Abstract
The storage of metastable compounds and modifications of elements are of great interest for synthesis and other, e.g., semiconductor, applications. Whereas white phosphorus is a metastable modification that can be stored under certain conditions, storage of the extremely (light- and air-)sensitive form of arsenic, yellow arsenic, is a challenge rarely tackled so far. Herein, we report on the facile storage and release of these tetrahedral E4 molecules (E = P, As) using activated carbon as a porous storage material. These loaded materials are air- and light-stable and have been comprehensively characterized by solid-state 31P{1H} MAS NMR spectroscopy, powder X-ray diffraction analysis, nitrogen adsorption measurements, and thermogravimetric analysis. Additionally, we show that these materials can be used as a suitable E4 source for releasing intact white phosphorus or yellow arsenic, enabling subsequent reactions in solution. Because the uptake and release of E4 are reversible, these materials are excellent carriers of these highly reactive modifications.
Similar content being viewed by others
Introduction
Since the discovery of elemental white phosphorus1 (P4) by Hennig Brand in 1669, it has served as a widely used reagent in academia and industry alike. While activation of the P4 tetrahedron is of great interest in academic research2,3,4,5, industry is especially interested in the production of organophosphorus compounds and the synthesis of highly pure phosphoric acid starting from white phosphorus. However, solid P4 has to be handled with great care because of its high reactivity, its metastable character and its toxicity, and shipping even small amounts of P4 is subject to severe restrictions. P4 combusts spontaneously in air and decomposes slowly under light. As a result, P4 is stored under water in the dark. In 2009, Nitschke et al. showed the encapsulation of P4 within a self-assembled host–guest complex of iron, which is air-stable in the solid state6. The guest can be extracted by better fitting molecules such as benzene and cyclohexane, whereas poor fitting guests, such as n-heptane, cannot be used for extraction. Recently, Wu et al. succeeded in storing P4 and, interestingly, also As4 within tetrahedrally shaped cage complexes in solution7, 8. However, in all these cases, no subsequent reactions with the host–guest complexes were examined due to the resulting multi-component mixtures of the overall systems (occurrence of side reactions). Moreover, the Kawano group showed the encapsulation of P4 in the reusable metal–organic framework (MOF) [(ZnI2)3(TPT)]n (TPT = 2,4,6-tris(4-pyridiyl)-1,3,5-triazine)9, again without using them for subsequent syntheses. Here, P4 was synthesized from red phosphorus and trapped by gas phase diffusion. Furthermore, we succeeded in the encapsulation and stabilization of intact tetrahedral E4 molecules (E = P, As) in polymeric and spherical aggregates constructed by copper halides and [Cp*Fe(η5-P5)] (Cp* = η5-C5Me5)10. Even if the corresponding crystals are air and light-stable, they are insoluble in common solvents and the group 15 tetrahedra are not releasable without the decomposition of the complexes. Moreover, together with other coinage metal P4 complexes, the air-sensitive silver(I) salts [Ag(η2-E4)2][pftb] (E = P, As; pftb = [Al{OC(CF3)3}4]) show to be suitable sources of intact E4 (E = P, As) for particular chemical reactions, however with a limited scope due to the need of initial AgCl elimination11,12,13,14. Recently, we found two other systems, [(LCu)2(μ,η2:2-E4)] (E = P, As; L = [{N(C6H3iPr2-2,6)C(Me)}2CH−]) and CpRE4 (E = P; CpR = Cp* (η5-C5Me5), Cp″′ (η5-C5H3tBu2), Cp4iPr (η5-C5HiPr4), CpBIG (η5-C5(4-nBuC6H4)5); E = As, CpR = CpPEt (η5-C5(4-EtC6H4)5)), which coordinate either side-on the intact E4 tetrahedra or radically opened an E–E bond to form a twofold organic-substituted compound with a tetraphospha/tetraarsabicyclo[1.1.0]butane as a central structural motif15,16,17. For both systems, we could show the release of intact E4 tetrahedra, irreversible in solution, but reversible in the solid state for the latter case (except for E = As). However, all known coordinating/encapsulating systems of intact E4 have the disadvantage of a multistep synthesis of the starting materials before the final E4 addition can be executed, which is preparatively time-consuming, expensive, and surely not feasible in large-scale batches. Moreover, the existence of additional reactive components in the solutions after the release of E4 makes these systems unusable for subsequent reactions.
