Abstract
Smoking during pregnancy is a risk factor for various adverse birth outcomes but lowers the risk of preeclampsia. Cardiovascular adaptations might underlie these associations. We examined the association of maternal smoking with the risk of hypertensive disorders of pregnancy (HDP) in a low-risk population-based cohort of 76,303 pregnant women. This study was a part of the Japan Environment and Children’s Study. Smoking status was assessed using questionnaires completed by participants. Information about HDP was assessed using questionnaires completed by doctors. Compared with that for women who did not smoke, women who continued smoking >10 cigarettes per day during pregnancy had a significantly higher risk of developing HDP (odds ratio: 1.58, 95% confidence interval: 1.11–2.25). In multivariate analyses with adjustment for possible confounding factors, the association still remained (odds ratio: 1.51, 95% confidence interval: 1.04–2.19). When we regarded the number of cigarettes as a continuous variable, there was a linear association between the number of cigarettes and risk of HDP, with an odds ratio of 1.02 per cigarette per day (95% confidence interval: 1.00–1.04). Smoking a greater number of cigarettes was associated with a higher risk of HDP after adjustment for possible confounding factors. Cigarette smoking cessation may avoid the complications of HDP. Our findings suggest that, in addition to the risk of small-for-gestational-age children, an increased risk of HDP should be considered in the management of pregnant women who smoke cigarettes.
Similar content being viewed by others
Introduction
Hypertensive disorders of pregnancy (HDP), which are observed in ~ 5–10% of pregnant women, are associated with poor maternal and neonatal prognoses owing to premature delivery, stillbirth, impaired fetal growth, and maternal death [1]. HDP has specific risk factors, including a first pregnancy; higher body mass index (BMI), or age; and pre-existing dyslipidemia, diabetes mellitus, or renal diseases [2, 3]. Several guidelines, including those of the International Society for the Study of Hypertension in Pregnancy (ISSHP) and Japan Society for the Study of Hypertension in Pregnancy (JSSHP), define HDP as hypertension (blood pressure ≥ 140/90 mmHg) with or without proteinuria ( ≥ 300 mg/24 h) between 20 weeks gestation and 12 weeks postpartum [4, 5]. HDP is also classified into several subtypes: chronic hypertension, gestational hypertension, and preeclampsia, de novo or superimposed on chronic hypertension. Although the cause of preeclampsia is unknown, current hypotheses postulate a placental pathogenesis. Abnormal placentation leading to preeclampsia is marked by the failure of trophoblasts to induce physiologic dilatation and remodeling of spiral arteries, resulting in reduced placental blood flow [6].
Smoking during pregnancy is one of the most important risk factors for various adverse birth outcomes, including low birth weight and preterm birth [7], but may also be associated with a lower risk of preeclampsia [8, 9]. However, data from the Generation R study indicated that there is no significant association between smoking and preeclampsia, whereas there is a significant association between smoking and high blood pressure [10]. Although Mattsson et al. [11] reported a possible weak positive association between heavy intrauterine smoking exposure and the risk of subsequent late-onset preeclampsia, the relationship was not significant after adjustment for confounders.
The above-mentioned studies were conducted in the United States and Europe. Chang et al. [12] reported that there may be differences in the relationship between cigarette use during pregnancy and HDP according to maternal race/ethnicity. Hayashi et al. [13] showed that smokers during pregnancy had a higher risk of HDP using data from the Japan Perinatal Registry Network Database; however, the authors only adjusted for maternal age at delivery. Furthermore, HDP was not subclassified (i.e., preeclampsia, gestational hypertension) in these two studies. Consequently, there is little information regarding the relationship between maternal smoking and HDP in Japan.
The Japanese Ministry of the Environment launched the Japan Environment and Children’s Study (JECS), a large-scale epidemiological investigation, in January 2011 [14]. The study plan involves inviting ~ 100,000 pregnant women and their partners to participate over a period of 3 years, collecting biological samples, and subsequently collecting data on their children until they reach 13 years of age. Using JECS data, Suzuki et al. reported an association between maternal smoking and birth weight [7]. In the present study, we analyzed JECS data to elucidate a possible link between smoking habits and HDP risk.
Methods
Study design
This study was part of the JECS, an ongoing nationwide birth cohort study. The JECS was approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies on 6 April 2010 (no. 100406001). Written informed consent was obtained from all participants. JECS recruitment was performed between January 2011 and March 2014. Participants were recruited through 15 regional centers located in Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu, and Okinawa. The JECS national center has released several data sets, allowing research groups to examine their own research theme. The present analysis is based on the all-birth fixed data set, “jecs-ag-20160424” and “allbirth_revice001_ver001”, which was released in June 2016 and October 2016, respectively. These data sets include information on 104,102 fetuses and their parents.
