Abstract
As a promising hydrogen carrier, formic acid (HCOOH) is renewable, safe and nontoxic. Although noble-metal-based catalysts have exhibited excellent activity in HCOOH dehydrogenation, developing non-noble-metal heterogeneous catalysts with high efficiency remains a great challenge. Here, we modulate oxygen coverage on the surface of Ti3C2Tx MXenes to boost the catalytic activity toward HCOOH dehydrogenation. Impressively, Ti3C2Tx MXenes after treating with air at 250 °C (Ti3C2Tx-250) significantly increase the amount of surface oxygen atoms without the change of crystalline structure, exhibiting a mass activity of 365 mmol·g−1·h−1 with 100% of selectivity for H2 at 80 °C, which is 2.2 and 2.0 times that of commercial Pd/C and Pt/C, respectively. Further mechanistic studies demonstrate that HCOO* is the intermediate in HCOOH dehydrogenation over Ti3C2Tx MXenes with different coverages of surface oxygen atoms. Increasing the oxygen coverage on the surface of Ti3C2Tx MXenes not only promotes the conversion from HCOO* to CO2* by lowering the energy barrier, but also weakens the adsorption energy of CO2 and H2, thus accelerating the dehydrogenation of HCOOH.
Similar content being viewed by others
Introduction
As an appealing hydrogen carrier in liquid state, formic acid (HCOOH) has attracted tremendous attention due to its great potential for applications in fuel cells and chemical engineering1,2,3. Noble-metal-based catalysts are widely used in the dehydrogenation of HCOOH, exhibiting remarkable activity and selectivity4,5,6,7,8,9,10,11,12,13. For instance, the turnover frequency (TOF) reached 2882 h−1 in pure HCOOH at 50 °C over an Au catalyst modified with Schiff base7. The well-dispersed NiPd nanocrystals supported on nitrogen-functionalized graphene displayed superior activity with the TOF of 954.3 h−1 for HCOOH decomposition at room temperature8. Very recently, our group developed Pt1/Te single-atom catalysts, which delivered the TOF of 1206 h−1 with 100% selectivity for H2 at 25 °C via a plasmon-enhanced catalytic process14. In spite of these advances, the expensive cost of noble-metal-based catalysts impels people to exploit non-noble-metal-based catalysts for HCOOH decomposition, which has achieved exciting advances recently, especially for homogeneous catalysts15,16,17,18,19,20,21,22,23,24. For example, Beller et al.17 reported that a Fe catalyst with an extra equivalent of tetradentate auxiliary ligand decomposed HCOOH at 80 °C with the TOF of 9425 h−1. In the presence of pincer ligands, Fe catalysts exhibited ~1,000,000 turnovers assisted by Lewis acid16. As for recyclable heterogeneous catalysts, the activity of non-noble-metal-based nanocrystals for HCOOH dehydrogenation was far below than that of their homogeneous counterparts. Therefore, developing non-noble-metal heterogeneous catalysts with high efficiency of HCOOH dehydrogenation is urgently desired but remains a great challenge.
Incorporation of nonmetallic elements, such as C, N, O, and S, into non-noble-metal heterogeneous catalysts has emerged as an effective approach to improve their catalytic performance from both theoretical and experimental perspectives14,25,26,27,28,29,30,31. The incorporation of nonmetallic elements regulates the electronic structure of catalysts. For example, the introducing of N atoms effectively manipulated the surface electron densities of NiCo2S4, leading to an outstanding activity comparable to platinum on carbon (Pt/C) in hydrogen evolution30. Besides, defective structures generate accompanied with the involvement of nonmetallic elements in nanocrystals. A notable example is the fabrication of Ti3+ species and oxygen vacancies in TiO2 nanocrystals by doping N atoms25,26,27. Moreover, the reaction paths are even altered after the introduction of nonmetallic elements. For instance, N atoms in Co-based nanosheets directly interacted with CO2 and H2 to form HCOO* intermediates in CO2 hydrogenation28. Hence, modification of non-noble-metal heterogeneous catalysts with nonmetallic atoms serves as a potential route to construct efficient catalysts in HCOOH dehydrogenation.
Herein we develop a highly active non-noble-metal heterogeneous catalyst for HCOOH dehydrogenation, prepared by modulating the oxygen coverage on the surface of Ti3C2Tx MXenes. Ti3C2Tx MXenes after treating with air at 250 °C (Ti3C2Tx-250) significantly increase the amount of surface oxygen atoms without the change of crystalline structure, delivering 94% of conversion for HCOOH with 100% of selectivity for H2 at 80 °C. In addition, the mass activity of Ti3C2Tx-250 is 365 mmol g−1 h−1, 2.2 and 2.0 times that of commercial palladium on carbon (Pd/C) and Pt/C, respectively. Further mechanistic studies demonstrate that HCOO* is the intermediate in HCOOH dehydrogenation over Ti3C2Tx MXenes with different coverages of surface oxygen atoms. Increasing the oxygen coverage on the surface of Ti3C2Tx MXenes not only promotes the conversion from HCOO* to CO2* by lowering the energy barrier but also weakens the adsorption energy of CO2 and H2, thus accelerating the dehydrogenation of HCOOH.