In contrast to white phosphorus, the heavier congener As4 has less been used in industry and academia so far because of its pronounced photosensitivity and its extremely difficult and inconvenient handling. Yellow arsenic, discovered as an element modification 150 years ago18, is a metastable compound rapidly decomposing in the solid state even in the dark at low temperatures. Additionally, exposed to light, it decomposes within minutes in solution at room temperature into the thermodynamically stable modification gray arsenic19. Whereas solid yellow arsenic can practically not be stored, only solutions of freshly prepared As4 can be used for subsequent reactions20, which can, however, only be performed in the absolute dark without any exposure to light whatsoever, and the used amount of As4 is not exactly quantifiable, due to its fast decomposition to gray arsenic. Therefore, the challenging quest arises to develop an easily accessible and light-stable storage and release material for yellow arsenic to open ample perspectives for the usage of this very reactive modification in syntheses and reactivity by a large chemistry community. Keeping in mind the extremely low solubility of As4 at low temperatures, the need for a usable As4 delivery source to overcome this difficulty is obvious. Moreover, for both modifications, the problem of a possible hazard-free shipping has to be solved, which would be a breakthrough regarding their application by both industry and academia.
Herein, we report on the facile storage and release of yellow arsenic and white phosphorus, respectively, using activated carbon as a porous storage material, and on the first use of these materials for subsequent reactions in solutions.
Results
Preparation
The loaded materials E4@C (E = P, As) can be easily prepared by adsorbing E4 (E = P, As) from a freshly prepared solution in tetrahydrofuran (THF) by an activated carbon material (C) with a defined pore size distribution resulting in an air- and light-stable black solid of E4@C after work-up (Supplementary Figs. 1 and 2).
Release of E4
To study the release of E4 molecules by sublimation, the E4@C (for E = P: 820 mg; As: 418 mg) material was sublimed in vacuo at oil bath temperature of 160 °C and cold finger temperature of −20 °C. After 2 days, a black solid remained in the sublimation apparatus (for E = P: 508 mg; E = As: 370 mg), whereas 300 mg of a white waxy solid or 28 mg of a gray amorphous solid was isolated from the cold finger for E = P or As, respectively. The white waxy solid was characterized by 31P NMR spectroscopy well corresponding to those of P4 (Supplementary Fig. 3). The gray solid was characterized by powder X-ray diffraction analysis proving that it is amorphous arsenic (Supplementary Figs. 4 and 5). Furthermore, the solid was identified as gray arsenic by ICP-OES (inductively coupled plasma-optical emission spectrometry, Supplementary Fig. 6).
As the P4@C material retained molecular P4, a standard procedure was used to extract it with common solvents as n-hexane, toluene, dichloromethane, and THF. For this reason, P4@C (100 mg) was dispersed in exactly 10 mL of each of these solvents and stirred for 24 h in the dark. A 31P NMR spectrum of a defined volume (the same in all cases) of the supernatant was recorded using the same standard C6D6 capillary with defined internal PPh3. In the case of CS2, 152 mg P4@C in 20 mL solvent were used (Supplementary Figs. 7–11).
Stability
The stability of P4@C was examined by storing some black solid on the bench in air and under light for 3 weeks. This sample neither ignites spontaneously when brought to air nor reacts with a tissue over time (Supplementary Fig. 17). The solid was then examined with 31P{1H} MAS NMR. A signal for P4 is only slightly (by 8 ppm) down-field shifted compared to the inert stored sample. This fact can be attributed to a slightly different interaction in air within the pores (Supplementary Fig. 18). Additionally, solely one sharp signal for a decomposition product at 1.5 ppm is detected. Presumably, it can be attributed to H3PO4 immobilized within the carbon scaffold, since the signal is in the known range for phosphate derivatives21. This may be a result of slow oxidation and hydrolysis to some extent on the surface of the scaffold bound P4. However, it has to be noted that a signal for white phosphorus can be still detected after storage for 3 weeks in air. Furthermore, P4@C (88 mg) was brought again under nitrogen atmosphere and slurried in 7 mL CS2 overnight followed by 31P NMR investigation of the colorless supernatant. The same standard C6D6 capillary with defined internal PPh3 content was used (Supplementary Fig. 19). An intense signal characteristic of free P4 was again monitored. These results clearly prove the stability of P4@C in light and in air to some extent for at least 3 weeks. However, storage in air is not recommended due to a possible decomposition over a long period of time.
