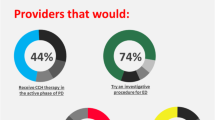
Abstract
Despite a well-documented increase in both the prevalence of Testosterone Deficiency (TD) and prescription of testosterone replacement therapy (TRT), few studies have investigated the preferences of patients receiving TRT and factors associated with increased treatment satisfaction. To investigate the preferences of patients receiving TRT and factors associated with improved treatment satisfaction, an open survey was completed by 140 men receiving TRT at a single institution. Survey questions investigated demographics, symptom burden of TD, TRT regimen, treatment preferences, and treatment satisfaction. 62.7% of patients were satisfied with their current TRT regimen. Those using auto-injectors (91.7%, odds ration [OR] = 9.3), subcutaneous pellets (90.0%, OR = 15.2), and intramuscular injections (67.5%, OR = 5.7), were with significantly increased satisfaction rates (p < 0.05). The majority of patients indicated that they would prefer to receive TRT injections when self-administered or administered at home. While patients noted that treatment efficacy was a significant driving factor when evaluating a TRT regimen, few patients felt that cost was the most significant factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Additional data are available from the corresponding author upon reasonable request.
References
Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA. et al. Evaluation and management of testosterone deficiency: AUA guideline. J Urol. 2018;200:423–32. https://doi.org/10.1016/j.juro.2018.03.115.
Tsametis CP, Isidori AM. Testosterone replacement therapy: for whom, when and how?. Metabolism. 2018;86:69–78. https://doi.org/10.1016/j.metabol.2018.03.007.
Jia H. Review of health risks of low testosterone and testosterone administration. World J Clin Cases. 2015;3:338. https://doi.org/10.12998/wjcc.v3.i4.338.
Kaltenboeck A, Foster S, Ivanova J, Diener M, Bergman R, Birnbaum H. et al. The direct and indirect costs among U.S. privately insured employees with hypogonadism. J Sex Med. 2012;9:2438–47. https://doi.org/10.1111/j.1743-6109.2012.02810.x.
Zarotsky V, Huang M-Y, Carman W, Morgentaler A, Singhal PK, Coffin D. et al. Systematic literature review of the risk factors, comorbidities, and consequences of hypogonadism in men. Andrology. 2014;2:819–34. https://doi.org/10.1111/andr.274.
Wu FCW, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD. et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. https://doi.org/10.1056/nejmoa0911101.
Anaissie J, DeLay KJ, Wang W, Hatzichristodoulou G, Hellstrom WJ. Testosterone deficiency in adults and corresponding treatment patterns across the globe. Transl Androl Urol. 2017;6:183–91. https://doi.org/10.21037/tau.2016.11.16.
Brawer MK. Testosterone replacement in men with andropause: an overview. Rev Urol. 2004;6:S9–15.
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR.Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2010;86:724–31. https://doi.org/10.1210/jcem.86.2.7219.
Layton JB, Li D, Meier CR, Sharpless JL, Stürmer T, Jick SS. et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000 to 2011. J Clin Endocrinol Metab. 2014;99:835–42. https://doi.org/10.1210/jc.2013-3570.
Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–6. https://doi.org/10.1001/jamainternmed.2013.6895.
Kovac JR, Rajanahally S, Smith RP, Coward RM, Lamb DJ, Lipshultz LI. Patient satisfaction with testosterone replacement therapies: the reasons behind the choices. J Sex Med. 2014;11:553–62. https://doi.org/10.1111/jsm.12369.
Zitzmann M, Mattern A, Hanisch J, Gooren L, Jones H, Maggi M. IPASS: a study on the tolerability and effectiveness of injectable testosterone undecanoate for the treatment of male hypogonadism in a worldwide sample of 1,438 men. J Sex Med. 2013;10:579–88. https://doi.org/10.1111/j.1743-6109.2012.02853.
Lee J, Brock G, Barkin J, Bryson N, Gronski MA, Ormsby R. The My-T study: patient satisfaction and preference comparing topical and nasal testosterone therapies. Can Urol Assoc J. 2019;13:384–9. https://doi.org/10.5489/cuaj.5680.
Schoenfeld MJ, Shortridge E, Cui Z, Muram D. Medication adherence and treatment patterns for hypogonadal patients treated with topical testosterone therapy: a retrospective medical claims analysis. J Sex Med. 2013;10:1401–9. https://doi.org/10.1111/jsm.12114.
Kang B, Noh M, Park HJ. Compliance with testosterone replacement therapy in patients with testosterone deficiency syndrome: a 10-year observational study in Korea. World J Mens Health. 2021;40:686–92. https://doi.org/10.5534/wjmh.200174.
Mohamed O, Freundlich RE, Dakik HK, Grober ED, Najari B, Lipshultz LI. et al. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Imp Res. 2010;22:20–24. https://doi.org/10.1038/ijir.2009.35.
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Imp Res. 1999;11:319–26. https://doi.org/10.1038/sj.ijir.3900472.
StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC.
Piecuch MJ, Patel BG, Hakim L, Wang R, Sadeghi-Nejad H. Testosterone pellet implantation practices: a sexual medicine society of North America (SMSNA) member questionnaire. J Sex Med. 2017;14:47–49. https://doi.org/10.1016/j.jsxm.2016.11.305.
Smith RP, Khanna A, Coward RM, Rajanahally S, Kovac JR, Gonzales MA. et al. Factors influencing patient decisions to initiate and discontinue subcutaneous testosterone pellets (Testopel) for treatment of hypogonadism. J Sex Med. 2013;10:2326–33. https://doi.org/10.1111/jsm.12226.
Author information
Authors and Affiliations
Contributions
The following authors were involved in the conception of the study (CL and FY), data collection (CL, JM, DS, LG, and MHM), data analysis and interpretation (JM and MHM), manuscript drafting (JM), manuscript revising (CL, JM, DS, LG, MHM, and FY), and approval of the final manuscript (CL, JM, DS, LG, MHM, and FY).
Corresponding author
Ethics declarations
Competing interests
FAY work has received funding from Viome. He has additionally received compensation as a member of the advisory board of Coloplast, Sprout, Cynosure, and Promescent, and has served as a speaker for Antares Pharma and Clarus Therapeutics. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loeb, C., Miller, J.A., Schneider, D. et al. Testosterone replacement therapy is associated with high satisfaction rates: results of a survey study. Int J Impot Res (2023). https://doi.org/10.1038/s41443-023-00724-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41443-023-00724-2