Abstract
Intrarenal renin–angiotensin system (RAS) activation plays an important role in the development of hypertension and renal damage. However, the association between daytime and night-time intrarenal RAS activation and renal structural damage in normotensive IgA nephropathy patients is unclear. We investigated the relationships between urinary angiotensinogen (U-AGT) excretion, which reflects intrarenal RAS activity, and renal structural damage (i.e., endocapillary and mesangial cell hypercellularity, arteriolar hyalinosis and arteriosclerosis levels, and global glomerulosclerosis and tubulointerstitial fibrosis percentages) in 27 normotensive IgA nephropathy patients (age 39.2 ± 13.6 years, 10 men and 17 women, estimated glomerular filtration rate (eGFR) 74.0 ± 17.3 ml/min/1.73 m2, urinary protein excretion 0.58 ± 0.50 g/day, and U-AGT excretion 64.9 ± 100.6 μg/day). The arteriosclerosis level had a significant positive association with the daytime and night-time U-AGT excretion levels. By contrast, the endocapillary and mesangial cell hypercellularity and arteriolar hyalinosis levels and global glomerulosclerosis and tubulointerstitial fibrosis percentages did not correlate with the daytime and night-time U-AGT excretion levels. The daytime and night-time U-AGT excretion levels were higher in patients with arteriosclerotic changes than in patients without these changes. Multiple linear regression analysis revealed that the arteriosclerosis levels had a significant positive association with the U-AGT excretion levels at night after adjusting for age, sex, body mass index, and the eGFR. However, when diastolic BP was added as an independent variable, the relationship between U-AGT excretion and arteriosclerosis at night disappeared. In normotensive IgA nephropathy patients, intrarenal RAS activation at night due to nocturnal hypertension may be associated with arteriosclerosis.
Similar content being viewed by others
Introduction
The circulating renin–angiotensin system (RAS) has a circadian rhythm and plays an important role in blood pressure (BP) and sodium homeostasis [1, 2]. Moreover, activation of intrarenal RAS plays a critical role in the pathophysiology of renal damage in some animal models and in patients with chronic kidney disease (CKD) or hypertension independent of circulating RAS [3,4,5,6,7,8,9,10,11]. However, few studies have investigated the pathological damages of all components in the kidney, including the glomerulus, tubulointerstitium and small arteries, at the same time in the animal models. In addition, renal damage is evaluated using urinary protein and/or albumin excretion as a surrogate marker in most clinical studies to examine the relationship between renal damage and intrarenal RAS activation [10, 11]. Even in the limited clinical studies that have evaluated renal damage using renal pathological findings, all components in the kidney have not been investigated similutaneously [12, 13].
Recently, we clarified that aggravation of intrarenal RAS activation led to renal damage and hypertension at night [14, 15]. However, the relationships between intrarenal RAS activation in each phase (daytime or night-time) and pathological damage of all components in the kidney are unknown.
IgA nephropathy is the most common type of chronic and progressive glomerular disease and is most frequently diagnosed by renal biopsy worldwide [16]. Intrarenal RAS is activated in IgA nephropathy patients [12, 13]. Moreover, Wu et al. [17] reported that IgA nephropathy patients with intrarenal arteriosclerosis and arteriolar hyalinosis but not patients without intrarenal arterial lesions were associated with hypertension.
The association between intrarenal RAS activation at each different time point and renal structural damages, including damage to the glomerulus, tubulointerstitium and small arteries, has not been clarified in clinically ill patients without the influence of hypertension. Therefore, this study aimed to clarify the associations in patients with normotensive IgA nephropathy, which is one of the most common chronic glomerular diseases.
Materials and methods
Patients
This study was approved by the ethics committee of Hamamatsu University School of Medicine (No. 24-171) and adhered to the principles of the Declaration of Helsinki. We recruited 27 consecutive normotensive patients who were admitted to our hospital for renal biopsies and diagnosed with IgA nephropathy from February 2012 to November 2016. Written informed consent was obtained from all patients. We excluded patients with factors associated with renal structural damage (e.g., diabetes mellitus, hypertension, or taking antihypertensives) [17, 18].