In the case of arsenic, no spontaneous ignition of As4@C was observed. The material was exposed to light for several days and stored in air, though in a fume hood due to its unknown toxicity. However, after extraction with CS2, no signal for As4 was monitored by 75As NMR spectroscopy. Consequently, the extracted As4 is likely harmed by slight oxygen and to some extent moisture uptake within the scaffold. Nevertheless, after sublimation for 48 h at the above-mentioned conditions, resulting gray solid being undoubtedly authenticated by ICP-OES as gray arsenic (Supplementary Figs. 20 and 21).
Recycling
The used storage material C can be recycled and used again for storage purposes, especially for phosphorus. After washing E4 out of E4@C, the regained storage material C is to be heated for several hours in high vacuum before it can be again loaded with solutions of E4.
Discussion
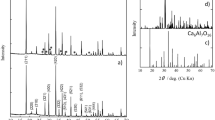
For P4@C, the decrease of the P4 concentration of the THF supernatant during the synthesis of the loaded material was determined by 31P NMR spectroscopy, indicating a minimum uptake of at least 18% phosphorus by mass. This result is in accordance with the elemental analysis of P4@C indicating ~20% phosphorus by mass, which is considerably more than for the tetranuclear iron complex described, e.g., by Nitschke with only 3.5% phosphorus by mass or for the P4-MOF complex by Kawano with 6.9% phosphorus by mass6, 9. The solid state 31P{1H} MAS NMR spectrum of P4@C reveals solely a broad singlet with a chemical shift of −506.4 ppm (ω1/2 = 1582 Hz) (Fig. 1a), which is in good agreement with the reported chemical shift of the encapsulated P4 molecule in spherical aggregates (−506 ppm)10. The signal is high-field shifted compared to P4 both as a solid (−462 ppm) and a liquid (−460 ppm), due to the interaction with its environment22,23,24,25. In contrast, the signal is low-field shifted compared to P4 in the gas phase (−553 ppm) and in solution (e.g., in benzene −522 ppm)23, 26. For the arsenic compound As4@C, no assignable signal can be detected in the solid-state 75As MAS NMR spectrum, which can be attributed to the low sensitivity of the quadrupolar nucleus 75As (Qspec = 0.314(6) b = 0.314(6) × 10−28 m2)27. However, a broad signal for free As4 can be detected in the 75As NMR spectrum at −863 ppm (ω1/2 = 2060 Hz) by extraction of As4@C with CS2 (Fig. 1b). Interestingly, this NMR spectrum was recorded after the storage of the As4 containing material in a glove box in the presence of light for more than 2 weeks. Consequently, As4@C can be seen as light-stable storage material of intact yellow arsenic. The chemical shift is also in good agreement with the reported values (−892 ppm for toluene/CD2Cl2 and −908 ppm for THF/CD2Cl2, respectively) considering the influence of the different solvents10.
The powder X-ray diffractograms of E4@C (E = P, As) show only two broad peaks arising from the amorphous carbon material and no diffraction pattern of E4 units (Fig. 2a). Thus, no crystalline E4 is present in the scaffold, indicating that the arsenic and phosphorus tetrahedra, respectively, are located in the pores (<5 nm). To clarify the distribution of the E4 (E = P, As) molecules, the nitrogen adsorption measurement at liquid nitrogen temperature of E4@C was examined and the DFT (density functional theory)-based pore size distribution was determined (Fig. 2b). As a result of the P4 storage, the specific surface area and the total pore volume of the carbon host decrease by 55% and 57%, respectively. Considering the bulk density of P4 (1.82 g cm−3)28, the maximum amount of P4 in P4@C is approximately 36%. The DFT pore size distribution displays the lack of the ultramicropores (<0.7 nm) as well as an intense decrease of supermicropores (0.7 < 2 nm) (Fig. 2c). Considering a P-P bond length of approx. 2.21 Å and a van der Waals radius of 1.80 Å for a phosphorus atom, the size of the P4 molecules (tetrahedrally shaped sphere with a diameter of about 0.61 nm) is similar to the pore size of the carbon host enabling a good fit into the ultramicropores29, 30. Additionally, the amount of meso pores (>2 nm) diminishes. These results prove the incorporation of the P4 molecules into the porous activated carbon. For As4@C, the specific surface area and the total pore volume decrease by 9% and 14%, respectively. Considering the bulk density of As4 (1.97 g cm−3)28, the maximum amount of As4 in As4@C is approximately 10%. With a diameter of about 0.65 nm, As4 molecules smoothly fit into the carbon scaffold. The thermogravimetric analysis of As4@C shows two steps of a loss of weight. In a first step, until 350 °C, the sample loses approximately 10% of its weight followed by an additional loss of 10% until 600 °C (Fig. 2d). In contrast, the thermogravimetric analysis of solid P4@C shows a loss of weight of about 10% until 180 °C followed by an unexpected, but reproducible increase of the sample mass by 10% (Fig. 2d). Between 400 and 800 °C, a loss of weight of 32% is again detected resulting from released phosphorus.