Results
Synthesis and structural characterizations
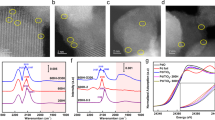
To begin with, Ti3C2Tx MXenes (Tx represents the adsorbed species on the surface) were synthesized by immersing commercial Ti3AlC2 powders in hydrofluoric acid (HF) for 72 h at room temperature, followed by ultrasonic treatment (Supplementary Fig. 1). As shown by the X-ray diffraction (XRD) patterns in Supplementary Fig. 2, the diffraction peaks for Ti3AlC2 disappeared after etching, with the generation of the peaks for Ti3C2Tx MXenes (denoted as Ti3C2Tx-25 for simplification). To modulate the coverage of oxygen atoms on the surface, we treated Ti3C2Tx-25 in a muffle furnace at 150, 250, and 350 °C for 1 h to acquire Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350, respectively. As shown in the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images (Fig. 1a and Supplementary Fig. 3), Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350 exhibited a nanosheet morphology which resembled that of Ti3C2Tx-25. Further observation in the magnified HAADF-STEM image of Ti3C2Tx-250 displayed that the lattice spacing was 2.6 Å, corresponding to the (0\(\overline1\)10) crystal plane of Ti3C2Tx MXenes (Fig. 1b). STEM energy-dispersive X-ray (EDX) elemental mapping images of an individual Ti3C2Tx-250 nanosheet indicated the homogeneous distribution of Ti, O, C, F, and Al elements (Fig. 1c). Inductively coupled plasma atomic emission spectroscopy (ICP-AES) result revealed that the mass ratio of Al in Ti3C2Tx-250 was about 2.0%. In addition, the crystalline structure of Ti3C2Tx-150 and Ti3C2Tx-250 was quite similar to that of Ti3C2Tx-25 based on XRD analysis (Supplementary Fig. 2). As a comparison, the XRD pattern of Ti3C2Tx-350 was indexed to a mixture of Ti3C2Tx MXene phase and tetragonal TiO2 phase (PDF#65-5714). Besides, the Brunauer–Emmett–Teller (BET) surface areas of Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350 were determined to be 16, 20, 21, and 44 m2 g−1, respectively (Supplementary Table 1). To determine the electronic and local structures of chemical nature of these samples, the X-ray absorption near-edge structure (XANES) spectrum and the extended X-ray absorption fine structure (EXAFS) spectrum were recorded. As shown in Ti K-edge XANES profiles (Fig. 1d), the absorption edge position of Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-250 were between those of Ti foil and TiO2. As a result, Ti species in Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-250 were partially oxidized. As for Ti3C2Tx-350, the edge position was similar to that of TiO2, proving that TiO2 phase made up a dominated part in Ti3C2Tx-350, consistent with the XRD results. This result was further confirmed by wavelet transform (WT) analysis (Fig.1e, f and Supplementary Fig. 4). Figure 1g presented Fourier-transformed EXAFS spectra in R space. The Ti-C coordination and Ti-O coordination were difficult to distinguish apart in EXAFS measurement due to the similar electron scattering abilities of O and C atoms. Thus we calculated Ti-C coordination plus Ti-O coordination (denoted it as Ti-C/O). As shown in Supplementary Table 2, the coordination numbers (CNs) for Ti-C/O shell in Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-250 were determined to be 4.5, 4.7, and 4.9, respectively. As for Ti3C2Tx-350, the CN for Ti-C/O shell was determined to be 2.9. Furthermore, we also performed Al K-edge XANES spectra of Ti3C2Tx-250 (Supplementary Fig. 5). Obviously, Al species in Ti3C2Tx-250 exhibited the characteristic of oxidation state.
a HAADF-STEM and b magnified HAADF-STEM images of Ti3C2Tx-250. c STEM-EDX elemental mapping of Ti3C2Tx-250. d Ti K-edge XANES spectra for Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350. The wavelet transform analysis in Ti K-edge for e Ti3C2Tx-250 and f Ti3C2Tx-350. g EXAFS spectra in R space for Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350. Ti foil, TiO2, and TiC were used as references.
We further explored the electronic properties of the catalysts by X-ray photoelectron spectroscopy (XPS) and the diffuse reflectance ultraviolet visible (UV-Vis) spectra. As shown in the O 1s spectra (Fig. 2a), four peaks centered at 533.7, 532.2, 531.7, and 529.7 eV were ascribed to the surface-adsorbed oxygen species, surface oxygen atoms bonded to titanium atoms (denoted as surface O-Ti species), surface OH groups, and lattice oxygen in TiO2, respectively32. The presence of surface OH groups was further confirmed by diffuse reflectance infrared Fourier transform (DRIFT) measurements (Supplementary Fig. 6). Surface-adsorbed oxygen species was considered as the physical adsorption. Thus surface hydroxyl groups and surface O-Ti species were regarded as the covered oxygen on the surface. Apparently, the O 1s spectrum of Ti3C2Tx-150 was quite similar to that of Ti3C2Tx-25, except for a slight increase in the area of the peak for surface O-Ti species. As for Ti3C2Tx-250, the peak intensity of surface O-Ti species was significantly strengthened. Besides, a dominated peak for TiO2 appeared for Ti3C2Tx-350. In addition, we also estimated the relative proportion of O-Ti species of Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-250 according to O 1s XPS spectra. As shown in Supplementary Table 3, after peak deconvolution, the percentages of O-Ti species were estimated to be 3.4, 7.3, and 26.5% for Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-250, respectively. Furthermore, Ti 2p XPS spectra were also carried out. As shown in Fig. 2b, the Ti 2p spectra of these samples were fitted with six main constituent peaks, corresponding to Ti-C at 461.1 and 455.3 eV, Ti-O at 464.4 and 458.8 eV, and Ti-F at 461.9 eV and 457.8 eV, respectively33,34,35,36,37. As for Ti3C2Tx-250, the peaks for Ti-O were significantly strengthened compared with those for Ti3C2Tx-25 and Ti3C2Tx-150. It is worth noting that the peaks for Ti-F decreased with the elevation of treatment temperatures for Ti3C2Tx MXenes. With regard to Ti3C2Tx-350, the peaks for Ti-O became the dominated ones with the neglectable peaks for Ti-C and Ti-F. As a result, the oxygen coverage on the surface of Ti3C2Tx MXenes could be facilely modulated by thermal treatment below 250 °C without the variation of crystalline structure.
Catalytic performance of different catalysts
The catalytic properties of the as-obtained Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350 were evaluated in comparison with commercial Pt/C and Pd/C toward dehydrogenation of HCOOH (Fig. 3a). Each reaction was performed in a home-made catalytic system containing 2.6 mmol of HCOOH and 10 mL of deionized water at 80 °C. A blank test was conducted without any catalyst, where almost no gas released. When the reaction was then catalyzed by Ti3C2Tx-25, Ti3C2Tx-150, and Ti3C2Tx-350, 16, 33, and 23 mL of gas generated after 40 min, respectively. Under the same reaction condition, the volume of the gas reached 120 mL over Ti3C2Tx-250. As a reference, commercial Pt/C (5% mass loading) and Pd/C (5% mass loading) were tested, where 60 and 65 mL of gas yielded after 40 min, respectively. Thus Ti3C2Tx-250 exhibited a significantly enhanced catalytic activity compared to Pt/C and Pd/C catalysts. Typically, HCOOH decomposition undergoes two distinct reaction pathways:
and
a Plots of the volume of gas formed from HCOOH decomposition over different catalysts versus time at 80 °C. b Comparison of mass activities for different catalysts. c Arrhenius plot for the dehydrogenation of HCOOH over different catalysts. d Catalytic performance of Ti3C2Tx-250 after successive rounds of reaction. The error bar was drawn based on the calculated standard error of three parallel tests.
