Abstract
Sulfur-sulfur motifs widely occur in vital function and drug design, which yearns for polysulfide construction in an efficient manner. However, it is a great challenge to install desired functional groups on both sides of sulfur-sulfur bonds at liberty. Herein, we designed a mesocyclic bilateral disulfurating reagent for sequential assembly and modular installation of polysulfides. Based on S-O bond dissociation energy imparity (mesocyclic compared to linear imparity is at least 5.34 kcal mol−1 higher), diverse types of functional molecules can be bridged via sulfur-sulfur bonds distinctly. With these stable reagents, excellent reactivities with nucleophiles including C, N and S are comprehensively demonstrated, sequentially installing on both sides of sulfur-sulfur motif with various substituents to afford six species of unsymmetrical polysulfides including di-, tri- and even tetra-sulfides. Life-related molecules, natural products and pharmaceuticals can be successively cross-linked with sulfur-sulfur bond. Remarkably, the cyclization of tri- and tetra-peptides affords 15- and 18-membered cyclic disulfide peptides with this reagent, respectively.
Similar content being viewed by others
Introduction
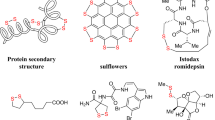
Sulfur–sulfur bond has unique and significant roles in biological, pharmaceutical, and material fields. In organism, tertiary structures of proteins are fixed and stabilized via the linkage of sulfur–sulfur bridge among secondary structures, contributing to the versatility of proteins with complex three-dimensional structure (Fig. 1a)1,2. Polysulfides such as trisulfides and tetrasulfides are primary H2S donors3, signaling of which endogenous gasotransmitter occurs via persulfidation of cysteine residues (RSH) to persulfides (RSSH) in proteins4 with the reduction of glutathione5 (Fig. 1b). As a powerful linker, sulfur–sulfur bridges cyclized peptide drugs with higher stability, activity, and potency compared with corresponding linear ones (Fig. 1c)6. Given the excellent metabolism of sulfur–sulfur bond in organism, cutting-edge drug design strategies of antibody–drug conjugates (ADCs)7,8,9 and small molecule-drug conjugates (SMDCs)10,11,12,13 involved disulfur extensively, such as Mylotarg14 and Vintafolide15, in which sulfur–sulfur bond serves as a reversible cross-linker. Cytotoxic drug molecule can be programmatically released relying on the reduction of glutathione when delivered to the target cells (Fig. 1d)16. Furthermore, polysulfides also possess high-capacity potential in cathode materials for rechargeable lithium battery, among which outstanding capacity of tetrasulfides are higher than that of trisulfides (Fig. 1e)17,18,19.
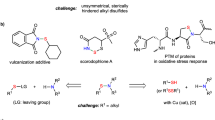
Despite of the great significance of disulfur, the construction of disulfide is not yet unhindered since of high reactivity from sulfur–sulfur bond20,21,22,23,24. Though both nucleophilic25 and electrophilic26 disulfurating reagents have been developed, free and flexible installation on both sides of sulfur–sulfur motif is still an insurmountable challenge. Based on our concept of mask effect27, we envision that disulfurating reagents with bilateral masks will cross-link two designated functional molecules with sulfur–sulfur bond sequentially and modularly (Fig. 2a). Dialkoxydisulfide28,29 and diaminodisulfide30,31,32 have been investigated as sulfur transfer reagents since 1970s. However, unsymmetrical polysulfidation with disulfanyl motif has never been achieved owing to the sharp contradiction raised by sequential and selective cleavage of dual S-O(N) bonds. Based on preliminary calculation of an assumed disulfurating process with molecular mechanics method (MM2, Fig. 2b), energy released from the first S-O cleavage is higher than the second due to the ring tension energy (>5.34 kcal mol−1) when application of mesocyclic bilateral disulfide, enabling to differentiate S-O bond cleavage (for details, see the Supplementary Figs. 7–9). Herein, we show a mesocyclic bilateral disulfurating reagent for sequential assembly and modular installation of unsymmetric polysulfides.
Results
Syntheses of diaza-disulfides 3 and aza-trisulfides 4
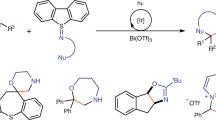
With this concept, a series of bilateral disulfurating reagents were synthesized (Fig. 3a), whose structures were further confirmed through X-ray analysis of 1f. In order to demonstrate the ladder-type reactivities of reagents, aniline was used as a nucleophile under the assistance of B(C6F5)3 as catalyst (Fig. 3b). As expected, linear disulfurating reagents 1a and 1b resulted in poor selectivities between two S-O/N bonds, bringing mixture when coupling with aniline. Cyclic diaminodisulfide 1c refused to transfer disulfur owing to week reactivity. Cyclic disulfane 1d and 1e failed to generate mono-coupling product 2 owing to the decomposition of starting material. Mono-aza-disulfide 2f was quantitatively obtained when 10-membered disulfane 1f was employed as a disulfurating reagent (for details, see the Supplementary Table 1).