Additionally, the suitability of E4@C for the preparative release of intact white phosphorus and yellow arsenic was investigated. By sublimation, a white waxy solid of P4 can be isolated within two days at 160 °C in vacuum. The 31P NMR spectrum of this solid, dissolved in benzene-d6 (singlet at −519.5 ppm), proves unambiguously that the P4 tetrahedra are still intact. The so obtained amount of phosphorus (36% by mass) indicates a maximum amount for the uptake of the porous carbon material and is in very good agreement with the calculated value of incorporated white phosphorus. The analogous sublimation with As4@C leads to the release of As4 and the isolation of at least 7% arsenic by mass. Due to the instability of yellow arsenic under light exposure, only a gray amorphous solid was isolated, which can be attributed to gray arsenic by ICP-OES. Furthermore, the extraction of P4 by different common solvents such as CS2, n-hexane, toluene, dichloromethane and THF was explored. A standard procedure for these solvents reveals that 17–21% P4 by mass can be extracted. In the case of yellow arsenic, extraction with common solvents as for instance n-pentane and toluene showed experimentally a release of about 5–10% arsenic by mass.
Additionally, we were interested in the use of E4@C for subsequent chemical reactions because activated carbon as an inert material should not harm chemical reactions by unintended contaminations in the way different metal ions do. Compared to known storage materials, which are based on multi-component systems containing metal ions, our system solely consists of an inert carbon host thus not resulting in any competitive reactions. That way, we succeeded in the synthesis of the reported compounds starting from E4@C (E = P, As). For example, the reaction of P4@C with [Cp*Fe(CO)2]2 in boiling decalin leads to the formation of the desired pentamethylcyclopentadienyl-pentaphosphaferrocene [Cp*Fe(η5-P5)] (Fig. 3a)31. Additionally, P4@C was reacted with [CpBIGFe(CO)2]2 (CpBIG = C5(4-nBuC6H4)5) at room temperature, analogously to the reported procedure32 starting from dissolved P4, resulting in the quantitative synthesis of the P4 butterfly complex [(CpBIGFe(CO)2)2(μ,η1:1-P4)] (Fig. 3b). The analogous reaction was also observed for As4@C. Therefore, E4@C has been shown to be a convenient source of white phosphorus and yellow arsenic for subsequent reactions. Because As4@C can also be used to deliver yellow arsenic at low temperatures for chemical syntheses, it becomes a useful synthetic tool and opens broad perspectives for its application.
Moreover, P4@C can be stored for at least four weeks in light and in air, without the black solid igniting spontaneously in air. After the storage of P4@C for three weeks in air on a bench, the solid-state 31P{1H} MAS NMR spectrum still reveals one broad singlet with a chemical shift of −498.4 ppm, which can be attributed to white phosphorus P4. This signal is only slightly shifted compared to the signal of P4@C stored in a glove box and is still in good agreement with the reported chemical shifts of P4. However, solely one additional very weak signal at 1.5 ppm was detected resulting presumably from traces of the oxidation of solid P4 and subsequent hydrolysis to some extent. Nevertheless, P4 can be still extracted with CS2 monitored by the 31P NMR spectroscopy and no decomposition product of P4 was observed by the solution 31P NMR spectroscopy in contrast to the solid-state NMR spectroscopy. Principally, As4@C can also be stored in light and in air, but the storage in air is not recommended due to its unknown toxicity. The stability of the E4 tetrahedra in the porous material might be attributed to its interaction within the pores of the carbon material since the tetrahedral structure and size of the E4 molecules match nearly perfectly the size of the pores and the cavities of the carbon scaffold, respectively. Anyhow, long-time storage of E4@C in air should be avoided due to a possible slow decomposition process.