Study protocols
We collected urine during the daytime (6.00 a.m. to 9.00 p.m.) and night-time (9.00 p.m. to 6.00 a.m.). Ambulatory BP monitoring (ABPM) was conducted at 30-min intervals during the day and night using an automatic device (TM-2431; A and D, Tokyo, Japan). We divided the daytime and night-time data for the 24-h ABPM using the sleeping and waking times recorded in the patients’ behavior records. Blood samples were also drawn at 9.00 p.m. and 6.00 a.m. the next day after the patients rested in a supine position for at least 15 min. The blood samples drawn at 9.00 p.m. and 6.00 a.m. were considered the samples at the ends of the daytime and night-time period, as described previously [14, 19,20,21].
Clinical data
The patients’ clinical data, including age, sex, and body mass index (BMI), were recorded at the time of admission. During the 24-h ABPM, the BP was measured noninvasively every 30 min as described above. Daytime BPs were calculated as the averages of the readings taken during waking hours, whereas night-time BPs were the averages of the remaining values. Circadian BP rhythms are classified as either extreme dipper, dipper, nondipper, or riser patterns when the night-to-day ratio of systolic BP is <0.80, 0.80 to <0.90, 0.90–1.00, and >1.00, respectively [22]. The serum creatinine and the urinary creatinine and protein (U-Pro) concentrations were measured in the clinical laboratory of the Hamamatsu University School of Medicine, University Hospital. The urinary angiotensinogen (U-AGT) level, which is a known surrogate marker of intrarenal RAS activity[10, 11], was measured using an enzyme-linked immunosorbent assay (ELISA) as described previously[23]. The plasma angiotensin II (Ang II) level, which is known to be an effector of circulating RAS activity, was determined using radioimmunoassay without special pretreatments (SRL, Tokyo, Japan). The serum creatinine concentrations were measured in blood drawn at 6:00 am, and the estimated glomerular filtration rate (eGFR) was calculated using the serum creatinine concentration with the Japanese eGFR equation [24]. The excretion ratios of U-Pro/creatinine (U-Pro/Cr) and U-AGT/creatinine (U-AGT/Cr) were calculated during both the daytime and night-time.
Histopathological data
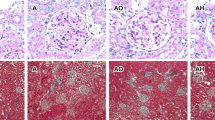
The degrees of mesangial cell hypercellularity were evaluated as follows: 0: <4 mesangial cells/mesangial area, 1: 4–5 mesangial cells/mesangial area, 2: 6–7 mesangial cells/mesangial area, and 3: 8 or more mesangial cells/mesangial area. The degrees in each glomerulus were averaged, and scores that were not greater than 0.5 or were greater than 0.5 were assigned a value of 0 or 1, respectively. The existence of endocapillary hypercellularity was scored (0: absent and 1: present). The arteriosclerosis levels (0: normal, 1: intima thickness and less than media thickness, and 2: intima thickness and more than media thickness) and arteriolar hyalinosis (the proportion of affected arterioles; 0: absent, 1: 1–25%, 2: 26–50%, and 3: 51–100%) of the intrarenal arteries were scored. These scores were evaluated using sections stained with periodic acid-Schiff (PAS) and Elastica van Gieson, respectively. The percentages of global glomerulosclerosis and tubulointerstitial fibrosis were evaluated through sections stained with PAS and Masson’s trichrome stain, respectively. The histopathological data were evaluated in accordance with our previous methods and the Oxford classification of IgA nephropathy [25, 26].