Since the first S-O cleavage was controllably realized, another nucleophile was subsequently subjected to aza-disulfide 2f under the assistance of weak base lithium carbonate, affording unsymmetrical diaza-disulfides 3 in Fig. 4. Diverse anilines bearing electron-withdrawing and electron-donating functional groups could be cross-linked with benzyl amines, straight-chain alkylamines, diallylamine, pyridyl methylamine, tryptamine, and even amino-acid esters at liberty (3a–3j). The unsymmetrical diaza-disulfide structure of 3a was further confirmed through X-ray analysis. Among them, compound 3d was afforded with a yield of 55% with occurrence of the polymerization of vinyl group accelerated by B(C6F5)333. The relative low efficiency of 3l, 3n, and 3o with tryptophan motif resulted from a cyclic disulfide intermediate generated from nucleophilic cyclization of 2-position of indole (for details, see the Supplementary Fig. 2). Amines with sensitive enamine structure, amino-acid esters and antibiotic sulfamethazine underwent the connection smoothly (3k–3m). Moreover, two different amino-acid esters could be cross-linked through sulfur–sulfur bond straightforward (3n and 3o). Notably, sulfamethazine and sulfamethoxazole could be successfully linked with different peptides in good yields (3p and 3q), which displayed a great potential for the synthesis of SMDCs drugs. Besides, the antibacterial sulfamethazine and cinacalcet, a kind of calcimimetics, could be connected efficiently in good yield (3r).
With this strategy, amines could be cross-linked with mercaptans via disulfur motif, affording aza-trisulfides 4 smoothly. Both electron donor and acceptor substituted anilines were applied in the connection compatibly (4a–4g). Weak nucleophilic thiophenol, straight-chain dodecamercaptan, electron-rich furfuryl mercaptan, electron-deficient 2-mercaptopyrimidine, and even cysteine could be successfully introduced in this connection to afford aza-trisulfides (4h–4m). Tryptamine, peptide, amines with sensitive enamine structure, even sulfonamides like sulfamethazine, sulfacetamide and sulfamethoxazole were cross-linked with thiols by disulfur perfectly (4n–4s), which supplies an efficient protocol for drug-linkage. Interestingly, we successfully synthesized an aza-trisulfide with a long chain of thirty-four-atoms via this method (4t). Tripeptides like H-Ala-Phe-Lys-OMe could be cyclized to form 15-membered cyclic peptides 5a and tetrapeptides like H-Ala-Phe-Trp-Lys-OMe could be cyclized to form 18-membered cyclic peptides 5b under the standard conditions (Fig. 5).
Syntheses of aza-disulfides 6, trisulfides 7 and disulfides 8
Furthermore, we established the cross-linkage between carbon and nucleophiles with phenyl boric acid and bilateral disulfurating reagent 1f as coupling partner first (Fig. 6). With the optimized conditions, mono-coupling was obtained in 84% yield (for details see the Supplementary Table 2). Investigating on nucleophiles, diverse aromatic rings were cross-linked with amines, mercaptans, and electron-rich aromatics modularly, affording aza-disulfides, trisulfides, and diaryl disulfides, respectively. The arylboronic acids substituted with electron-withdrawing and -donating functional groups afforded the corresponding aza-disulfides readily (6a–6f, 7b–7g, and 8h). Arylboronic acids derived from l-tyrosine and estrone were compatible in the cross-linkage, affording a pathway to late-stage modification of natural products (6g and 7h), though the slow rate of transmetallation of boric acid with Cu[III] brought about insufficient efficiency in the first step. The scope of amine is quite broad when it is served as a nucleophile. Anilines (6a and 6b), aliphatic amines (6c–6e), amino-acid esters (6f and 6g), and antibiotic sulfamethazine (6h) were all efficiently transformed to the corresponding aza-disulfides in moderate yields. Trisulfides could be easily obtained when mercaptans were applied in the cross-coupling. Arylthiophenol like 2-mercaptopyrimidine provided diaryl trisulfide (7a). Other thiols even containing hydroxyl (7b) and triethoxylsilyl ether (7h) could afforded trisulfides in moderate yields. Sterically bulky aliphatic thiols, such as tert-butylthiol and 1-adamantanethiol, showed great reactivity in this reaction (7e and 7f). Furthermore, cysteine derivatives were successfully converted to trisulfide derivatives (7d). Diaryl disulfides were generated when electron-rich aromatics were accommodated under the standard conditions. (+)-δ-Tocopherol, a kind of vitamin E, could be disulfurated directly despite of the presence of free hydroxyl group (8b) under nitrogen atmosphere. Indole derivatives were excellent reactants even there is a free amino group (8c). Heterocycles like thiophene could be connected in the reaction as well (8g). The 2-position of N-methyl pyrrole possesses sufficient reactivity in the reaction (8h). The structure of 8f was further confirmed through X-ray analysis.