In summary, we have shown the facile storage and release of yellow arsenic and white phosphorus in an inert and low-cost activated carbon-based porous material. Because of the stabilization of these metastable compounds in a porous framework, we succeeded in synthesizing a light-stable, storable and conveyable P4 and As4 source. This allows for example unproblematic shipping, especially of white phosphorus, which is hindered by law for small batches, and renders possible the shipping of yellow arsenic as a stabilized material. Additionally, these sources can be used directly for chemical reactions. As a result, new applications for phosphorus and arsenic chemistry are opened up for academia and industry. That way, the As4 containing material is especially regarded as a suitable arsenic source with a high potential, e.g., for new approaches in arsenic chemistry and, for instance, in semiconductor technologies e.g., for chemical vapor deposition (CVD) processes. Because of the multiple use of the carbon-based material for storage and release, it becomes an extremely useful and low-cost solid for these purposes. The uploading of other unstable molecules might create important perspectives thus representing a topic of future investigations.
Methods
General
All manipulations were performed under an atmosphere of dry nitrogen and under exclusion of oxygen and moisture using standard Schlenk and glove box techniques. All solvents were dried using conventional techniques, degassed and saturated with nitrogen prior to use.
Activation of porous carbon material
The porous carbon material (C, commercially available Norit DLC Super 50 of the Cabot Norit Nederland BV) was activated in vacuo at 140 °C before utilization.
Characterization
NMR spectroscopy: 1H, 31P{1H} and 31P NMR spectra were recorded on a Bruker Avance 400 (1H: 400.130 MHz, 31P: 161.976 MHz) or on a Bruker Avance 300 (1H: 300.132 MHz, 31P: 121.495 MHz) spectrometer. The chemical shifts are reported in ppm relative to external TMS (1H) and 85% H3PO4 (31P). The 75As NMR spectra were recorded on a Bruker Avance III HD 600 (600.13 MHz) spectrometer, equipped with a 5 mm TBI-probehead (1H, X, 19F) and (1H, X, 31P) with Z-gradient, respectively, and externally referenced to Na[AsF6]. The 31P{1H} MAS NMR spectra were recorded on a Bruker Avance 300 MHz device and referenced to external NaH2PO4 (2.3 ppm).
IR spectroscopy: The IR spectra were measured on a VARIAN FTS-800 FT-IR spectrometer.
Raman spectroscopy: The Raman spectra were recorded on a Thermo Fisher Scientific DXR-Smart-Raman spectrometer (excitation laser 532 nm; Supplementary Fig. 22).
EPR spectroscopy: The X-Band EPS spectra were recorded on a Magnettech MiniScope MS400 spectrometer with 9 GHz microwave frequency (Supplementary Fig. 23).
Elemental analysis: The elemental analysis was performed by the Catalysis Research Center of the Technical University of Munich. Phosphorus was thereby photometrically determined by the P-vanadate method. C, H and N were determined by combustion analysis. The values are seen as benchmarks since the sample was not totally stable during weighing.
Powder X-ray diffraction analysis: Powder X-ray data were collected using a XʹPert Pro diffractometer from PANalytical with CuKα1 radiation (λ = 1.5406 Å) in reflection mode from 4° to 60° 2θ.
Physisorption and DFT pore size distribution: Prior to porosity analysis, porous samples were degassed under vacuum at room temperature for 24 h. Nitrogen physisorption measurements at −196 °C were carried out on a Quadrasorb apparatus (Quantachrome Instruments) and an Autosorb 1 C (Quantachrome Instruments) for low-pressure experiments. Specific surface areas were calculated using the micropore BET assistant of the ASiQwin 3.01 software from Quantachrome to find the optimum P/P0. Total pore volumes were determined from the amount of adsorbed nitrogen at 0.95 P/P0. Pore size distributions (PSDs) were calculated using the quenched solid density functional theory method for carbon (slit/cylindrical pores, adsorption branch). Micropore volumes are estimated from the cumulative pore volumes at a diameter of 2 nm.
Thermogravimetric analysis: TG-DTA under argon atmosphere was measured with a Netzsch STA 409 PC/PG. An empty corundum crucible was used as a reference and the heating ramp was 1 K min−1.