Data collection
This data set included data obtained using the MT1 questionnaire, which was completed on enrollment. For the second and third trimester, data were gathered using the MT2 questionnaire. Mean (standard deviation) gestational age at the time of the completion of the MT1 and MT2 questionnaires was 16.4 (8.0) and 27.9 (6.5) weeks gestation, respectively. We obtained during-pregnancy information, as well as the weight before pregnancy, from these two self-reported questionnaires (MT1 and MT2). As far as possible, incomplete questionnaires were filled by face-to-face or telephone interviews.
This data set also included data obtained using the Dr0m questionnaire, which was designed to gather data on the outcomes of the pregnancy and offspring, such as the maternal age and weight at delivery, complications during pregnancy (HDP, gestational diabetes mellitus (GDM), preterm birth, etc.), anomalies of the offspring, birth weight, and length of gestation. The Dr0m questionnaire was obtained from medical records after the delivery by cooperating health care providers or research coordinators from each unit center; the medical records were transcribed by instructed physicians, midwives/nurses, and/or research coordinators.
Table 1 provides the baseline characteristics. We obtained data at enrollment, particularly parity, BMI (kg/m2), smoking, and drinking status of participants and their partners, K6 score, and complications before pregnancy (i.e., hypertension, renal disease, dyslipidemia, and diabetes mellitus) from the MT1 questionnaire; and socioeconomic status, such as family income and educational level of participants and their partners from the MT2 questionnaire. The K6 has been widely used as a screening scale for psychological distress in the general population [15]. The Japanese version of the K6 was recently developed using the standard back-translation method [16]. The details of the K6 have been described previously [17].
Participants and analyzed subjects
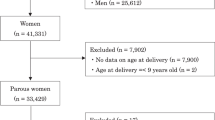
Of 104,102 records in the jecs-ag-20160424 data set, we excluded women who were entered into the JECS twice or more (n = 5687), or had missing information regarding smoking status (n = 5850) and baseline characteristics (such as the information in Table 1) (n = 12,104). Since our main interest was in low-risk pregnancies, we subsequently excluded women with multiple pregnancy (n = 1590) or miscarriage or stillbirth (n = 121). Finally, we also excluded women with specified comorbidities (n = 2447), such as diagnosed hypertension (n = 363), renal diseases (n = 1581), diabetes mellitus (n = 163), and dyslipidemia (n = 403), with some overlap between conditions in single participants. The records of the remaining 76,303 women were analyzed (Fig. 1).
Smoking during pregnancy
Information on smoking was obtained by self-administered MT1 questionnaires. Smoking at enrollment was assessed in the questionnaire by asking each woman whether she smoked before and during pregnancy (categories: no smoking, quit before pregnancy, quit during pregnancy, and continued smoking). Women who smoked were asked to complete a questionnaire regarding the average number of cigarettes smoked per day. We classified the number of cigarettes into three categories ( ≤ 5 cigarettes/day, 6–10 cigarettes/day, and > 10 cigarettes/day).
HDP
Information on HDP was obtained from the Dr0m questionnaire. This questionnaire only revealed whether the participant was diagnosed with HDP or not; HDP was not classified (i.e., gestational hypertension, preeclampsia, superimposed preeclampsia or eclampsia). In this study, we analyzed the incidence of HDP without a previous history of hypertension before pregnancy.
HDP was defined as hypertension (blood pressure ≥ 140/90 mmHg) with or without proteinuria ( ≥ 300 mg/24 h) emerging after 20 weeks gestation but resolving up to 12 weeks postpartum [5].
Statistical analysis
Baseline characteristics were evaluated based on percentage, mean, and standard deviation. These characteristics were compared between groups using a chi-squared test for categorical variables or Kruskal–Wallis for continuous variables. We performed crude analyses using univariate logistic regression analyses. We also performed multivariate logistic regression analyses with adjustment for other risk factors for HDP, such as age, parity, BMI before pregnancy, family income, maternal educational level, drinking status, and K6 score (model 1). Model 2 adjusted for a history of HDP and GDM in addition to the factors in model 1 (model 2). Model 3 further adjusted for the partner’s smoking habits and educational level. When evaluating the effect of the number of cigarettes smoked on the risk of HDP, the composite of never-smokers and participants who quit smoking before or after becoming pregnant was set as the reference in the crude and multivariate analyses. SAS software (version 9.4, SAS Institute Inc., Cary, NC) was used for statistical analysis.
