Abstract
Plants have a perception system triggered by pathogen and pest signals to initiate defense. These signals include evolutionarily conserved molecules from microbes and insects termed pathogen/herbivore-associated molecular patterns (PAMPs/HAMPs). Here we showed that hexaacetyl-chitohexaose (HC), an oligosaccharide from chitin, a structural component in insect exoskeletons and fungi cell walls, upregulated defense-associated genes WRKY22, GST1, RAR1, EDS1, PAL1 and NPR2, and downregulated ICS1 at 1 h after HC treatment in Sun Chu Sha mandarin leaves. The effect was transient as defense gene transcriptional changes were not observed at 18 h after the treatment. Electrical penetration graph (EPG) recordings were used to study the feeding behavior of Asian citrus psyllid (ACP) following the HC treatment. ACP is the hemipteran vector of Candidatus Liberibacter asiaticus (CLas), the pathogen associated with huanglongbing (HLB). Adult ACP displayed reduced intercellular probing, reduced xylem feeding count and duration, and increased non-probing activity on HC-treated citrus compared to controls. During an 18-h recording, percentage for total duration of xylem ingestion, phloem ingestion, intercellular probing were lower, and the percentage of non-probing behavior was higher in HC-treated leaves than in controls. In host-selection behavior studies, HC treatment did not alter the attractiveness of citrus leaves under light or dark conditions. In addition, ACP feeding on HC-treated leaves did not show differences in mortality for up to 10 day of exposure. In summary, we report that HC induced a transient defense in citrus and an inhibitory effect on ACP feeding but did not affect host selection or the insect fitness under the tested conditions.
Similar content being viewed by others
Introduction
The bacterial disease Huanglongbing (HLB) or citrus greening is endemic and significantly impacting the citrus industries in the United States and worldwide1,2,3. The associated pathogen, Candidatus Liberibacter asiaticus (CLas), is a Gram-negative, intracellular bacterium residing in the phloem cells of the plant host. CLas is transmitted by the Asian citrus psyllid (ACP, Diaphorina citri), an insect capable of acquiring the bacterium from infected trees and transmitting to healthy ones through phloem feeding. Citrus infected by CLas display typical HLB symptoms, including leaf blotchy mottle and yellow shoots. A severe infection can result in stunted and declining trees along with fruit drop and/or small misshaped fruits that significantly reduce citrus yield and commercial value. Understanding the HLB pathosystem has been hindered by the fastidious nature of the bacterium, which makes studies related to bacterial pathogenicity and development of control strategies challenging. Genetic resistance to the bacterium is lacking in cultivated Citrus; however, HLB tolerance has been identified in some citrus species and relatives, evidenced by reduced developmental impairment by CLas infection4,5,6,7,8,9. Therefore, it is important to monitor and control the insect vector as major part of disease management.
Insecticidal suppression of ACP has been the major tactic for insect control. In Florida, the application of broad-spectrum pesticides during the winter has been recommended as it can suppress ACP populations and reduce need for insecticide during citrus flushing when beneficial insects are present10. During leaf flushing, additional sprays of selective insecticides further reduce ACP adults though nymphs are affected to a lesser degree11. Broader efficacy testing of insecticides with different modes of action may enhance insecticide rotation to avoid resistance development12. Biological control may serve as a sustainable ACP management strategy. Naturally occurring predators, including ladybeetles (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae), predatory mites (Acari: Phytoseiidae), and syrphid flies (Diptera: Syrphidae), have been shown to contribute to ACP mortality13,14,15,16. The exotic parasitoid Tamarixia radiata Waterston (Eulophidae), has been imported into several countries, including the United States, and it has been widely established in Florida and California citrus growing areas16,17,18. Utilization of host resistance is another long-term strategy for insect pest management. Studies have shown that the citrus relative Poncirus trifoliata displayed relatively low ACP colonization, and possesses both antixenosis and antibiosis resistance characteristics19,20,21,22, which may contribute to breeding for ACP resistance.