Syntheses
Synthesis of P4@C: A solution of P4 (595 mg, 4.80 mmol) in 40 mL THF was added to activated C (975 mg) and stirred for 17 h in the dark. Afterward, the black slurry was centrifuged and the supernatant was decanted. The resulting black solid was washed with n-pentane (2 × 10 mL) and dried in vacuo. Yield: 1.46 g. For the determination of the decrease of white phosphorus, 31P NMR spectra of the supernatant before and after addition of the solution to the activated carbon were recorded (Supplementary Figs. 1 and 2). Hereby, defined sample volumes were used and a standard C6D6 capillary with defined amount of PPh3 as internal standard was utilized. 31P{1H} MAS NMR (121 MHz, 300 K): δ [ppm] = −506.4 (s, br, ω1/2 = 1582 Hz, P4); elemental analysis (%): found: C, 72.66; H, 0.93; N, 0.18; P, 20.45.
Synthesis of yellow arsenic: Solutions of yellow arsenic were prepared according to literature procedures19, 33.
Synthesis of As4@C: A freshly prepared solution of As4 (ca. 150 mg, starting from ~6–8 g gray arsenic) in 250 mL THF was filtered over diatomaceous earth and added to activated C (1.0 g). The black suspension was then stirred for ~17 h in the dark. Subsequently, the slurry was centrifuged and the supernatant was decanted. Afterward, the black solid was washed with n-pentane (1 × 30 mL) and dried in vacuo. Yield: 1.1 g.
Synthesis of [Cp*Fe(η5-P5)]: P4@C (661 mg) and [Cp*Fe(CO)2]2 (50 mg, 0.10 mmol) were refluxed in boiling decalin (30–40 mL) for 2 h. Subsequently, the solvent was removed resulting in a gray black solid. The latter was slurried in dichloromethane and filtered over diatomaceous earth and then over SiO2. The green filtrate was brought to dryness resulting in a dark green solid. The NMR chemical shifts (NMR spectra: Supplementary Figs. 12 and 13) are in very good agreement with the literature values31. Yield: 5 mg (0.014 mmol, 7%). 1H NMR (CD2Cl2, 400 MHz, 300 K): δ [ppm] = 1.43 (s, 15 H, C5(CH3)5); 31P{1H} NMR (CD2Cl2, 161 MHz, 300 K): δ [ppm] = 152.2 (s, P5); 31P NMR (CD2Cl2, 161 MHz, 300 K): δ [ppm] = 152.2 (s, P5).
Synthesis of [{CpBIGFe(CO)2}2(μ,η1:1-P4)]: A green solution of [CpBIGFe(CO)2]2 (CpBIG = C5(4-nBuC6H4)5) (100 mg, 0.06 mmol) in toluene (10 mL) was added to solid P4@C (40 mg). Immediately, the supernatant of the black suspension turned orange. The suspension was brought to dryness and slurried in dichloromethane. Then, the slurry was filtered over diatomaceous earth. The orange filtrate was brought to dryness. The analytical data are in very good agreement with the reported values (NMR spectra: Supplementary Figs. 14 and 15)32. Yield: 98 mg (0.054 mmol, 91% based on [CpBIGFe(CO)2]2). 1H NMR (C6D6, 400 MHz, 300 K): δ [ppm] = 0.80 (t, 3J(H,H) = 7.3 Hz, 30 H, CH3), 1.16 (m, 20 H, CH2), 1.35 (m, 20 H, CH2), 2.29 (t, 3J(H,H) = 7.8 Hz, 20 H, CH2), 6.72 (d, 3J(H,H) = 8.0 Hz, 20 H, C6H4), 7.32 (d, 3J(H,H) = 8.0 Hz, 20 H, C6H4); 31P NMR (C6D6, 161 MHz, 300 K): δ [ppm] = −54.1 (t, 1J(P,P) = 187 Hz, 2 P, Fe–P–P), −317.2 (t, 1J(P,P) = 187 Hz, 2 P, Fe–P–P); 31P{1H} NMR (C6D6, 161 MHz, 300 K): δ [ppm] = −54.1 (t, 1J(P,P) = 187 Hz, 2 P, Fe–P–P), −317.2 (t, 1J(P,P) = 187 Hz, 2 P, Fe–P–P); IR (toluene): \(\tilde v\) [cm−1] = 2002 (s), 1955 (s).