Results
The baseline characteristics of the pregnant women are shown in Table 1. The numbers of women in the no smoking, quit before pregnancy, quit during pregnancy, and continued smoking groups were 44,998 (59.0%), 17,992 (23.6%), 9899 (13.0%), and 3414 (4.5%), respectively. Among all pregnant women in this study, those who continued smoking were less educated and had a smaller income. The prevalence of pregnant women with HDP in these groups was as follows: no smoking, 2.8%; quit before pregnancy 3.0%; quit during pregnancy, 3.3%; and continued smoking, 3.4% (p = 0.04).
The results of the univariate analyses for covariates are shown in Table 2. Compared with that for never-smokers, pregnant women who quit during pregnancy and those who continued smoking had a higher risk of developing HDP (OR: 1.16, 95% CI: 1.02–1.31; and OR: 1.21, 95% CI: 1.00–1.47, respectively). In the multivariate analysis with adjustment for baseline characteristics including age, parity, BMI before pregnancy, family income, maternal educational level, drinking status, K6 score, history of HDP and GDM, and partner’s smoking habits and drinking status, the association between smoking status and HDP was diminished (OR: 1.08, 95% CI: 0.94–1.24; OR: 1.17, 95 % CI: 0.95–1.45, respectively).
Next, we compared the HDP risk between women who continued smoking, classified by the number of cigarettes smoked daily (categories: ≤ 5 cigarettes/day, 6–10 cigarettes/day, > 10 cigarettes/day), and the other groups (no smoking, quit before pregnancy, and quit during pregnancy) (Table 3). In the univariate analysis, women who continued smoking <10 cigarettes per day had a significantly higher risk of developing HDP (OR: 1.58, 95% CI: 1.11–2.25) than did women who did not continue to smoke. In the multivariate analyses with adjustment for maternal confounding factors (model 1), previous history of GDM and HDP (model 2), and partner’s factors (model 3), the association still remained (model 1, OR: 1.55, 95% CI: 1.08–2.23; model 2, OR: 1.54, 95% CI: 1.07–2.23; and model 3, OR: 1.51, 95% CI: 1.04–2.19). When we regarded the number of cigarettes as a continuous variable, there was a significant linear association between the number of cigarettes and risk of HDP, with an OR of 1.02 per cigarette per day (95% CI: 1.00–1.04). After repeating these analyses with adjustment for miscarriage, abortions, and women with diagnosed complications (hypertension, renal diseases, diabetes mellitus, and dyslipidemia), we found similar results (data not shown). The results of analyses evaluating the association between the number of cigarettes and risk of HDP stratified according to body mass index (Supplementary Table 1), parity, (Supplementary Table 2), high age at delivery (Supplementary Table 3), and a history of HDP (Supplementary Table 4) are shown in the Supplementary File.
Discussion
Our nationwide prospective cohort study showed that smoking >10 cigarettes per day is associated with a significantly higher risk of developing HDP in Japan. This finding in Japan differs from those in previous studies conducted in Europe and the United States. Prior studies have observed no significant relationship or a decreased risk of both preeclampsia and gestational hypertension among women who smoked during pregnancy compared with those who did not smoke during pregnancy in Europe and the United States [3, 18]. Furthermore, Lindqvist et al. [19] reported that moderate smoking during pregnancy seemed to protect against the development of preeclampsia in Sweden. In contrast, the Generation R study indicated that there was no significant association between smoking and preeclampsia in the Netherlands [10].
The association between cigarette use during pregnancy and HDP might differ according to maternal race/ethnicity. Chang et al. [12] reported that non-Hispanic Asian/Pacific Islander women who smoked during pregnancy had increased risk of HDP, whereas a reduced risk of HDP among pregnant smokers was evident for non-Hispanic white and non-Hispanic American Indian women. A retrospective case-cohort study in Japan showed that maternal smoking during pregnancy was associated with HDP [13]. One hypothesis is that genetic variations among race/ethnicity might underlie the observed differences in the relationship between smoking and HDP. Recently, novel and original variations in single-nucleotide polymorphisms were identified in Japanese subjects [20]. As cigarette smoking and hypertension are reported to be moderated by CYP2A6 genotypes [21, 22], a genotype analysis might reveal additional confounding factors and may clarify the mechanism underlying the observed differences in the relationship between smoking and HDP according to maternal race/ethnicity. However, the genetic factors underlying this association remain unclear and further investigations are required.