Statistical analyses
The results are expressed as the means ± SD. The significance of differences between daytime and night-time was determined using Student’s t-test for paired samples. Because U-Pro/Cr and U-AGT/Cr did not show a normal distribution, logarithmic transformation was applied for these values prior to the Student’s t-test evaluation. The correlations between U-AGT/Cr and renal structural damage (hyalinosis, arteriosclerosis, mesangial cell hypercellularity, endocapillary hypercellularity, global glomerulosclerosis, and tubulointerstitial fibrosis) and the clinical parameters were evaluated using Pearson’s product-moment correlation test. The significance of the differences between the patients with or without arteriosclerosis in relation to their clinical data was determined using Student’s t-test for unpaired samples or the Chi-square test for categorical variables. Multiple linear regression analyses were conducted to evaluate the relationships between arteriosclerosis and U-AGT/Cr. Age, sex, BMI, the eGFR and the systolic or diastolic BPs were selected as independent variables because these parameters were common variables in multiple linear regression analyses. In addition, age and the systolic or diastolic BPs were correlated with urinary AGT excretion and age, the eGFR and the systolic or diastolic BPs were significantly different between the patients with and without arteriosclerosis. We considered a value of p < 0.05 significant. The statistical analyses were performed using the IBM® SPSS® software, version 20 (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
Twenty-seven patients who underwent renal biopsy at our hospital during the study period and were diagnosed with IgA nephropathy were included in this study. The baseline characteristics are presented in Table 1. Most patients were young to middle age (39.2 ± 13.6 years), and their BPs were well-controlled without antihypertensives (112.0 ± 11.8/70.0 ± 9.5 mmHg). The circadian BP rhythms of the patients were as follows: dipper: 8, nondipper: 16, and riser: 3. No patients with the extreme dipper pattern were observed in this study. The patients’ renal functions were within normal limits (eGFR: 74.0 ± 17.3 ml/min/1.73 m2), and the daily urinary protein excretion levels were low (0.58 ± 0.50 g/day). The U-AGT excretion levels were 64.9 ± 100.6 μg/day. The pathological damage to the glomerulus, tubulointerstitium and small arteries was mild.
Changes in each parameter between daytime and night-time
Table 2 shows the changes in each parameter between daytime and night-time in normotensive IgA nephropathy patients. The BPs and urinary protein excretion levels were significantly higher during daytime than during night-time. In addition, urinary AGT excretion showed the same circadian rhythm as urinary protein excretion. However, the plasma Ang II levels were the same during daytime and night-time. These results coincided with our previous data [14, 19].
Relationships between the urinary AGT excretion levels and the clinical parameters and levels of pathological damage
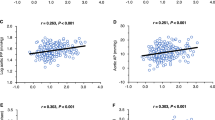
We investigated the correlations between urinary AGT excretion during the daytime and night-time and the clinical parameters and levels of pathological damage. The arteriosclerosis levels had a significant positive correlation with the urinary AGT excretion levels during the daytime and night-time (daytime; r = 0.41, p = 0.036, and night-time; r = 0.46, p = 0.016). However, no significant relationships were found among the hyalinosis, mesangial cell hypercellularity and endocapillary hypercellularity levels, the global glomerulosclerosis and tubulointerstitial fibrosis percentages and the urinary AGT excretion levels during the day and night. In addition, a significant positive relationship was found between age and urinary AGT excretion at night. The night-time systolic BPs had a significant positive correlation with urinary AGT excretion at night, and the diastolic BPs during the daytime and night-time had significant positive correlations with urinary AGT excretion during the daytime and night-time, respectively. Moreover, urinary protein excretion during the daytime and night-time had significant positive correlations with urinary AGT excretion during these time period (Table 3).
Clinical characteristics in the presence and absence of arteriosclerosis in the kidney
Because the arteriosclerosis levels seemed to be associated with the urinary AGT excretion levels, we evaluated clinical parameters, including the urinary AGT excretion levels, in the presence and absence of arteriosclerosis. The patients with arteriosclerosis were significantly older and had lower eGFRs and higher systolic and diastolic BPs at night and urinary protein and AGT excretion during the daytime and night-time than those without arteriosclerosis. In contrast, no significant differences were found between the patients who did and did not have arteriosclerosis in relation to sex, BMI, systolic and diastolic BP during the day, the circadian BP rhythm, and the plasma Ang II level (Table 4).
Multiple linear regression analyses of arteriosclerosis in the kidney and the clinical parameters
We performed multiple linear regression analyses to evaluate the relationships between arteriosclerosis in the kidney and the clinical parameters. The multiple linear regression analyses revealed that the arteriosclerosis levels had a significant positive correlation with the urinary AGT excretion levels at night even after adjusting for age, sex, BMI, and the eGFR (β = 0.39, p = 0.043; Model 4). In addition, although no significant relationships were found between the arteriosclerosis levels and the urinary AGT excretion levels during the day, positive relationships similar to those found at night were observed between these variables after their adjustment during the daytime (β = 0.31, p = 0.092; Model 1). However, when systolic or diastolic BPs were added in addition to these variables as an independent variable, the relationship between the arteriosclerosis levels and urinary AGT excretion levels was not observed during the daytime and night-time (Table 5).