Synthesis of [{CpBIGFe(CO)2}2(μ,η1:1-As4)]: A green solution of [CpBIGFe(CO)2]2 (CpBIG = η5-C5(4-nBuC6H4)5) (100 mg, 0.06 mmol) in toluene (10 mL) was added to solid As4@C (400 mg). Thereby, the supernatant of the black suspension turned red-orange. The suspension was brought to dryness and slurried in dichloromethane. Then, the slurry was filtered over diatomaceous earth. The red-orange filtrate was brought to dryness. The analytical data are in very good agreement with the reported values (NMR spectra: Supplementary Fig. 16)32. Yield: 93 mg (0.047 mmol, 78% based on [CpBIGFe(CO)2]2). 1H NMR (C6D6, 400 MHz, 300 K): δ [ppm] = 0.80 (t, 3J(H,H) = 7.2 Hz, 30 H, CH3), 1.15 (m, 20 H, CH2), 1.33 (m, 20 H, CH2), 2.27 (t, 3J(H,H) = 7.8 Hz, 20 H, CH2), 6.71 (d, 3J(H,H) = 8.0 Hz, 20 H, C6H4), 7.32 (d, 3J(H,H) = 8.0 Hz, 20 H, C6H4); IR (toluene): \(\tilde v\) [cm−1] = 1993 (s), 1947 (s).
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request; see author contributions for specific data sets.
References
Krafft, F. Phosphorus. From elemental light to chemical element. Angew. Chem. Int. Ed. Engl. 8, 660–671 (1969).
Cossairt, B. M., Piro, N. A. & Cummins, C. C. Early-transition-metal-mediated activation and transformation of white phosphorus. Chem. Rev. 110, 4164–4177 (2010).
Caporali, M., Gonsalvi, L., Rossin, A. & Peruzzini, M. P4 activation by late-transition metal complexes. Chem. Rev. 110, 4178–4235 (2010).
Scheer, M., Balázs, G. & Seitz, A. P4 activation by main group elements and compounds. Chem. Rev. 110, 4236–4256 (2010).
Giffin, N. A. & Masuda, J. D. Reactivity of white phosphorus with compounds of the p-block. Coord. Chem. Rev. 255, 1342–1359 (2011).
Mal, P., Breiner, B., Rissanen, K. & Nitschke, J. R. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science 324, 1697–1699 (2009).
Yang, D. et al. Air- and light-stable P4 and As4 within an anion-coordination-based tetrahedral cage. J. Am. Chem. Soc. 139, 5946–5951 (2017).
Yang, D. et al. Correction to “air- and light-stable P4 and As4 within an anion-coordination-based tetrahedral cage”. J. Am. Chem. Soc. 139, 7130–7130 (2017).
Choi, W., Ohtsu, H., Matsushita, Y. & Kawano, M. Safe P4 reagent in a reusable porous coordination network. Dalton Trans. 45, 6357–6360 (2016).
Schwarzmaier, C. et al. Stabilization of tetrahedral P4 and As4 molecules as guests in polymeric and spherical environments. Angew. Chem. Int. Ed. 52, 10896–10899 (2013).
Krossing, I. Ag(P4)2+: the first homoleptic metal–phosphorus cation. J. Am. Chem. Soc. 123, 4603–4604 (2001).
Forfar, L. C. et al. White phosphorus as a ligand for the coinage metals. Chem. Commun. 48, 1970–1972 (2012).
Schwarzmaier, C., Sierka, M. & Scheer, M. Intact As4 tetrahedra coordinated side-on to metal cations. Angew. Chem. Int. Ed. 52, 858–861 (2013).
Schwarzmaier, C., Timoshkin, A. Y. & Scheer, M. An end-on-coordinated As4 tetrahedron. Angew. Chem. Int. Ed. 52, 7600–7603 (2013).
Spitzer, F. et al. Fixation and release of intact E4 tetrahedra (E = P, As). Angew. Chem. Int. Ed. 54, 4392–4396 (2015).
Heinl, S., Reisinger, S., Schwarzmaier, C., Bodensteiner, M. & Scheer, M. Selective functionalization of P4 by metal-mediated C-P bond formation. Angew. Chem. Int. Ed. 53, 7639–7642 (2014).
Heinl, S., Balázs, G., Stauber, A. & Scheer, M. CpPEt2As4 - an organic-substituted As4 butterfly compound. Angew. Chem. Int. Ed. 55, 15524–15527 (2016).
Bettendorff, A. Allotropische zustände des arsens. Justus. Liebigs. Ann. Chem. 144, 110–114 (1867).
Erdmann, H. & Unruh, M. V. VI. Über gelbes arsen. Z. Anorg. Chem. 32, 437–452 (1902).
Spinney, H. A., Piro, N. A. & Cummins, C. C. Triple-bond reactivity of an AsP complex intermediate: synthesis stemming from molecular arsenic, As4. J. Am. Chem. Soc. 131, 16233–16243 (2009).