Cigarette smoking is associated with both lower maternal soluble fms-like tyrosine kinase 1 (sFlt-1) concentrations during pregnancy and preeclampsia [23]. sFlt-1 is an antagonist of vascular endothelial growth factor and placental growth factor, and high levels of sFlt-1 have been observed in women with preeclampsia [24]. Chen et al. [25] reported that the endothelial nitric oxide (NO) system might be damaged in women with preeclampsia, although NO is known as a vasodilating factor [26]. The authors isolated and cultured human umbilical vein endothelial cells from normal and preeclamptic pregnancies. Compared with that for control cells, NO production was markedly decreased in preeclamptic cells. There are several hypotheses regarding the association between smoking and preterm birth, which could overlap the mechanisms for hypertension during pregnancy. Llurba et al. [27] reported that although smoking during pregnancy increases the placental growth factor/sFlt-1 ratio at 24 weeks of gestation, intrauterine growth restriction is significantly higher in smokers. Although smoking during pregnancy might induce less preeclampsia with the inhibition of a reasonable reaction against fetal ischemia, as in non-pregnant settings, endothelial dysfunction, macrophage recruitment, and arterial inflammation might increase the blood pressure in pregnant women. Furthermore, a higher incidence of preterm birth among women who smoke during pregnancy might terminate the pregnancy before the onset of proteinuria. Further investigations on the mechanisms of the association between cigarette smoking and the risk of hypertension or HDP are required.
There are some limitations to the present study. First, we could not classify HDP as gestational hypertension, preeclampsia, and other specific conditions owing to the questionnaires employed in this study. Therefore, we could not assess the effect of cigarette smoking during pregnancy on each type of HDP. Goel et al. [28] suggested that postpartum hypertension may represent a group of women with subclinical or unresolved preeclampsia. We could not consider this possibility because of the utilized questionnaires. Second, for clinical settings, biochemical and physiological data are important for understanding the relationship between smoking exposure and maternal outcomes. The JECS study is biobanking serum samples collected during pregnancy. In short, the analysis of such data will play a key role in understanding the causal associations. Third, although we adjusted for the possible confounding factors available from the questionnaires, unmeasured confounding factors might also affect the risk of HDP. Fourth, information about smoking during pregnancy was collected by questionnaires. Under-reporting of maternal smoking across the various smoking categories may be present and lead to misclassification. Fifth, we could not examine the genetic background of participants.
Conclusion
Smoking a greater number of cigarettes was associated with a higher risk of HDP after adjustment for possible confounding factors. Cigarette smoking cessation may avoid the complications of HDP. Our findings suggest that, in addition to the risk of small-for-gestational-age newborns, an increased risk of HDP should be considered in the management of pregnant women who smoke cigarettes.
References
Zhang J, Zeisler J, Hatch MC, Berkowitz G. Epidemiology of pregnancy-induced hypertension. Epidemiol Rev. 1997;19:218–32.
Kaaja R. Predictors and risk factors of preeclampsia. Minerva Ginecol. 2008;60:421–9.
Magnussen EB, Vatten LJ, Lund-Nilsen TI, Salvesen KA, Davey Smith G, Romundstad PR. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;10:978.
Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:9–14.
Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y. Outline of definition and classification of “pregnancy induced hypertension (PIH)”. Hypertens Res Pregnancy. 2013;1:3–4.
Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi JM. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. 2011;7:467–74.
Suzuki K, Shinohara R, Sato M, Otawa S, Yamagata Z. Association between maternal smoking during pregnancy and birth weight: an appropriately adjusted model from the Japan Environment and Children’s Study. J Epidemiol. 2016;26:371–7.
Wikström AK, Stephansson O, Cnattingius S. Tobacco use during pregnancy and preeclampsia risk effects of cigarette smoking and snuff. Hypertension. 2010;55:1254–9.
Ness RB, Zhang J, Bass D, Klebanoff MA. Interactions between smoking and weight in pregnancies complicated by preeclampsia and small-for-gestational-age birth. Am J Epidemiol. 2008;168:427–33.
Bakker R, Steegers EA, Mackenbach JP, Hofman A, Jaddoe VW. Maternal smoking and blood pressure in different trimesters of pregnancy: the Generation R Study. J Hypertens. 2010;28:2210–8.
Mattsson K, Källén K, Rignell-Hydbom A, Hansson SR, McElrath TF, Cantonwine DE et al. Maternal smoking during pregnancy and daughters’ preeclampsia risk. PLoS ONE. 2015;10:e0144207.
Chang JJ, Strauss JF 3rd, Deshazo JP, Rigby FB, Chelmow DP, Macones GA. Reassessing the impact of smoking on preeclampsia/eclampsia: are there age and racial difference? PLoS ONE. 2014;9:e106446.