Discussion
Herein, we report that the arteriosclerosis level had a significant positive correlation with intrarenal RAS activation and that the hyalinosis, mesangial cell hypercellularity and endocapillary hypercellularity levels and global glomerulosclerosis and tubulointerstitial fibrosis percentages were not associated with intrarenal RAS activation. In addition, the patients with arteriosclerosis had significantly higher systolic and diastolic BPs at night but not during the day than the patients without arteriosclerosis. Moreover, when systolic or diastolic BP was added as an independent variable in addition to age, sex, BMI and the eGFR, the relationship between arteriosclerosis and the urinary AGT excretion level was not observed during the daytime and night-time. These results indicate that intrarenal RAS activation at night due to nocturnal hypertension may be associated with renal arteriosclerosis in normotensive IgA nephropathy patients and may support the evidence that nocturnal BP treatment improves hypertension and reduces cardiovascular and stroke risks [27].
Some confounding factors may affect the arteriosclerosis and intrarenal RAS activation levels. In fact, a positive relationship was obtained between age and the urinary AGT excretion levels at night, and significant differences in age and the eGFR were observed between the patients with and without arteriosclerosis. A population-based autopsy study showed that the prevalence of arteriosclerosis increased with increasing age [28]. However, the relationships between the arteriosclerosis and urinary AGT excretion levels at night were maintained after adjusting for age, sex, BMI and the eGFR. These data suggested that age and the eGFR did not affect the arteriosclerosis and intrarenal RAS activation levels in this study.
Kimura et al. [29] reported that nocturnal hypertension was induced by an impaired renal capacity to excrete sodium due to a reduced ultrafiltration capacity and enhanced tubular sodium reabsorption [29]. A reduced ultrafiltration capacity is indicative of renal dysfunction, and a reduction in renal function is associated with intrarenal RAS activation [11]. In addition, enhanced tubular reabsorption correlates with intrarenal RAS activation [30]. These findings indicate that nocturnal hypertension is induced by intrarenal RAS activation due to renal damage. Many factors are associated with intrarenal RAS activation due to renal damage, among which reactive oxygen species and inflammation due to interleukin 6 are the main contributors [5,6,7, 31]. In fact, abnormalities in circadian BP patterns (nondipper and riser) were found in more than 70% of patients despite normal BP levels. We also showed that the urinary AGT levels were not decreased at night compared with the levels measured during the day in CKD patients showing the circadian BP riser pattern, that circadian fluctuations in proteinuria occurred parallel to fluctuations in the urinary AGT levels and that circadian fluctuations in the urinary AGT levels were correlated with diurnal BP changes [14, 15]. Moreover, Matsusaka et al. reported that AGT produced in the liver and filtered through the glomerular basement membrane was the primary source of intrarenal RAS activation. Therefore, glomerular permeability augmented in the setting of glomerular injury and glomerular hypertension increases the AGT excretion levels into the tubular lumen and activates intrarenal RAS at night when systemic or glomerular hypertension is induced, eventually resulting in renal damage. These concepts coincided with our results showing that although a significant positive relationship was not found between the arteriosclerosis and urinary AGT excretion levels during the day, a significant positive relationship was maintained after adjusting for age, sex, BMI, and the eGFR. Furthermore, a positive relationship between the arteriosclerosis and urinary AGT excretion levels was not observed during the night-time when systolic or diastolic BP was considered as an independent variable.
Wu et al. reported that the prevalence of arterial lesions was more prominent in IgA nephropathy patients than in non-IgA nephropathy and membranous nephropathy patients, although the IgA nephropathy patients were younger when the renal biopsies were performed [17]. In addition, intrarenal RAS activation is more enhanced in IgA nephropathy patients than in patients with minor glomerular abnormalities [32]. These previous data and our data from this study suggest that the intrarenal RAS activation levels vary in accordance with each kidney disease and that the differences in intrarenal RAS activation influence the arteriosclerosis levels. In the future, we intend to examine this association between intrarenal RAS activation and the arteriosclerosis levels for each kidney disease.
Activation of intrarenal RAS plays a critical role in the pathophysiology of renal damage in some animal models and patients [3,4,5,6,7,8,9,10,11]. Moreover, activated intrarenal RAS is associated with renal structural damage in IgA nephropathy model animals and patients [5, 6, 13, 32], and the increase in the afferent arteriolar thickness is caused by intrarenal RAS activation but not BP elevation in spontaneous hypertension rat models [8]. However, to the best of our knowledge, this report is the first to clarify the association between intrarenal RAS activation and the arteriosclerosis levels in normotensive IgA nephropathy patients.