Synthesis of tetrasulfides 9
Unsymmetrical tetrasulfides was a challenging subject in organic synthesis, but the connection between two different mercaptans with 1d as a disulfurating reagent afforded the desired tetrasulfides highly efficiently, owing to large difference betwen two S-O bonds of eight-membered 1d (9.53 kcal mol−1) (for details see the Supplementary Table 3). The unsymmetrical tetrasulfide linkage was comprehensively investigated in Fig. 7. Pyrimidine and pyrazine can be easily accomodated under the standard conditions (9a–9c). The structure of 9a was further confirmed through X-ray analysis as a linear tetrasulfide. Penicillamine and cysteine, two different amino acids, were cross-linked with tetrasulfur fragment via this method (9d). Cysteine (9e), tripeptide (9f), and even glucosinolate (9g) could be cross-linked with 1-adamantanethiol, forming unsymmetrical tetrasulfides. Sensitive thiols, which even contained hydroxyl and triethoxylsilyl ether, could afforded tetrasulfides (9h). Remarkably, volatile and low-polar allicin analog was modularly provided when propanethiol and allyl mercaptan were used as nucleophiles (9i).
Bilateral reagents 1d and 1f are odorless and stable solid stored at −10 °C regardless of air and water. No decomposition was observed even after 5 months, whereas they will deteriorate at room temperature after 24 h. With these designed bilateral reagents, we have established six different kinds of polysulfides, most of which are quite stable under room temperature except aza-trisulfides. They need to be stored in fridge (−10 °C) for long-term preservation. Diaza-disulfides, aza-trisulfide, aza-disulfide, and tetrasulfides are fragile to acidic conditions.
S2Cl2, a common disulfur structure, hardly achieves multiple heteroatom hybrid connection on account of its fractious activity and strong acidity. Taking synthesis of 9a as an example, the selectivity of 9a to 9a-S5 is 2.5:1 when S2Cl2 was involved, much lower than 15:1 afforded by our reagent 1d. Besides, there is a huge gap between the efficiency afforded by S2Cl2 and 1d (8% vs 70%) (Fig. 8a). Di(1-phthalimidyl)disulfane (1b) reagent developed by Harpp30, which avoids the disadvantage of acidity with S2Cl2, still remains less selective and efficient owing to the nondistinctive S-N bonds. For instance, Harpp’s reagent gave a mono-coupling product only in 30% and a bis-coupling byproduct in 60% in the first step coupling with aniline. Optimistically, quantitative yield of 2f could be obtained with our reagent 1f (Fig. 8b).
Discussion
In summary, based on S-O bond dissociation energy nuance, a series of mesocyclic bilateral disulfurating reagents were designed for constructing six species of unsymmetrical polysulfides. Disulfides, trisulfides and tetrasulfides can be accurately achieved with amines, mercaptans, arylboronic acids, and electron-rich aromatic molecules. A considerable range of significant life-related molecules, such as sulfonamides, amino acids, peptides, glucosinolate, vitamin E, and estrone could be cross-linked at will with disulfur bridge to form varieties of diverse functional molecules, which showcases great potential for application of SMDCs and ADCs. Readily available linear peptide precursors can be tied up with disulfur fragment to form unique cyclic peptides with a tetraheteroatomic motif. Drug discovery with polysulfides is undergoing in our laboratory.
Methods
General procedure for syntheses of diaza-disulfides 3 and aza-trisulfides 4
To a Schlenk tube were added amine (0.1 mmol, 1.0 equiv), B(C6F5)3 (0.5 mg, 1 mol%),1f (29.0 mg, 0.105 mmol), and 1,4-dioxane (0.25 mL), the mixture was stirred at r.t. for 4 h to obtain 2. After amine was consumed, another amine (0.12 mmol, 1.2 equiv) or thiol (0.11 mmol, 1.1 equiv) and Li2CO3 (7.4 mg, 0.1 mmol) were added to the mixture. The mixture was stirred at r.t. for 8–24 h before it was concentrated under vacuum. Purification by column chromatography afforded the desired product 3 or 4.