Plants exploit an arsenal of structural, chemical and biochemical defenses against herbivore attacks. Morphological features, such as trichomes, spines, cuticles, thorns, and lignified cell walls, can directly deter the feeding of herbivores23,24,25. Plant secondary metabolites that either function as phytoanticipins or phytoalexins render plant tissue toxic or impart an antifeedant effect26. Among the plant defensive chemicals, phenols27,28,29, flavonoids30, and tannins31 are well documented secondary metabolites with roles in insect defense. Ingestion of various defensive proteins disrupts insect digestion and contributes to plant protection. Examples include lectins which are carbohydrate-binding proteins that survive insect digestive systems and are insecticidal32; proteinase inhibitors (PIs) that bind insect digestive enzymes and impair protein digestion33, disrupting insect growth, development, reproduction, and even survival34,35,36,37; and anti-oxidative enzymes such as peroxidases (PODs), polyphenol oxidases (PPOs), and lipoxygenases (LOXs) which have roles in insect deterrence via either direct toxicity or host defense activation38.
Plants can perceive microbial and insect molecules as danger signals and mount effective defense against invasions. Studies on interactions with phytopathogens have established that plants have a layered innate immune system which responds to different microbial elicitors39, and these early signaling events are similar to those induced by insects40,41,42,43. Well-studied pathogen-associated molecular patterns (PAMPs) include chitin from fungal cell wall, epitopes of bacterial flagellin (flg22) and elongation factor Tu (efl18), which can elicit plant defenses that protect from subsequent pathogen infections44,45,46. Although the identities of many insect-derived elicitors (herbivore-associated molecular patterns/HAMPs) remains unclear, the host defenses can be triggered by oral secretions, saliva, and fluid from oviposition47. The recognition of such insect elicitors affects the outcome of plant-insect interactions. For example, leaf infiltration with crude extracts from green peach aphids (GPA) triggered innate immune responses resembling those against phytopathogens and resulted in increased insect mortality48. Treatment of plants with the protein extracted from GPA saliva-induced expression of defense genes and local resistance that reduced insect productivity49.
A previous investigation of citrus defense against bacterial pathogens indicated that flg22-associated PAMP-triggered immunity (PTI) played an important role in resistance to citrus canker50. In this study, we showed that hexaacetyl-chitohexaose (HC), an oligosaccharide derived from chitin that has been established as the elicitor in other plants51,52, induced the expression of defense-associated genes in Sun Chu Sha mandarin as an herbivore-associated molecular pattern, a response similar to the one triggered by flg22. Using the electrical penetration graph (EPG) method, the feeding behavior of ACP was monitored and HC treatment displayed an antifeedant effect against ACP. Further studies indicated that HC treatment did not appear to modify host attractiveness, nor the survival of psyllids as observed by reduced feeding activities in the EPG analysis. The impact of HC-induced transient defense on the citrus–ACP interactions is discussed.
Results
HC-induced transient activation of defense genes in Sun Chu Sha mandarin
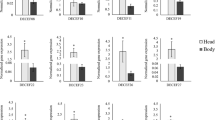
A previous investigation indicated that citrus varieties, including Sun Chu Sha mandarin, were sensitive to bacterial flg22 and can activate the expression of a suite of defense-associated genes representing key functional nodes of defense pathways50. These genes were PTI markers (WRKY22 and GST1)53, PTI and effector-triggered immunity perception and signaling genes (RAR1, SGT1, NDR1, and EDS1)54,55,56,57, genes encoding salicylic acid (SA) signaling (NPR2, NPR3, and AZI1)58,59,60 and biosynthesis (PAL1 and ICS1)61,62, and jasmonic acid (JA) perception and metabolism genes (COI1 and JAR1)63,64. Here, the effect of HC on the expression of citrus defense-associated genes was studied in Sun Chu Sha mandarin at 1 h and 18 h after treatment. Results indicated significantly increased expression of WRKY22, GST1, RAR1, EDS1, PAL1, and NPR2, and reduced expression of ICS1 at 1 h in the plants treated with HC (Fig. 1a). This effect was not observed at 18 h after the treatment and none of the genes studied showed expression level changes compared with water controls at this time point (Fig. 1b).