To investigate the selectivity of the catalysts, the generated gases were analyzed by chromatograph (gas chromatography) tests. Only H2 and CO2 were detected without the signal of CO (Supplementary Fig. 7). According to Eq. (1), the conversion of HCOOH over Ti3C2Tx-250 reached 94% within 40 min. Figure 3b illustrates the mass activities for each catalyst within the initial 10 min. The mass activity of Ti3C2Tx-250 was determined to be 365 mmol g−1 h−1, 7.3, 3.5, 5.7, 2.2, and 2.0 times than that of Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-350, Pd/C, and Pt/C, respectively. Besides, the turnover frequencies of Ti3C2Tx-250, Pd/C, and Pt/C were calculated to be 960, 2679, and 4585 h−1, respectively (Supplementary Table 5). Representative works on the dehydrogenation of HCOOH by homogeneous and heterogeneous non-noble-metal catalysts over past few years are listed in Supplementary Table 415,16,17,20,22,24,38,39,40,41,42,43,44. Ti3C2Tx-250 was among the best in heterogeneous catalysts. Considering the remaining F− adsorbed on Ti3C2Tx MXenes, we conducted HCOOH dehydrogenation over Ti3C2Tx-250 in NaF aqueous solution with different concentrations to investigate the influence of fluoride species. Despite the varied concentrations of F−, a similar mass activity of ~340 mmol g−1 h−1 was obtained (Supplementary Fig. 8). To further investigate the effect of the residual Al species on HCOOH dehydrogenation, Ti3C2Tx-250-20h and Ti3C2Tx-250-96h were prepared with the similar method except for varying the etching time from 72 h to 20 and 96 h, respectively. ICP-AES result revealed that the mass ratios of Al element in Ti3C2Tx-250-20h and Ti3C2Tx-250-96h were about 3.6 and 1.2%, respectively. When using Ti3C2Tx-250-20h and Ti3C2Tx-250-96h as the catalysts in HCOOH dehydrogenation, the mass activities were similar to that of Ti3C2Tx-250 with the values of 345 and 356 mmol g−1 h−1 under the same reaction conditions, respectively (Supplementary Fig. 9). Thus the influence of fluoride and the residual Al species on HCOOH dehydrogenation over Ti3C2Tx-250 was negligible. Oxygen coverage on the surface of Ti3C2Tx MXenes was regarded as the predominant factor for varied catalytic activity. This point was further confirmed by the relationship between the percentage of O-Ti species and the activity of HCOOH dehydrogenation. As shown in Supplementary Fig. 10a, linear correlation was obtained between the percentage of O-Ti species and the activity of HCOOH dehydrogenation. In addition, the mass activities of Ti3C2Tx-250 at different temperatures were further measured to construct the Arrhenius plot (Fig. 3c). The activation energy for the decomposition of HCOOH over Ti3C2Tx-250 were calculated to be 31.4 kJ mol−1, which was lower than Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-350, Pt/C, and Pd/C (Supplementary Table 6)3,4,6,7,10. Moreover, the stability of Ti3C2Tx-250 was also studied by performing successive rounds of reaction. After four rounds, almost 90% of the original activity was preserved, indicating the high stability of Ti3C2Tx-250 (Fig. 3d). This high stability of Ti3C2Tx-250 is of crucial importance for potential applications in industrial processes. In addition, we also performed HAADF-STEM, EDX mapping, BET surface areas, and DRIFT measurement of Ti3C2Tx-250 after catalytic tests (Ti3C2Tx-250-used). As shown in Supplementary Fig. 11, the nanosheet morphology of Ti3C2Tx-250-used was well maintained. Besides, the BET surface areas of Ti3C2Tx-250 before and after catalytic tests were 21 and 22 m2/g, respectively. In addition, the DRIFT spectrum of Ti3C2Tx-250-used still exhibited an obvious absorption peak assigning to the ν(O–H) stretching mode. These results further proved the high stability of Ti3C2Tx-250 during catalytic process. Besides, Ti3C2Tx-25 was able to be prepared in large-scale, where 6.3305 g of Ti3C2Tx-25 was successfully acquired in one pot (Supplementary Fig. 12). Those results provided the possibility for industrial application of Ti3C2Tx MXenes in HCOOH dehydrogenation.
Furthermore, we also investigated the catalytic performance over Ti2CTx MXenes. Ti2CTx MXenes were prepared similar to the procedure for Ti3C2Tx MXenes, expect for the use of commercial Ti2AlC powder as the precursor (Supplementary Fig. 13). Ti2CTx-25, Ti2CTx-150, Ti2CTx-250, and Ti2CTx-350 were obtained by thermal treatment at 25, 150, 250, and 350 °C, respectively. Supplementary Fig. 14a showed their catalytic activities in HCOOH dehydrogenation. When the reaction was catalyzed by Ti2CTx-25, Ti2CTx-150, Ti2CTx-250, and Ti2CTx-350, 15, 33, 80, and 42 mL of gas generated after 30 min, respectively. Supplementary Fig. 14b illustrated their mass activities within the initial 10 min. The mass activity of Ti2CTx-250 was determined to be 234 mmol g−1 h−1, 6.3, 2.3, and 1.8 times as high as that of Ti2CTx-25, Ti2CTx-150, and Ti2CTx-350, respectively. The results indicated that the catalytic activity of Ti2CTx MXenes in HCOOH dehydrogenation was able to be modulated by varying oxygen coverage on the surface of Ti2CTx MXenes. In addition, Ti2CTx-250 exhibited a comparable catalytic activity with that of Ti3C2Tx-250 in HCOOH dehydrogenation.
Investigation of the mechanism for HCOOH dehydrogenation
To investigate the reaction mechanism in HCOOH dehydrogenation, we investigated the kinetic isotope effect (KIE) of dehydrogenation of HCOOH over Ti3C2Tx-250. As for HCOOH, the hydrogen in the carboxyl group is able to be exchanged with deuterium oxide at room temperature, while the hydrogen in the methyl group is nearly unable to be exchanged with deuterium oxide under the reaction conditions45,46,47. Thus, to investigate the KIE, we performed the dehydrogenation of HCOOH over Ti3C2Tx-250 by employing different substrates, including HCOOH, formic-d acid (DCOOH), formic acid-d (HCOOD), and formic acid-d2 (DCOOD, and solvents, including H2O and D2O. The experiment by using specific HCOOH and water as the substrate and solvent was abbreviated as “formic acid+water.” In this case, we implemented the following experiments: HCOOH + H2O, DCOOH + H2O, HCOOD + D2O, and DCOOD + D2O. As the rate of HCOOH dehydrogenation is generally constant at the beginning of the reaction, the initial rate is used in KIE studies for HCOOH dehydrogenation5. In this work, the initial rate was named as k(formic acid+water) by using specific HCOOH and water as the substrate and solvent, respectively. The reaction profiles versus reaction time are shown in Fig. 4a, where gases were all gradually generated at different reaction conditions. The values of k(HCOOH+H2O)/k(DCOOH+H2O), k(HCOOD+D2O)/k(DCOOD+D2O), k(HCOOH+H2O)/k(HCOOD+D2O), k(DCOOH+H2O)/k(DCOOD+D2O), and k(HCOOH+H2O)/k(DCOOD+D2O) were calculated to be 2.64, 3.61, 1.38, 1.41, and 4.41, respectively (Fig. 4b). Generally speaking, a first-order KIE is obtained when the value of kH/kD was greater than ~1.548,49. Thus the first-order KIE was attributed to the dissociation of H/DCOO* to form COO* over Ti3C2Tx-250, which was regarded as the rate-determining step. Besides, the released gas was also analyzed by mass spectroscopy. As shown in Fig. 4c, d, H2 and D2 were detected as the sole product in the cases of HCOOH + H2O and DCOOD + D2O, respectively. As for the cases of DCOOH + H2O and HCOOD + D2O, HD was detected as the dominated product, as well as a small amount of H2 and D2, respectively (Fig. 4e, f). Accordingly, surface hydride and hydrogen atom from HCOO* combined to get H2. A nearly mono-molecular concerted mechanism rather than β-hydride elimination mechanism was regarded to have occurred over Ti3C2Tx-250 toward the dehydrogenation of HCOOH.