General procedure for syntheses of aza-disulfides 6, trisulfides 7, and disulfides 8
To a Schlenk tube were added arylboronic acid (0.15 mmol, 1.5 equiv), 1f (29 mg, 0.10 mmol), Cu(MeCN)4PF6 (3.7 mg, 10 mol%), 2,2′-bpy (3.1 mg, 20 mol%), and redistilled CH2Cl2 (1 mL), the mixture was stirred at r.t. for 10 h under N2 atmosphere. After 1f was consumed, another nucleophile (0.12 mmol, 1.2 equiv) was added to the mixture. The mixture was stirred at r.t. for 8 h under air before it was concentrated under vacuum. Purification by column chromatography afforded the desired product 6, 7, or 8.
General procedure for syntheses of tetrasulfides 9
To a solution of 1d (24.0 mg, 0.12 mmol) in MeOH (1 mL) was added thiol (0.1 mmol, 1.0 equiv) in MeOH (1 mL) dropwise at −78 °C, then the mixture was stirred at −78 °C for 30 min before MeOH was removed under vacuum. CH2Cl2 (1 mL), another thiol (0.11 mmol, 1.1 equiv) and B(C6F5)3 (0.5 mg, 0.001 mmol, 1 mol%) was added to the mixture at r.t. for 4 h under air before it was concentrated under vacuum. Purification by column chromatography afforded the desired product 9.
Data availability
The X-ray crystallographic coordinates for the structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC-1941481 (1f), 1941479 (3a), 1941480 (4d), 1941478 (8f), and 1941482 (9a). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Source data are provided with this paper.
References
Alegre-Cebollada, J., Kosuri, P., R.-Pardo, J. A. & Fernández, J. M. Direct observation of disulfide isomerization in a single protein. Nat. Chem. 3, 882–887 (2011).
Lu, S. et al. Mapping native disulfide bonds at a proteome scale. Nat. Methods 12, 329–331 (2015).
Benavides, G. A. et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl Acad. Sci. USA 104, 17977–17982 (2007).
Filipovic, M. R., Zivanovic, J., Alvarez, B. & Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 118, 1253–1337 (2018).
Kaplowitz, N. The importance and regulation of hepatic glutathione. Yale J. Biol. Med. 54, 497–502 (1981).
Góngora-Benítez, M., Tulla-Puche, J. & Albericio, F. Multifaceted roles of disulfide bonds. Peptides as therapeutics. Chem. Rev. 114, 901–926 (2014).
Staben, L. R. et al. Targeted drug delivery through the traceless release of tertiary and heteroaryl amines from antibody-drug conjugates. Nat. Chem. 8, 1112–1119 (2016).
Chari, R. V. J., Miller, M. L. & Widdison, W. C. Antibody–drug conjugates: an emerging concept in cancer therapy. Angew. Chem. Int. Ed. 53, 3796–3827 (2014).
Beck, A., Goetsch, L., Dumontet, C. & Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 16, 315–337 (2017).
Krall, N., Pretto, F. & Neri, D. A bivalent small molecule-drug conjugate directed against carbonic anhydrase IX can elicit complete tumour regression in mice. Chem. Sci. 5, 3640–3644 (2014).
Krall, N. et al. A small-molecule drug conjugate for the treatment of carbonic anhydrase IX expressing tumors. Angew. Chem. Int. Ed. 53, 4231–4235 (2014).
Cazzamalli, S. et al. Enhanced therapeutic activity of non-internalizing small-molecule-drug conjugates targeting carbonic anhydrase IX in combination with targeted interleukin-2. Clin. Cancer Res. 24, 3656–3667 (2018).
Cazzamalli, S., Corso, A. D., Widmayer, F. & Neri, D. Chemically defined antibody– and small molecule–drug conjugates for in vivo tumor targeting applications: a comparative analysis. J. Am. Chem. Soc. 140, 1617–1621 (2018).
Richard, A. et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33–positive acute myeloid leukemia in first recurrence. Cancer 104, 1442–1452 (2005).
Razzak, M. Vintafolide–targeting the folate receptor with a cytotoxic offers hope. Nat. Rev. Clin. Oncol. 10, 668 (2013).
Couto, N., Malys, N., Gaskell, S. J. & Barber, J. Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: a proteomic approach. J. Proteome Res. 12, 2885–2894 (2013).
Wu, M. et al. Organotrisulfide: a high capacity cathode material for rechargeable lithium batteries. Angew. Chem. 128, 10181–10185 (2016).