The kidneys have an autoregulatory vasoconstrictor response, and an increase in the systemic BP does not directly influence the intraglomerular pressure [33]. Therefore, pressure-associated arteriolar injury is thought to occur before glomerular injury. When renal injury progresses and the number of nephrons decreases, the intraglomerular pressure increases, causing global glomerulosclerosis. Thereafter, the increase in glomerular permeability due to glomerular hypertension causes an increased filtered protein load in tubular cells. Hence, excessive reabsorption and catabolism of filtered proteins, increased nuclear signaling for gene expression, and activation of complement, the inflammasome and autophagy are induced in tubular cells, causing fibroblast proliferation and accumulation of extracellular matrix in tubulointerstitial lesions. Finally, tubular atrophy and interstitial fibrosis are induced [34]. In this study, the amount of urinary protein was small, and the renal function was almost within normal limits. Hence, intrarenal RAS activation is probably correlated with the arteriosclerosis levels but not with the global glomerulosclerosis or tubulointerstitial fibrosis levels.
This study has some limitations. First, because the BP values were obtained at 30-min intervals, even one missing value might have a significant effect on the results. However, because the rates of the time points when the BPs could be measured were 99.2% in this study and the frequency of missing values was very low (data not shown), we dealt with the data that could be measured. In addition, many researchers, including ourselves, measure BP values at 30-min intervals for clinical studies [14, 19,20,21]. Second, a low-salt diet was not given to some patients in this study (salt intake: 10 g/day to 11 patients (40.7%) and 6 g/day to 16 patients [59.3%]) because the renal function levels and BPs were within their normal limits, and the protein excretion levels in the urine were low. The difference in salt intake may have influenced the results of this study. However, because the renal biopsy was performed immediately after admission, whether the difference in salt intake after admission influenced pathological damage could not be determined. Moreover, the amounts of salt included in the normal and low-salt diets in our hospital are 10 and 6 g/day, respectively, indicating a small difference in salt intake between the diets. Third, several analyses did not show significant results, possibly due to the small number of patients included. However, we confirmed the relationships between intrarenal RAS activation at night due to nocturnal hypertension and renal arteriosclerosis. Finally, the urinary AGT excretion levels may not reflect RAS activation in the kidney. However, this method of collecting urinary AGT during the daytime and night-time has been used for evaluation of intrarenal RAS activation at day and night, respectively, in previous studies [14, 19]. In addition, when we collected and measured the urinary AGT excretion levels and the AGT and Ang II expression levels in the kidneys of anti-thymocyte serum nephritis rats every 6 h, the fluctuations in the values were parallel [9]. Therefore, we consider that the temporal accuracy of urinary collection is not a major limitation.
In conclusion, intrarenal RAS activation at night due to nocturnal hypertension may be associated with renal arteriosclerosis in normotensive IgA nephropathy patients.
References
Kala R, Fyhrquist F, Eisalo A. Diurnal variation of plasma angiotensin II in man. Scand J Clin Lab Invest. 1973;31:363–5.
Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87.
Shao W, Seth DM, Prieto MC, Kobori H, Navar LG. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. Am J Physiol Ren Physiol. 2013;304:F505–514.
Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29:716–23.
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Activation of reactive oxygen species and the renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:509–15.
Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Urushihara M, Kobori H. Role of activated intrarenal reactive oxygen species and renin-angiotensin system in IgA nephropathy model mice. Clin Exp Pharmacol Physiol. 2009;36:750–5.
Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–7.
Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–80.
Isobe S, Ohashi N, Ishigaki S, Tsuji T, Sakao Y, Kato A, Miyajima H, Fujigaki Y, Nishiyama A, Yasuda H. Augmented circadian rhythm of the intrarenal renin-angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens Res. 2016;39:312–20.
Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–50.
Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–54.
Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, Kobori H, Kagami S. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–67.
Kobori H, Katsurada A, Ozawa Y, Satou R, Miyata K, Hase N, Suzaki Y, Shoji T. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;22:156–63.
Isobe S, Ohashi N, Fujikura T, Tsuji T, Sakao Y, Yasuda H, Kato A, Miyajima H, Fujigaki Y. Disturbed circadian rhythm of the intrarenal renin-angiotensin system: relevant to nocturnal hypertension and renal damage. Clin Exp Nephrol. 2015;19:231–9.