To further investigate the reaction intermediates in HCOOH dehydrogenation, we performed in situ DRIFT measurements. After treatment of the catalysts with a flowing N2 (1 bar) for 20 min at 25 °C, the background spectrum was acquired. Then 1 bar of N2 was allowed to bubble in HCOOH solution and flowed into the cell for 20 min at 25 °C, followed by in situ DRIFT measurements, which was regarded as the in situ DRIFT spectrum at 0 min. Then the cell was sealed and heated to 80 °C within ca. 20 s. In situ DRIFT spectra were then recorded at interval at 80 °C. As for Ti3C2Tx-250 shown in Fig. 5a, three sets of frequencies were observed once HCOOH was introduced. The set of frequencies at 2945, 1790, 1208, and 1100 cm−1 were assigned to the stretching vibration of C-H, the stretching vibration of C = O, the coupled stretching vibration of C-O with the deformation vibration of O-H, and the stretching vibration of C-OH in HCOOH* species, respectively50. The set of typical frequencies at 2882, 1555, and 1455 cm−1 corresponded to the stretching vibration of C-H, the asymmetrical stretching vibration of COO, and the symmetrical stretching vibration of COO in HCOO* species, respectively51,52,53. After reaction for 5 min at 80 °C, the peaks for HCOOH* species and HCOO* species significantly attenuated (Fig. 5a). More importantly, the peaks for physisorbed CO2* species at 2334 and 2364 cm−1 strengthened tremendously. Further prolonging the reaction time to 10 and 20 min, the peaks for HCOOH* species and HCOO* species vanished. Meanwhile, the peaks for physisorbed CO2* species became the dominated ones. In comparison, as for Ti3C2Tx-25, the peaks for HCOOH* species slightly weakened while the peaks for HCOO* species remained almost unchanged (Fig. 5b). In addition, no obvious enhancement in peaks for physisorbed CO2* species was observed for Ti3C2Tx-25 (Fig. 5b). Thus, HCOO* species was regarded as reaction intermediate in HCOOH dehydrogenation. Moreover, it was assumed that HCOOH* could be consumed swiftly while the conversion of HCOO* to CO2* was blocked. Therefore, Ti3C2Tx-250 performed with high efficiency in the transformation from HCOO* to CO2*, gaining the highest activity in dehydrogenation of HCOOH. By contrast, catalytic reactions over Ti3C2Tx-25 were obstructed by HCOO*-to-CO2* conversion. As for the role of OH groups in catalytic reaction, XPS spectra and DRIFT spectra proved the presence of OH groups on the surface of Ti3C2Tx MXenes (Fig. 2a and Supplementary Fig. 6). More importantly, the H atom of HCOOH* was able to dissociate to form HCOO* and OH* on the surface of Ti3C2Tx MXenes. Thus the absorption peak ranging from 3000 to 3700 cm−1 was obviously observed from in situ DRIFT measurements for both Ti3C2Tx-25 and Ti3C2Tx-250 (Fig. 5a, b). As for Ti3C2Tx-25, the peak of OH* species slightly weakened while the peaks of HCOO* species remained almost unchanged from 0 to 20 min at 80 °C. With regard to Ti3C2Tx-250, the peak of OH* species vanished from 0 to 20 min at 80 °C with the increasing of the peaks of CO2* species, because surface OH* and hydrogen atom from HCOO* combined to get H2 and CO2. Thus the surface OH* groups were nearly unable to promote the dehydrogenation of HCOOH.
To gain atomic-level insight into the origin of remarkable activity for Ti3C2Tx-250, density functional theory (DFT) calculations were carried out. Based on the experimental characterization results, three models were constructed to mimic different oxygen coverages, i.e., Ti3C2 without surface oxygen atoms, Ti3C2O2-V with oxygen vacancies on the surface, and Ti3C2O2 with saturated oxygen coverage (Supplementary Fig. 15), respectively. We screened possible intermediates and optimal paths for HCOOH decomposition over three models. HCOOH adsorbed on Ti3C2 with an adsorption energy of −3.05 eV (Supplementary Fig. 16), followed by spontaneous dissociation to generate HCOO* and H* with the energy barrier (Ea) of only 0.25 eV. Nevertheless, the dissociation of the H atom from HCOO* was impeded thermodynamically with the Ea of 1.65 eV. With regard to Ti3C2O2-V, the dissociation of HCOO* species was also inhibited with the Ea of 2.50 eV when the oxygen vacancy served as the active site (Supplementary Fig. 17). As for Ti3C2O2, HCOOH exhibited a bidentate adsorption configuration with an adsorption energy of −0.35 eV, followed by the dissociation of H atom to form -OH group (Fig. 5c). Afterwards, HCOO* adjusted the adsorption configuration to form meta-stable state with an increased energy of 0.68 eV. The Ea for the dissociation of HCOO* over Ti3C2O2 through meta-stable state was only 0.61 eV, much lower than that over Ti3C2 and Ti3C2O2-V. Due to the presence of the same active sites, HCOOH was speculatively decomposed to CO2 and H2 over Ti3C2O2-V similar to the channel over Ti3C2O2 when oxygen vacancy was not selected as the reactive site. Besides, we also considered the β-hydride elimination mechanism by calculating the formation of H2 from two surface H atoms bonded to the surface of Ti3C2O2. The result indicated that this process was highly impeded thermodynamically with a reaction energy of 1.10 eV. Based on the above analysis, HCOO* was the intermediate in HCOOH dehydrogenation over all three models with different coverages of surface oxygen atoms, consistent with the results of in situ DRIFT. Moreover, the energy barrier was lowered with the increasing of coverages of surface oxygen atoms. In addition, the oxygen atoms on the surface of Ti3C2O2 also benefited to the desorption of CO2 and H2 with the adsorption energies of −0.16 and −0.06 eV, respectively (Supplementary Table 7). By contrast, the adsorption energies for CO2 and H2 on Ti3C2 were up to −2.93 and −0.51 eV, respectively (Supplementary Table 7). As a result, the surface oxygen atoms on Ti3C2Tx MXenes facilely regulate the reaction energy barriers as well as the adsorption energies of intermediate species, contributing to the enhanced catalytic activity toward HCOOH dehydrogenation. Based on the above analysis, the surface Ti species were regarded as the active sites. Surface oxygen coverage on the surface of Ti3C2Tx MXenes regulated the catalytic properties of surface Ti species. Therefore, the possible active species in this case were regarded as surface [O-Ti-C] species.
