–414 (1995).
Scott, T. E., LaMorte, W. W., Gorin, D. R. & Menzoian, J. O. Risk factors for chronic venous insufficiency: a dual case-control study. J. Vasc. Surg. 22, 622–628 (1995).
Zöller, B., Ji, J., Sundquist, J. & Sundquist, K. Family history and risk of hospital treatment for varicose veins in Sweden. Br. J. Surg. 99, 948–953 (2012).
Fiebig, A. et al. Heritability of chronic venous disease. Hum. Genet. 127, 669–674 (2010).
Ellinghaus, E. et al. Genome-wide association analysis for chronic venous disease identifies EFEMP1 and KCNH8 as susceptibility loci. Sci. Rep. 7, 45652 (2017).
Fukaya, E. et al. Clinical and genetic determinants of varicose veins. Circulation 1–12 https://doi.org/10.1161/CIRCULATIONAHA.118.035584 (2018).
Baylis, R. A., Smith, N. L., Klarin, D. & Fukaya, E. Epidemiology and genetics of venous thromboembolism and chronic venous disease. Circ. Res. 128, 1988–2002 (2021).
Bulik-Sullivan, B. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Watanabe, K., Taskesen, E., Van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Ng, P. C. & Henikoff, S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Lukacs, V. et al. Impaired PIEZO1 function in patients with a novel autosomal recessive congenital lymphatic dysplasia. Nat. Commun. 6, 1–7 (2015).
Rentzsch, P., Witten, D., Cooper, G. M., Shendure, J. & Kircher, M. CADD: predicting the deleteriousness of variants throughout the human genome. Nucleic Acids Res. 47, D886–D894 (2018).
Boyle, A. P. et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 22, 1790–1797 (2012).
Weissbrod, O. et al. Functionally informed fine-mapping and polygenic localization of complex trait heritability. Nat. Genet. 52, 1355–1363 (2020).
Aguet, F. et al. Genetic effects on gene expression across human tissues. Nature 550, 204–213 (2017).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
Zhu, Z. et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 48, 481–487 (2016).
Fang, H., Knezevic, B., Burnham, K. L. & Knight, J. C. XGR software for enhanced interpretation of genomic summary data, illustrated by application to immunological traits. Genome Med. 8, 1–20 (2016).
Morris, D. L. et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat. Genet. 48, 940–946 (2016).
Ghoussaini, M. et al. Open targets genetics: Systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 49, D1311–D1320 (2021).
Carvalho-Silva, D. et al. Open targets platform: new developments and updates two years on. Nucleic Acids Res. 47, D1056–D1065 (2019).
Oklu, R. et al. Pathogenesis of varicose veins. J. Vasc. Interv. Radiol. 23, 33–39 (2012).
Segiet, O. A. et al. Biomolecular mechanisms in varicose veins development. Ann. Vasc. Surg. 29, 377–384 (2015).
Gandhi, R. H. et al. Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J. Vasc. Surg. 18, 814–820 (1993).
Pace, J. M., Corrado, M., Missero, C. & Byers, P. H. Identification, characterization and expression analysis of a new fibrillar collagen gene, COL27A1. Matrix Biol. 22, 3–14 (2003).
Markovic, J. N. & Shortell, C. K. Genomics of varicose veins and chronic venous insufficiency. Semin. Vasc. Surg. 26, 2–13 (2013).
Pietzner, M. et al. Mapping the proteo-genomic convergence of human diseases. Science (80-.) 374, 1–20 (2021).
Livingstone, I., Uversky, V. N., Furniss, D. & Wiberg, A. The pathophysiological significance of fibulin-3. Biomolecules 10, 1–24 (2020).
Jorgenson, E. et al. A genome-wide association study identifies four novel susceptibility loci underlying inguinal hernia. Nat. Commun. 6, 10130 (2015).
Albig, A. R., Neil, J. R. & Schiemann, W. P. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 66, 2621–2629 (2006).
Parra, J. R. et al. Tissue inhibitor of metalloproteinase-1 is increased in the saphenofemoral junction of patients with varices in the leg. J. Vasc. Surg. 28, 669–675 (1998).
Gao, L.-B., Tian, S., Gao, H.-H. & Xu, Y.-Y. Metformin inhibits glioma cell U251 invasion by downregulation of fibulin-3. Neuroreport 24, 504–508 (2013).
Engelhardt, K. R. et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J. Allergy Clin. Immunol. 124, 1289–302.e4 (2009).
Okada, Y. et al. A genome-wide association study identified AFF1 as a susceptibility locus for systemic lupus eyrthematosus in Japanese. PLoS Genet. 8, e1002455 (2012).
Lewis, R. S. Calcium signaling mechanisms in T lymphocytes. Annu. Rev. Immunol. 19, 497–521 (2001).
Sayer, G. L. & Smith, P. D. C. Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur. J. Vasc. Endovasc. Surg. 28, 479–483 (2004).
Chang, M. Y. et al. Apoptosis and angiogenesis in varicose veins using gene expression profiling. Fooyin J. Heal. Sci. 1, 85–91 (2009).
Ferrara, N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 77, 527–543 (1999).
Kowalewski, R., Małkowski, A., Sobolewski, K. & Gacko, M. Vascular endothelial growth factor and its receptors in the varicose vein wall Czynnik wzrostu śródbłonka naczyniowego i jego receptory w ścianie żylaków kończyn. Acta Angiol. 17, 141–149 (2011).
Howlader, M. H. & Coleridge Smith, P. D. Relationship of plasma vascular endothelial growth factor to CEAP clinical stage and symptoms in patients with chronic venous disease. Eur. J. Vasc. Endovasc. Surg. 27, 89–93 (2004).