Ohashi N, Isobe S, Ishigaki S, Yasuda H. Circadian rhythm of blood pressure and the renin-angiotensin system in the kidney. Hypertens Res. 2017;40:413–22.
Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–14.
Wu J, Chen X, Xie Y, Yamanaka N, Shi S, Wu D, Liu S, Cai G. Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant. 2005;20:719–27.
Lin L, Zhang H, Yang J, Zhang J, Li K, Huo B, Dai H, Zhang W, Yang J, Tan W, He Y. Nocturnal and circadian rhythm of blood pressure is associated with renal structure damage and function in patients with IgAN. Arch Med Res. 2016;47:25–32.
Ishigaki S, Ohashi N, Isobe S, Tsuji N, Iwakura T, Ono M, Sakao Y, Tsuji T, Kato A, Miyajima H, Yasuda H. Impaired endogenous nighttime melatonin secretion relates to intrarenal renin-angiotensin system activation and renal damage in patients with chronic kidney disease. Clin Exp Nephrol. 2016;20:878–84.
Ohashi N, Isobe S, Ishigaki S, Suzuki T, Motoyama D, Sugiyama T, Nagata M, Kato A, Ozono S, Yasuda H. The Effects of Unilateral Nephrectomy on Blood Pressure and Its Circadian Rhythm. Intern Med. 2016;55:3427–33.
Fukuda M, Mizuno M, Yamanaka T, Motokawa M, Shirasawa Y, Nishio T, Miyagi S, Yoshida A, Kimura G. Patients with renal dysfunction require a longer duration until blood pressure dips during the night. Hypertension. 2008;52:1155–60.
Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004;26:177–89.
Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Ren Physiol. 2007;293:F956–960.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Isobe S, Ohashi N, Ishigaki S, Tsuji N, Tsuji T, Kato A, Yasuda H. Increased nocturnal blood pressure variability is associated with renal arteriolar hyalinosis in normotensive patients with IgA nephropathy. Hypertens Res. 2017;40:921–6. Pubmed in press
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–56.
Hermida RC, Ayala DE, Smolensky MH, Fernández JR, Mojón A, Portaluppi F. Chronotherapy with conventional blood pressure medications improves management of hypertension and reduces cardiovascular and stroke risks. Hypertens Res. 2016;39:277–92.
Ninomiya T, Kubo M, Doi Y, Yonemoto K, Tanizaki Y, Tsuruya K, Sueishi K, Tsuneyoshi M, Iida M, Kiyohara Y. Prehypertension increases the risk for renal arteriosclerosis in autopsies: the Hisayama Study. J Am Soc Nephrol. 2007;18:2135–42.
Kimura G, Dohi Y, Fukuda M. Salt sensitivity and circadian rhythm of blood pressure: the keys to connect CKD with cardiovascular events. Hypertens Res. 2010;33:515–20.
Fukuda M, Urushihara M, Wakamatsu T, Oikawa T, Kobori H. Proximal tubular angiotensinogen in renal biopsy suggests nondipper BP rhythm accompanied by enhanced tubular sodium reabsorption. J Hypertens. 2012;30:1453–9.
Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Urushihara M, Acres OW, Navar LG, Kobori H. IL-6 augments angiotensinogen in primary cultured renal proximal tubular cells. Mol Cell Endocrinol. 2009;13:24–31.
Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–7.
Griffin KA, Picken MM, Bidani AK. Deleterious effects of calcium channel blockade on pressure transmission and glomerular injury in rat remnant kidneys. J Clin Invest. 1995;96:793–800.
Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2015;30:706–12.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ohashi, N., Isobe, S., Matsuyama, T. et al. Night-time activation of the intrarenal renin–angiotensin system due to nocturnal hypertension is associated with renal arteriosclerosis in normotensive IgA nephropathy patients. Hypertens Res 41, 334–341 (2018). https://doi.org/10.1038/s41440-018-0026-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-018-0026-4
This article is cited by
-
Normotensive non-dipping blood pressure profile does not predict the risk of chronic kidney disease progression
Hypertension Research (2019)
-
Increased heart rate is associated with intrarenal renin–angiotensin system activation in chronic kidney disease patients
Clinical and Experimental Nephrology (2019)