Besides, considering the presence of other species on the surface of Ti3C2Tx-250, such as OH, F, and Al, we also investigated their possible effect on HCOOH dehydrogenation from theoretical perspectives. To investigate the effect of surface OH groups, we put a single H atom to pre-adsorb on Ti3C2O2 surface (Ti3C2O2-H1) as a model (Supplementary Fig. 18a). As shown in Supplementary Fig. 18b, Ti3C2O2-H1 exhibited a stronger adsorption for HCOOH with an adsorption energy of −0.53 eV than that of Ti3C2O2 with the adsorption energy of −0.35 eV. In addition, we also simulated the process of H2 formation on Ti3C2O2-H1, where the activation energy was computed to be 0.57 eV, very close to that over Ti3C2O2 with the value of 0.61 eV (Supplementary Fig. 18c). Thus the surface OH groups on Ti3C2O2 contributed little to the dehydrogenation of HCOOH.
Furthermore, we also investigated the effect of F species on HCOOH dehydrogenation by DFT calculations. In the simulation, two models were constructed, i.e., single F atoms adsorbed on Ti3C2O2 and Ti3C2O2-V (F1@Ti3C2O2 and F1@Ti3C2O2-V) (Supplementary Fig. 19a, b). As shown in Supplementary Fig. 19c, F1@Ti3C2O2 exhibited a weak adsorption for HCOOH with the adsorption energy of −0.17 eV. As for F1@Ti3C2O2-V, a similar adsorption energy of −0.36 eV was observed compared with that for Ti3C2O2-V with the value of −0.35 eV (Supplementary Fig. 19d). Therefore, the influence of F species on HCOOH dehydrogenation was negligible in this case, which was also supported by our experiment of conducting HCOOH dehydrogenation over Ti3C2Tx-250 in NaF aqueous solution with different concentrations (Supplementary Fig. 8).
The influence of the residual Al species on HCOOH dehydrogenation was also investigated in detail. In the simulation, two models were constructed, i.e., Al1@Ti3C2O2 and Al1@Ti3C2O2-V, which represent a single Al atom adsorbed on the surface of Ti3C2O2 and Ti3C2O2-V, respectively (Supplementary Figs. 20a and 21a). As shown in Supplementary Figs. 20b and 21b, HCOOH exhibited a dissociation adsorption on the surface of both Al1@Ti3C2O2 and Al1@Ti3C2O2-V to generate HCOO-Al* and H* with the adsorption energies of −3.29 and −3.26 eV, respectively. The adsorption of HCOOH was too much strong in the presence of Al element, which was unfavorable for the next reaction. More importantly, the subsequent dissociation of HCOO-Al* to COO-Al* over Al1@Ti3C2O2 and Al1@Ti3C2O2-V was severely impeded thermodynamically with the reaction energies up to 2.46 and 2.54 eV, respectively (Supplementary Fig. 20c and Supplementary Fig. 21c). The Ea for the dissociation of HCOO* over Ti3C2O2 through meta-stable state was only 0.61 eV on the surface of Ti3C2O2. With regard to Ti3C2O2-V, the Ea of the dissociation of HCOO* was 2.5 eV. Therefore, the theoretical results as well as experimental findings indicated that the promotional role of the residual Al species in Ti3C2Tx-250 could be neglected for HCOOH dehydrogenation.
Discussion
In conclusion, we have constructed non-noble-metal heterogeneous catalysts with high efficiency for HCOOH dehydrogenation by modulating the surface oxygen coverage of Ti3C2Tx MXenes. Ti3C2Tx-250 exhibited a mass activity of 365 mmol g−1 h−1 with 100% of selectivity for H2, much higher than that of commercial Pd/C and Pt/C. The remarkable catalytic performance of Ti3C2Tx MXenes originates from the lowered reaction energy barrier for HCOO*-to-CO2* step and adsorption energy for CO2 and H2 by the regulation of surface oxygen atoms. This work not only provides a strategy for developing efficient non-noble-metal catalysts but also advances the understanding in precise control of surface structure of catalyst from atomic insight.
Methods
Chemicals and materials
Ti3AlC2 powder, HCOOH (98%), and HF (≥40%) were obtained from Sinopharm Chemical Reagent Co., Ltd. Pt/C (5% mass loading) and Pd/C (5% mass loading) were obtained from Sigma-Aldrich. DCOOH (95 wt.% in H2O, 98 atom% D), HCOOD (95 wt.% in D2O, 98 atom% D), and DCOOD (95 wt.% in D2O, 98 atom% D) were purchased from Sigma-Aldrich Co. All solvents and chemicals were of analytical grade and used as received without further purification. All aqueous solutions were prepared using deionized water with a resistivity of 18.2 MΩ cm−1.
Preparation of Ti3C2Tx-25
In all, 1.225 g of Ti3AlC2 powder was immersed in 15 mL of 40% HF solution, followed by stirring for 72 h at room temperature. Al species in Ti3AlC2 powder were selectively etched in this process. After that, the obtained powder was washed with ethanol and water for several times until the pH of the supernatant was elevated to 6.0. Then the product was collected by centrifugation at ~2739 × g for 10 min and dried at 60 °C under vacuum for 24 h. Finally, the as-obtained powder was dispersed into 50 mL of water and subjected to ultra-sonication for 24 h to gain Ti3C2Tx-25.
Preparation of Ti3C2Tx-25 in large scale
In all, 8.0 g of Ti3AlC2 powder was immersed in 120 mL of 40% HF solution, followed by stirring for 72 h at room temperature. Al species in Ti3AlC2 powder was selectively etched in this process. After that, the obtained powder was washed with ethanol and water for several times until the pH of the supernatant was elevated to 6.0. Then the product was collected by centrifugation at ~2739 × g for 10 min and dried at 60 °C under vacuum for 24 h. Finally, the as-obtained powder was dispersed into 400 mL of water and subjected to ultra-sonication for 24 h to gain 6.3305 g of Ti3C2Tx-25. This successful large-scale preparation of Ti3C2Tx-25 provided the possibility for industrial application.
Preparation of Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350
The as-obtained Ti3C2Tx-25 powder was treated in a muffle furnace with air at 150, 250, and 350 °C for 1 h to gain Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350, respectively. Ti3C2Tx-250-20h and Ti3C2Tx-250-96h were prepared with the similar method except for varying the etching time from 72 h to 20 and 96 h, respectively.
Preparation of Ti2CTx-25
In all, 5.0 g of Ti2AlC powder was immersed in 30 mL of 16% HF solution, followed by stirring for 72 h at room temperature. Al species in Ti2AlC powder was selectively etched in this process. After that, the obtained powder was washed with ethanol and water for several times until the pH of the supernatant was elevated to 6.0. Then the product was collected by centrifugation at ~2739 × g for 10 min and dried at 60 °C under vacuum for 24 h. Finally, the as-obtained powder was dispersed into 50 mL of water and subjected to ultra-sonication for 24 h to gain Ti2CTx-25.