Hollingsworth, S. J., Powell, G. L., Barker, S. G. E. & Cooper, D. G. Primary varicose veins: Altered transcription of VEGF and its receptors (KDR, flt-1, soluble flt-1) with sapheno-femoral junction incompetence. Eur. J. Vasc. Endovasc. Surg. 27, 259–268 (2004).
Kim, I. et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-κB activation in endothelial cells. J. Biol. Chem. 276, 7614–7620 (2001).
Harvey, N. L. et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 37, 1072–1081 (2005).
Wigle, J. T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Bazigou, E. et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J. Clin. Invest. 121, 2984–2992 (2011).
Sathish Srinivasan, R. & Oliver, G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 25, 2187–2197 (2011).
Sabine, A. et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 22, 430–445 (2012).
Fang, J. et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 67, 1382–1388 (2000).
Mellor, R. H. et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation 1912–1921 https://doi.org/10.1161/CIRCULATIONAHA.106.675348 (2007).
Gohel, M. S. et al. A randomized trial of early endovenous ablation in venous ulceration. N. Engl. J. Med. 378, 2105–2114 (2018).
Allen, N. E., Sudlow, C., Peakman, T. & Collins, R. UK biobank data: come and get it. Sci. Transl. Med. 6, 224ed4 (2014).
Bycroft, C. et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209 (2018).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007).
Wiberg, A. et al. A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat. Commun. 10, 1030 (2019).
Loh, P. R. et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat. Genet. 47, 284–290 (2015).
Durand, E. Y., Do, C. B., Mountain, J. L. & Macpherson, J. M. Ancestry composition: a novel, efficient pipeline for ancestry deconvolution. Preprint at bioRxivhttps://doi.org/10.1101/010512 (2014).
O’Connell, J. et al. Haplotype estimation for biobank-scale data sets. Nat. Genet. 48, 817–820 (2016).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Walter, K. et al. The UK10K project identifies rare variants in health and disease. Nature 526, 82–89 (2015).
Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
Loh, P. R., Palamara, P. F. & Price, A. L. Fast and accurate long-range phasing in a UK Biobank cohort. Nat. Genet. 48, 811–816 (2016).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. 48, 1284–1287 (2016).
Mägi, R. & Morris, A. P. GWAMA: Software for genome-wide association meta-analysis. BMC Bioinformatics 11, 288 (2010).
Ernst, J. & Kellis, M. Chromatin-state discovery and genome annotation with ChromHMM. Nat. Protoc. 12, 2478–2492 (2017).
Schilder, B., Humphrey, J. & Raj, T. echolocatoR: an automated end-to-end statistical and functional genomic fine-mapping pipeline. Bioinformatics 38, 536–539 (2021).
Liberzon, A. et al. The molecular signatures database hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Loh, P. R. et al. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat. Genet. 47, 1385–1392 (2015).
Zheng, J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017).
Pirastu, N. et al. Genetic analyses identify widespread sex-differential participation bias. Nat. Genet. 53, 663–671 (2021).
Privé, F., Arbel, J. & Vilhjálmsson, B. J. LDpred2: better, faster, stronger. Bioinformatics 36, 5424–5431 (2020).
Wu, P. et al. Mapping ICD-10 and ICD-10-CM codes to phecodes: workflow development and initial evaluation. JMIR Med. Informatics 7, 1–13 (2019).
Kleeman, S. O. et al. Cystatin C is a glucocorticoid response gene predictive of cancer immunotherapy failure. Preprint at medRxivhttps://doi.org/10.1101/2021.08.17.21261668 (2021).
Elsworth, B. et al. The MRC IEU OpenGWAS data infrastructure. Preprint at bioRxivhttps://doi.org/10.1101/2020.08.10.244293 (2020).
Acknowledgements
The data analysed in the present study was in part provided by the UK Biobank (www.ukbiobank.ac.uk), received under UK Biobank application no. 22572. The replication cohort was provided by 23andMe, Inc. (California). W.A. is supported by the Aziz Foundation, Wolfson Foundation, Royal College Surgeons of England and Oxford NIHR Biomedical Research Centre (Musculoskeletal theme). W.A. was previously supported by grants from the British Association of Plastic and Reconstructive Surgeons (BAPRAS) and Royal College Surgeons of Edinburgh. R.L. is funded by the UK Research and Innovation Future Leaders Fellowship. M.N. and D.F. are supported by the Oxford NIHR Biomedical Research Centre (BMC). A.W. is an NIHR Academic Clinical Lecturer and was supported by an MRC Clinical Research Training Fellowship (MR/N001524/1). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results. This represents independent research funded by the NIHR. The view expressed are the authors’ own and are not necessarily those of the NIHR, NHS or Department of Health and Social Care.
Author information
Authors and Affiliations
Consortia
Contributions
D.F, A.W. and K.Z. contributed to the conception, study design, and supervision of this work. W.A., S.K., A.W. and M.N. contributed to data analysis. W.W., A.A. and the 23andMe Research Team contributed to the replication study and analysis. R.L. and A.H. contributed to case ascertainment. W.A., A.W., D.F. prepared the first draft of the manuscript. All co-authors made substantial contributions to data acquisition, data interpretation, and revised the work critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
W.W., A.A. and members of the 23andMe Research Team disclose a relationship with 23andMe, Inc. as employees, and hold stock or stock options in 23andMe, Inc. All other authors have no competing interests to disclose.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, WUR., Kleeman, S., Ng, M. et al. Genome-wide association analysis and replication in 810,625 individuals with varicose veins. Nat Commun 13, 3065 (2022). https://doi.org/10.1038/s41467-022-30765-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-30765-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.