Wu, M. et al. Highly reversible diphenyl trisulfide catholyte for rechargeable lithium batteries. ACS Energy Lett. 1, 1221–1226 (2016).
Guo, W. et al. Bis(aryl) tetrasulfides as cathode materials for rechargeable lithium batteries. Chem. Eur. J. 23, 16941–16947 (2017).
Wang, M. & Jiang, X. Sulfur-sulfur bond construction. Top. Curr. Chem. 376, 14 (2018).
Park, C. M. et al. 9-Fluorenylmethyl (Fm) disulfides: biomimetic precursors for persulfides. Org. Lett. 18, 904–907 (2016).
Wang, W. et al. Cu-catalyzed electrophilic disulfur transfer: synthesis of unsymmetrical disulfides. Org. Lett. 20, 3829–3832 (2018).
Zou, J. et al. Phthalimide-carried disulfur transfer to synthesize unsymmetrical disulfanes via copper catalysis. ACS Catal. 9, 11426–11430 (2019).
Gao, W., Tian, J., Shang, Y. & Jiang, X. Steric and stereoscopic disulfide construction for cross-linkage via N-dithiophthalimides. Chem. Sci. 11, 3903–3908 (2020).
Xiao, X., Feng, M. & Jiang, X. New design of disulfurating reagent: facile and straightforward pathway to unsymmetrical disulfanes via Cu-catalyzed oxidative cross coupling. Angew. Chem. Int. Ed. 55, 14121–14121 (2016).
Xiao, X., Xue, J. & Jiang, X. Polysulfurating reagent design for unsymmetrical polysulfide construction. Nat. Commun. 9, 2191 (2018).
Li, Y., Wang, M. & Jiang, X. Controllable sulfoxidation and sulfenylation with organic thiosulfate salts via dual electron- and energy-transfer photocatalysis. ACS Catal. 7, 7587–7592 (2017).
Tardif, S. L., Williams, C. R. & Harpp, D. N. Diatomic sulfur transfer from stable alkoxy disulfides. J. Am. Chem. Soc. 117, 9067–9068 (1995).
Zysman-Colman, E. et al. Crossover point between dialkoxy disulfides (ROSSOR) and thionosulfites ((RO)2S=S): prediction, synthesis, and structure. J. Am. Chem. Soc. 128, 291–304 (2006).
Harpp, D. N., Steliou, K. & Chan, T. H. Synthesis and reactions of some new sulfur transfer reagents. J. Am. Chem. Soc. 100, 1222–1228 (1978).
Huang, N., Lakshmikantham, M. V. & Cava, M. P. Thiation reactions of some active carbonyl compounds with sulfur transfer reagents. J. Org. Chem. 52, 169–172 (1987).
Graf, T. A. et al. New polymers possessing a disulfide bond in a unique environment. Macromolecules 45, 8193–8200 (2012).
Yang, X., Stern, C. L. & Marks, T. J. Cation-like homogeneous olefin polymerization catalysts based upon zirconocene alkyls and tris(pentafluorophenyl)borane. J. Am. Chem. Soc. 113, 3623–3625 (1991).
Acknowledgements
We are grateful for financial support provided by the National Key Research and Development Program of China (2017YFD0200500), NSFC (21971065, 21722202, 21672069), the S&TCSM of Shanghai (18JC1415600), Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, National Program for Support of Top-notch Young Professionals, and Innovative Research Team of High-Level Local Universities in Shanghai.
Author information
Authors and Affiliations
Contributions
X.J. conceived the idea and supervised the whole project. J.X. designed and carried out the experiments. X.J. and J.X. discussed the results, contributed to writing the manuscript, and commented on the manuscript. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Zhen Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xue, J., Jiang, X. Unsymmetrical polysulfidation via designed bilateral disulfurating reagents. Nat Commun 11, 4170 (2020). https://doi.org/10.1038/s41467-020-18029-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-020-18029-z
This article is cited by
-
Bioinspired zinc-mediated umpolung thiolation of alkyl electrophiles: reaction development, scope and mechanism
Science China Chemistry (2024)
-
Radical approaches to C–S bonds
Nature Reviews Chemistry (2023)
-
Synthesis of N-acyl sulfenamides via copper catalysis and their use as S-sulfenylating reagents of thiols
Nature Communications (2022)
-
Decatungstate-catalyzed radical disulfuration through direct C-H functionalization for the preparation of unsymmetrical disulfides
Nature Communications (2022)
-
Efficient preparation of unsymmetrical disulfides by nickel-catalyzed reductive coupling strategy
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.