Preparation of Ti2CTx-150, Ti2CTx-250, and Ti2CTx-350
The as-obtained Ti2CTx-25 powder was treated in a muffle furnace with air at 150, 250, and 350 °C for 1 h to gain Ti2CTx-150, Ti2CTx-250, and Ti2CTx-350, respectively.
X-ray absorption fine structure (XAFS) measurements for Ti element
The XAFS spectra at Ti K-edge (E0 = 4966 eV) were performed at BL14W1 beamline of Shanghai Synchrotron Radiation Facility (SSRF) operated at 3.5 GeV under “top-up” mode with a constant current of 220 mA. The XAFS data of Ti3C2Tx-25, Ti3C2Tx-150, Ti3C2Tx-250, and Ti3C2Tx-350 samples were recorded under transmission mode with two ion chambers. The white light was monochromatized by a Si (111) double-crystal monochromator and calibrated with a Ti foil Athena and Artemis codes were used to extract the data and fit the profiles. For the XANES part, the experimental absorption coefficients as a function of energies μ(E) were processed by background subtraction and normalization procedures. We refer to this process as “normalized absorption.” For the EXAFS part, the Fourier-transformed (FT) data were fitted in R space. The passive electron factors, S02, were determined by fitting the experimental TiO2 data and fixing the Ti-O CN to be 6 and then fixed for further analysis of the measured samples. The parameters describing the local structure environment including CN, bond distance (R), and Debye–Waller factor around the absorbed atoms were allowed to vary during the fit process. WT analysis was employed using the Igor pro script developed by Funke et al.54. The Morlet wavelet was chosen as basis mother wavelet and the parameters (η = 8, σ = 1) were used for a better resolution in the wave vector k.
XPS measurements
XPS experiments were performed at the Photoemission End-station connected to the BL10B beamline in the National Synchrotron Radiation Laboratory in Hefei, China. The beamline is connected to an in-vacuum undulator and equipped with two gratings that offer soft X-rays from 100 to 1000 eV with a typical photon flux of 1 × 1010 photons s−1. The analysis chamber is connected to the beamline and equipped with a VG Scienta R3000 electron energy analyzer, a twin anode X-ray source (Mg Kα and Al Kα), a UV light source, a rear-view optics for low-energy electron diffraction, and a high-precision manipulator with four degree of freedom. All of the XPS data were fitted by “XPS Peak Fitting Program for WIN95/98 XPSPEAK Version 4.1” developed by Raymund W.M. Kwok. During the process of fitting, the peak is determined by the parameters of peak position, area, full width at half maximum (FWHM), and %Gaussian–Lorentzian. For the fitting peaks, the values of FWHM for one species in different samples should be kept the same, at least <10% (user manual written by Raymund W.M. Kwok, July 1, 1999).
Catalytic tests
Catalytic reactions were carried out in a 100-mL three-neck flask immersed into an oil bath. The volume of the gas generated during the catalytic reaction was monitored by a gas burette system. In a typical reaction, 30 mg of catalysts were added into a 100-mL three-neck flask containing 2.6 mmol of HCOOH and 10 mL of deionized water, followed by magnetic stirring at 80 °C for 40 min. The composition of released gas was determined by gas chromatograph (Shimadzu GC-2014C). The mass activity of each catalyst was calculated within the initial 10 min. Besides, after finishing the reaction, the catalyst was recollected by centrifugation for 10 min and dried at 60 °C under vacuum for 12 h, which was named as Ti3C2Tx-250-used.
Isotope-labeled experiments
To acquire KIE data, we performed the dehydrogenation of HCOOH over Ti3C2Tx-250 under the same conditions excepting for employing different substrates, including HCOOH, DCOOH, HCOOD, and DCOOD, and solvents, including H2O and D2O. The experimental process is described in detail. (1) 30 mg of Ti3C2Tx-250 were added into a 100-mL three-neck flask containing 2.6 mmol of HCOOH and 10 mL of deionized water (H2O), followed by magnetic stirring at 80 °C for 10 min. The volume of the released gas was determined by a gas burette system. (2) 30 mg of Ti3C2Tx-250 were added into a 100-mL three-neck flask containing 2.6 mmol of DCOOH and 10 mL of deionized water (H2O), followed by magnetic stirring at 80 °C for 10 min. The volume of the released gas was determined by a gas burette system. (3) 30 mg of Ti3C2Tx-250 were added into a 100-mL three-neck flask containing 2.6 mmol of HCOOD and 10 mL of D2O, followed by magnetic stirring at 80 °C for 10 min. The volume of the released gas was determined by a gas burette system. (4) 30 mg of Ti3C2Tx-250 were added into a 100-mL three-neck flask containing 2.6 mmol of DCOOD and 10 mL of D2O, followed by magnetic stirring at 80 °C for 10 min. The volume of the released gas was determined by a gas burette system. To analyze the generation of H2, HD, and D2, we performed the experiments under the same reaction conditions except for using a 100-mL home-made reactor with gas inlet. The formed H2, D2, and HD were analyzed by mass spectrometry (THERMOStar gas analysis system).
In situ DRIFT measurements
In situ DRIFT experiments were conducted in an elevated-pressure cell (Harrick DRK-4-BR4) with a FT infrared spectrometer (Bruker TENSOR II). After treatment of the catalysts with a flowing of N2 (1 bar) for 20 min at 25 °C, the background spectrum was acquired. Then 1 bar of N2 was allowed to bubble in HCOOH solution and flowed into the cell for 20 min at 25 °C, followed by in situ DRIFT measurements, which was regarded as the in situ DRIFT spectrum at 0 min. Then the cell was sealed and heated to 80 °C within ca. 20 s. In situ DRIFT spectra were then recorded at interval at 80 °C.
DFT calculations
The calculations were done using the periodic DFT method in the Vienna ab initio simulation package55. The electron ion interaction was treated with the projector augmented wave method56. The electron exchange and correlation energy was described using the generalized gradient approximation-based Perdew–Burke–Erzenhorf functional57. The van der Waals correction was considered within the empirical Grimme scheme (vdW-D3)58. The cut-off energy was set up to 500 eV. The force convergence was set to be <0.02 eV Å−1, and the total energy convergence was set to be <10−5 eV. Electron smearing of σ = 0.1 eV was used following the Gaussian scheme. Brillouin zone sampling was employed using a Monkhorst–Pack grid59. The nudged elastic band method was used to locate the transition state between the initial and final states60.
A single-layer periodic slab with (3 × 3) supercell Ti3C2 and Ti3C2O2 and Ti3C2O2-V with exposed (111) facet was employed to model the experimental catalyst, and all the atoms were fully relaxed during the calculations. The vacuum slab was set up to 15 Å. It is noted that Ti3C2 and Ti3C2O2 represent the catalysts without surface oxygen atoms and surface with saturated oxygen coverage, respectively. Ti3C2O2-V is the model that has one O atom missing at each surface of the model Ti3C2O2, representing a catalyst with less oxygen coverage compared to Ti3C2O2.