Hayashi K, Matsuda Y, Kawamichi Y, Shiozaki A, Saito S. Smoking during pregnancy increases risks of various obstetric complications: a case-cohort study of the Japan Perinatal Registry Network Database. J Epidemiol. 2011;21:61–66.
Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M et al. Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health. 2014;14:25.
Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL et al. Short screening scales to monitor population prevalence and trends in non-specific psychological distress. Psychol Med. 2002;32:959–76.
Furukawa TA, Kawakami N, Saito M, Ono Y, Nakane Y, Nakamura Y et al. The performance of the Japanese version of the K6 and K10 in the World Mental Health Survey Japan. Int J Methods Psychiatr Res. 2008;17:152–8.
Watanabe Z, Iwama N, Nishikori H, Nishikori T, Mizuno S, Sakurai K et al. Psychological distress during pregnancy in Miyagi after the Great East Japan Earthquake: The Japan Environment and Children’s Study. J Affect Disord. 2016;190:341–8.
Aliyu MH, Lynch O, Wilson RE, Alio AP, Kristensen S, Marty PJ et al. Association between tobacco use in pregnancy and placenta-associated syndromes: a population-based study. Arch Gynecol Obstet. 2011;283:729–34.
Lindqvist PG, Marsál K. Moderate smoking during pregnancy is associated with a reduced risk of preeclampsia. Acta Obstet Gynecol Scand. 1999;78:693–7.
Kawai Y, Mimori T, Kojima K, Nariai N, Danjoh I, Saito R et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J Hum Genet. 2015;60:581–7.
Liu T, Tyndale RF, David SP, Wang H, Yu XQ, Chen W et al. Association between daily cigarette consumption and hypertension moderated by CYP2A6 genotypes in Chinese male current smokers. J Hum Hypertens. 2013;27:24–30.
Loukola A, Buchwald J, Gupta R, Palviainen T, Hällfors J, Tikkanen E et al. A genome-wide association study of a biomarker of nicotine metabolism. PLoS Genet. 2015;11:e1005498.
Jeyabalan A, Powers RW, Durica AR, Harger GF, Roverts JM, Ness RB. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertes. 2008;21:943–7.
Genbacev O, DiFederico E, McMaster M, Fisher SJ. Invasive cytotrophoblast apoptosis in pre-eclampsia. Hum Reprod. 1999;14:59–66.
Chen J, Gao Q, Jiang L, Feng X, Zhu X, Fan X et al. the NOX2-derived reactive oxygen species damaged endothelial nitric oxide system via suppressed BKCa/SKCa in preeclampsia. Hypertens Res. 2017;40:457–64.
Weiner CP, Thompson LP. Nitric oxide and pregnancy. Semin Perinatol. 1997;21:367–80.
Llurba E, Sánchez O, Domínguez C, Soro G, Goya M, Alijotas-Reig J et al. Smoking during pregnancy: changes in mid-gestation angiogenic factors in women at risk of developing preeclampsia according to uterine artery Doppler findings. Hypertens Pregnancy. 2013;32:50–59.
Goel A, Maski MR, Bajracharya S, Wenger JB, Zhang D, Salahuddin S et al. Epidemiology and mechanisms of de novo and persistent hypertension in the postpartum period. Circulation. 2015;132:1726–33.
Acknowledgements
Members of the Japan Environment and Children’s Study (JECS) as of 2016 (principal investigator, Toshihiro Kawamoto): Hirohisa Saito (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Michihiro Kamijima (Nagoya City University, Nagoya, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Yasuaki Hirooka (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan). Grants for Scientific Research (No. 16H05243) from the Ministry of Japan Grants from the Astellas Research Support, the Pfizer Academic Contributions, and Chugai Pharmaceutical Co., Ltd. This research was also supported by Research Promotion and Practical Use for Women’s Health, AMED. We thank Editage (www.editage.jp) for English language editing. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the government.
Funding information
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. This study is partly supported by Grants for Scientific Research (16H05243) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, K., Nishigori, H., Watanabe, Z. et al. Higher prevalence of hypertensive disorders of pregnancy in women who smoke: the Japan environment and children’s study. Hypertens Res 42, 558–566 (2019). https://doi.org/10.1038/s41440-019-0206-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0206-x
Keywords
This article is cited by
-
Hypertensive disorders of pregnancy: definition, management, and out-of-office blood pressure measurement
Hypertension Research (2022)
-
Acute myocardial infarction and stoke after the enactment of smoke-free legislation in public places in Bibai city: data analysis of hospital admissions and ambulance transports
Hypertension Research (2019)