Mudrakovskii, I. L., Shmachkova, V. P., Kotsarenko, N. S. & Mastikhin, V. M. 31P nmr study of I–IV group polycrystalline phosphates. J. Phys. Chem. Solids 47, 335–339 (1986).
Siebert, H., Eints, J. & Fluck, E. Kernresonanzspektrum und Struktur des Trisilylphosphins. Z. Nat. B 23, 1006 (1968).
Heckmann, G. & Fluck, E. 31P-Kernresonanzdaten von weißem phosphor und Lösungen des weißen phosphors. Z. Nat. B 24, 1092–1094 (1969).
Oschatz, M. et al. in Annual Reports on NMR Spectroscopy Vol. 87 (ed. Webb Graham, A.) 237–318 (Academic Press, Oxford, 2016).
Borchardt, L., Oschatz, M., Paasch, S., Kaskel, S. & Brunner, E. Interaction of electrolyte molecules with carbon materials of well-defined porosity: characterization by solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 15, 15177–15184 (2013).
Heckmann, G. & Fluck, E. 31P nuclear magnetic resonance chemical shifts of elemental phosphorus in the gas phase. Mol. Phys. 23, 175–183 (1972).
Effenberger, B. et al. Determination of the spectroscopic quadrupole moments of 3375As and 2963Cu. Z. Phys. A 309, 77–81 (1982).
Schenk, P. W. in Handbook of Preparative Inorganic Chemistry 2nd edn (ed. Brauer, G.) 591–629 (Academic Press, New York, 1963).
Maxwell, L. R., Hendricks, S. B. & Mosley, V. M. Electron diffraction by gases. J. Chem. Phys. 3, 699–709 (1935).
Bondi, A. van der Waals volumes and radii. J. Chem. Phys. 68, 441–451 (1964).
Scherer, O. J. & Brück, T. [(η5-P5)Fe(η5-C5Me5)], a pentaphosphaferrocene derivative. Angew. Chem. Int. Ed. Engl. 26, 59 (1987).
Heinl, S. & Scheer, M. Activation of group 15 based cage compounds by [CpBIGFe(CO)2] radicals. Chem. Sci. 5, 3221–3225 (2014).
Scherer, O. J., Sitzmann, H. & Wolmershäuser, G. (E2)2-Einheiten (E = P, As) als clusterbausteine. J. Organomet. Chem. 309, 77–86 (1986).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG). A.E.S. is grateful for a PhD fellowship of the Fonds der Chemischen Industrie. The results are also part of the doctoral thesis of A.E.S., University of Regensburg, 2017. D. Fiedler (powder X-ray diffraction analysis of sublimed gray solid), J. Rewitzer (ICP-OES measurements), F. Hastreiter (measurement of the 75As NMR spectra), the Catalysis Research Center of the Technical University of Munich (elemental analysis) and M. Modl (supply of [CpBIGFe(CO)2]2) are thankfully acknowledged.
Author information
Authors and Affiliations
Contributions
Syntheses and characterizations of E4@C were performed by A.E.S., except the powder X-ray diffraction analyses, thermogravimetric analyses as well as the investigations of the nitrogen adsorption measurement at liquid nitrogen temperature and the DFT pore size distribution of E4@C (F.H.). 31P{1H} MAS NMR spectra were recorded by W.K. Experiments on the release of E4, reactivity studies of E4@C, and the studies on the air stability of E4@C were performed by A.E.S. Figures and schemes were made by A.E.S., except the figures of the powder X-ray diffraction analyses, thermogravimetric analyses as well as the investigations of the nitrogen adsorption measurement at liquid nitrogen temperature and the DFT pore size distribution (F.H.). S.K. and M.S. originated the central idea, supervised the work, analyzed the data, and A.E.S. and M.S. wrote the manuscript with contributions from all the co-authors.
Corresponding author
Ethics declarations
Competing interests
This publication concerns the patent: EP 17 163 405.8 from 28.03.2017 filed by A.E.S., F.H., S.K. and M.S. The remaining author declares no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seitz, A.E., Hippauf, F., Kremer, W. et al. Facile storage and release of white phosphorus and yellow arsenic. Nat Commun 9, 361 (2018). https://doi.org/10.1038/s41467-017-02735-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-02735-2
This article is cited by
-
φ-Aromaticity in prismatic {Bi6}-based clusters
Nature Chemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.