Instrumentations
TEM, HAADF-STEM, and STEM-EDX images were collected on a JEOL ARM-200F field-emission transmission electron microscope operating at 200 KV accelerating voltage. XRD pattern was recorded by using a Philips X’Pert Pro Super diffractometer with Cu-Kα radiation (λ = 1.54178 Å). ICP-AES (Atomscan Advantage, Thermo Jarrell Ash, USA) was used to determine the concentration of Al. The BET surface areas of the samples were measured on a Micromeritics ASAP 2460 adsorption apparatus. The numbers of surface Pd and Pt in Pd/C and Pt/C were determined by CO pulse chemisorption (VDsorb-91i). UV-Vis tests were conducted on a TU-1901 at room temperature. The Al K-edge XANES was performed at 08U1A beamline of SSRF.
Data availability
All data generated and analyzed during this study are included in this article and its Supplementary Information or are available from the corresponding authors upon reasonable request.
Code availability
All the codes used in the simulations supporting this article are available from the corresponding authors upon request.
References
Mellmann, D., Sponholz, P., Junge, H. & Beller, M. Formic acid as a hydrogen storage material - development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 45, 3954–3988 (2016).
Grasemann, M. & Laurenczy, G. Formic acid as a hydrogen source - recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012).
Zhu, Q.-L. & Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 8, 478–512 (2015).
Bi, Q.-Y. et al. Dehydrogenation of formic acid at room temperature: boosting palladium nanoparticle efficiency by coupling with pyridinic-nitrogen-doped carbon. Angew. Chem. Int. Ed. 55, 11849–11853 (2016).
Celaje, J. J. A. et al. A prolific catalyst for dehydrogenation of neat formic acid. Nat. Commun. 7, 11308 (2016).
Chen, Y., Zhu, Q.-L., Tsumori, N. & Xu, Q. Immobilizing highly catalytically active noble metal nanoparticles on reduced graphene oxide: a non-noble metal sacrificial approach. J. Am. Chem. Soc. 137, 106–109 (2015).
Liu, Q. et al. A Schiff base modified gold catalyst for green and efficient H2 production from formic acid. Energy Environ. Sci. 8, 3204–3207 (2015).
Yan, J.-M. et al. Anchoring and upgrading ultrafine NiPd on room-temperature-synthesized bifunctional NH2-N-rGO toward low-cost and highly efficient catalysts for selective formic acid dehydrogenation. Adv. Mater. 30, 1703038 (2018).
Zheng, Z., Tachikawa, T. & Majima, T. Plasmon-enhanced formic acid dehydrogenation using anisotropic Pd-Au nanorods studied at the single-particle level. J. Am. Chem. Soc. 137, 948–957 (2015).
Zhu, Q.-L., Tsumori, N. & Xu, Q. Immobilizing extremely catalytically active palladium nanoparticles to carbon nanospheres: a weakly-capping growth approach. J. Am. Chem. Soc. 137, 11743–11748 (2015).
Barnard, J. H., Wang, C., Berry, N. G. & Xiao, J. L. Long-range metal-ligand bifunctional catalysis: cyclometallated iridium catalysts for the mild and rapid dehydrogenation of formic acid. Chem. Sci. 4, 1234–1244 (2013).
Guan, C. et al. Dehydrogenation of formic acid catalyzed by a ruthenium complex with an N,N’-diimine ligand. Inorg. Chem. 56, 438–445 (2017).
Hull, J. F. et al. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 4, 383–388 (2012).
Han, L. et al. Anchoring Pt single atoms on Te nanowires for plasmon‐enhanced dehydrogenation of formic acid at room temperature. Adv. Sci. 6, 1900006 (2019).
Bertini, F., Mellone, I., Ienco, A., Peruzzini, M. & Gonsalvi, L. Iron(II) complexes of the linear rac-tetraphos-1 ligand as efficient homogeneous catalysts for sodium bicarbonate hydrogenation and formic acid dehydrogenation. ACS Catal. 5, 1254–1265 (2015).
Bielinski, E. A. et al. Lewis acid-assisted formic acid dehydrogenation using a pincer-supported iron catalyst. J. Am. Chem. Soc. 136, 10234–10237 (2014).
Boddien, A. et al. Efficient dehydrogenation of formic acid using an iron catalyst. Science 333, 1733–1736 (2011).
Cao, J. et al. Hydrogen production from formic acid over morphology-controllable molybdenum carbide catalysts. J. Alloy. Compd. 735, 1463–1471 (2018).
Flaherty, D. W., Berglund, S. P. & Mullins, C. B. Selective decomposition of formic acid on molybdenum carbide: a new reaction pathway. J. Catal. 269, 33–43 (2010).
Koroteev, V. O., Bulushev, D. A., Chuvilin, A. L., Okotrub, A. V. & Bulusheva, L. G. Nanometer-sized MoS2 clusters on graphene flakes for catalytic formic acid decomposition. ACS Catal. 4, 3950–3956 (2014).
Luo, Q., Wang, T., Walther, G., Beller, M. & Jiao, H. Molybdenum carbide catalysed hydrogen production from formic acid - a density functional theory study. J. Power Sources 246, 548–555 (2014).
Tang, C. et al. A stable nanocobalt catalyst with highly dispersed CoNx active sites for the selective dehydrogenation of formic acid. Agnew. Chem. Int. Ed. 56, 16616–16620 (2017).
Wang, J. et al. Decomposition of formic acid for hydrogen production over metal doped nanosheet-like MoC1−x catalysts. Energy Convers. Manag. 147, 166–173 (2017).
Zell, T., Butschke, B., Ben-David, Y. & Milstein, D. Efficient hydrogen liberation from formic acid catalyzed by a well-defined iron pincer complex under mild conditions. Chem. Eur. J. 19, 8068–8072 (2013).
Lynch, J. et al. Substitutional or interstitial site-selective nitrogen doping in TiO2 nanostructures. J. Phys. Chem. C 119, 7443–7452 (2015).
Patel, N. et al. Experimental and theoretical investigations on the activity and stability of substitutional and interstitial boron in TiO2 photocatalyst. J. Phys. Chem. C 119, 18581–18590 (2015).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
Wang, L. et al. Incorporating nitrogen atoms into cobalt nanosheets as a strategy to boost catalytic activity toward CO2 hydrogenation. Nat. Energy 2, 869–876 (2017).
Yang, W. et al. Enhanced photoexcited carrier separation in oxygen-doped ZnIn2S4 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 55, 6716–6720 (2016).
Wu, Y. et al. Electron density modulation of NiCo2S4 nanowires by nitrogen incorporation for highly efficient hydrogen evolution catalysis. Nat. Commun. 9, 1425 (2018).
Zhu, M. et al. New method to synthesize S-doped TiO2 with stable and highly efficient photocatalytic performance under indoor sunlight irradiation. ACS Sustainable Chem. Eng. 3, 3123–3129 (2015).
Banger, K. K. et al. Low-temperature, high-performance solution-processed metal oxide thin-film transistors formed by a ‘sol-gel on chip’ process. Nat. Mater. 10, 45–50 (2011).
Chen, S. et al. Polyoxometalate-coupled MXene nanohybrid via poly(ionic liquid) linkers and its electrode for enhanced supercapacitive performance. Nanoscale 10, 20043–20052 (2018).
Kang, K. M. et al. Selective molecular separation on Ti3C2Tx-graphene oxide membranes during pressure-driven filtration: comparison with graphene oxide and mXenes. ACS Appl. Mater. Interfaces 9, 44687–44694 (2017).
Kim, S. J. et al. Metallic Ti3C2TX MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 12, 986–993 (2018).
Li, J. et al. Improved sodium-ion storage performance of Ti3C2Tx MXenes by sulfur doping. J. Mol. Catal. A 6, 1234–1243 (2018).
Cao, S., Shen, B., Tong, T., Fu, J. & Yu, J. 2D/2D heterojunction of ultrathin MXene/Bi2WO6 nanosheets for improved photocatalytic CO2 reduction. Adv. Funct. Mater. 28, 1800136 (2018).
Wang, J. L. et al. Non-precious molybdenum-based catalyst derived from biomass: CO-free hydrogen production from formic acid at low temperature. Energy Convers. Manag. 164, 122–131 (2018).
Bide, Y., Nabid, M. R. & Etemadi, B. Facile synthesis and catalytic application of selenium doped graphene/CoFe2O4 for highly efficient and noble metal free dehydrogenation of formic acid. Int. J. Hydrog. Energy 41, 20147–20155 (2016).
Kuehnel, M. F., Wakerley, D. W., Orchard, K. L. & Reisner, E. Photocatalytic formic acid conversion on CdS nanocrystals with controllable selectivity for H2 or CO. Agnew. Chem. Int. Ed. 54, 9627–9631 (2015).
Enthaler, S. et al. Exploring the reactivity of nickel pincer complexes in the decomposition of formic acid to CO2/H2 and the hydrogenation of NaHCO3 to HCOONa. ChemCatChem 7, 65–69 (2015).
Myers, T. W. & Berben, L. A. Aluminium-ligand cooperation promotes selective dehydrogenation of formic acid to H2 and CO2. Chem. Sci. 5, 2771–2777 (2014).
Boddien, A. et al. ortho-Metalation of iron(0) tribenzylphosphine complexes: homogeneous catalysts for the generation of hydrogen from formic acid. Angew. Chem. Int. Ed. 49, 8993–8996 (2010).
Boddien, A. et al. Catalytic generation of hydrogen from formic acid and its derivatives: useful hydrogen storage materials. J. Am. Chem. Soc. 132, 8924–8934 (2010).
Millikan, R. C. & Pitzer, K. S. Infrared spectra and vibrational assignment of monomeric formic acid. J. Chem. Phys. 27, 1305–1308 (1957).
Leonard, N. J. & Sauers, R. R. Unsaturated amines. XI. the course of formic acid reduction of enamines. The Course of Formic Acid Reduction of Enamines, 6210-6214 (1957).
Style, D. W. G. & Ward, J. C. The origins of the fluorescences obtained from formic acid and methylene iodide. J. Chem. Soc. 2125–2127 (1952).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Hou, T. et al. Yin and yang dual characters of CuOx clusters for C–C bond oxidation driven by visible light. ACS Catal. 7, 3850–3859 (2017).
Luo, Q. Q., Feng, G., Beller, M. & Jiao, H. J. Formic acid dehydrogenation on Ni(111) and comparison with Pd(111) and Pt(111). J. Phys. Chem. C 116, 4149–4156 (2012).
Graciani, J. et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 345, 546–550 (2014).
Khan, M. U. et al. Pt3Co octapods as superior catalysts of CO2 hydrogenation. Angew. Chem. Int. Ed. 55, 9548–9552 (2016).
Zhang, W. B. et al. Integration of quantum confinement and alloy effect to modulate electronic properties of RhW nanocrystals for improved catalytic performance toward CO2 hydrogenation. Nano Lett. 17, 788–793 (2017).
Funke, H., Scheinost, A. C. & Chukalina, M. Wavelet analysis of extended x-ray absorption fine structure data. Phys. Rev. B 71, 094110 (2005).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901 (2000).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant nos. 51801235, 11875258, 11505187, 51374255, 51802356, 51572299, 21688102, 21703222, and 11874334), Innovation-Driven Project of Central South University (No. 2018CX004), the Start-up Funding of Central South University (No. 502045005), the Fundamental Research Funds for the Central South Universities (Nos. WK2310000066, WK2060190081, and WK2060190103), China Postdoctoral Science Foundation (No. 2019M652797), Central South University Postdoctoral Research Opening Fund, Youth Innovation Promotion Association CAS (2020458), joint funding supports from National Synchrotron Radiation Laboratory (KY2340000115), National Key Research, Development Program of China (2019YFA0307900), and Natural Science Foundation of Hunan Province (2020JJ5690). We acknowledge the staff of 08U1A at Shanghai Synchrotron Radiation Facility. The calculations were performed on the supercomputing system at the Supercomputing Center of University of Science and Technology of China and the High-performance Computing Platform of Anhui University.
Author information
Authors and Affiliations
Contributions
T.H. and L.W. designed the studies and wrote the paper. T.H. and Q. Li synthesized catalysts and performed catalytic tests and in situ DRIFT experiment. Q. Luo, J.C., and J.Y. performed DFT calculations. Y.L. performed the aberration-corrected STEM characterization. P.C. and X.Z. conducted XPS and XAFS measurement. H.Z. and S.C. performed XRD experiment. W.Z. and S.L. revised the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Dmitri Bulushev and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, T., Luo, Q., Li, Q. et al. Modulating oxygen coverage of Ti3C2Tx MXenes to boost catalytic activity for HCOOH dehydrogenation. Nat Commun 11, 4251 (2020). https://doi.org/10.1038/s41467-020-18091-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-18091-7
This article is cited by
-
Highly sensitive and reversible MXene-based micro quartz tuning fork gas sensors with tunable selectivity
npj 2D Materials and Applications (2024)
-
Acidic CO2-to-HCOOH electrolysis with industrial-level current on phase engineered tin sulfide
Nature Communications (2023)
-
2D MXenes polar catalysts for multi-renewable energy harvesting applications
Nature Communications (2023)
-
Defect-driven nanostructuring of low-nuclearity Pt-Mo ensembles for continuous gas-phase formic acid dehydrogenation
Nature Communications (2023)
-
Asymmetrically coordinated single-atom iron nanozymes with Fe-N1C2 structure: A peroxidase mimetic for melatonin detection
Nano Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.