(a) and 18 h (b) after the treatment. HC solution at 100 µg/mL was infiltrated into young fully expanded Sun Chu Sha mandarin leaves and infiltration of water was used as control. Gene relative quantification values were normalized with the endogenous gene GAPDH and calculated in reference to a randomly selected control sample. The genes analyzed are indicated on the x axis. An asterisk stands for significant difference between HC treatment and control for the gene studied (p < 0.05). Bars are means ± standard error (n = 3)
HC modified ACP feeding behavior in Sun Chu Sha mandarin
ACP behavior was studied on HC-treated vs. control citrus leaves to determine if HC-induced defense gene activation corresponded to plant resistance to insects. Insect feeding behavior was monitored using EPG recordings, which generated waveforms representing specific feeding activities, including intercellular probing (C), phloem penetration (D), phloem salivation (E1), phloem ingestion (E2), xylem ingestion (G), and non-probing (Np). EPG recording was conducted for 18 h starting at 1 h after HC or control treatment, which were the same time points studied for defense gene induction. For the 18 h recording period, the mean number of waveform bouts (count), mean duration, and total duration for each type of feeding activity are shown in Table 1. Results indicated that there was significantly less intercellular probing (C) for psyllids feeding on HC-treated leaves than the controls (Table 1). Significant reductions in the count, mean, and total duration of xylem feeding activities were observed for HC-treated leaves compared with controls (Table 1). Psyllids feeding on HC-treated leaves spent significantly longer durations of time on non-probing (Np) activities than on the controls (Table 1). Over the total duration of feeding activity studied (18 h), HC treatment resulted in 60% of total time in non-probing (Np) compared with 23% in controls (Fig. 2). The percentages of xylem ingestion (G) (17% vs. 42%), phloem ingestion (E2) (4% vs. 9%), and intercellular probing (C) (19% vs. 26%) were lower in the HC treatment than in controls.
Young fully-expanded leaves were infiltrated with 100 µg/mL of HC solution or water as the Control. Psyllids were placed on citrus leaves 1 h after infiltration and feeding behavior was recorded by EPG for an 18 h duration. The total duration of intercellular probing (C), phloem penetration (D), phloem salivation (E1), phloem ingestion (E2), xylem ingestion (G), and non-probing (Np) are indicated by percentage in the pie charts. The data represent the average of 10 biological replicates for each treatment
The effect of HC on ACP host-selection behavior in Sun Chu Sha mandarin
The choice assay comparing HC- and control-treated leaves was conducted to determine if HC-induced defense affected host selection, perhaps through modification of volatile releases. The experiments were compared in both light and dark conditions. There was no significant difference between the numbers of ACP entering vials containing HC-treated leaves or control leaves under either light or dark conditions, indicating no effect of HC treatment on host preference (Fig. 3).
Young fully expanded leaves were infiltrated with 100 µg/mL of HC or water as controls. The injected leaves were detached from plants and placed in vials with an opening on top. Each setup is a cage containing 50 adult D. citri with two vials inside for 24 h. The number of psyllids that entered each vial was recorded. The experiments were carried out 14 times in a chamber with light (n = 14) and 20 times without light (n = 20). Statistical significance was determined by Student’s t using JMP Genomics. Bars are means ± standard error
The effect of HC on ACP mortality in Sun Chu Sha mandarin
Leaf infiltration of HC resulted in transient induction of defense-associated genes and reduced xylem and phloem feeding by ACP. To determine if this effect had any long-term influence on ACP fitness, the mortality of psyllids feeding on HC-treated citrus was compared with those feeding on the control plants. In detached leaf assays, the ACP mortality was counted at 1, 3, and 4 days after the treatment, and there was no difference between HC and control treatments (Fig. 4a). Longer period of observation of ACP mortality was performed using intact plants. Results showed no difference in mortality between HC and control treatments at 2, 6, or 10 days after leaf infiltrations (Fig. 4b).
(a) and attached leaves (a) of Sun Chu Sha mandarin. Young fully expanded leaves were infiltrated with 100 µg/mL of HC or water as controls. In the detached leaf assay, injected leaves were removed from the plants and each exposed to 10 adult psyllids for 4 days (n = 10 per time point and treatment). For a longer period of observation, attached leaves on greenhouse plants received HC or water treatment and were enclosed in a Petri dish with 10 psyllids/leaf, and the mortality was monitored for 10 days (n = 8 per time point and treatment). Statistical significance was determined by Student’s t using JMP Genomics. Bars are means ± standard error
Discussion
Chitin is comprised of N-acetylchitooligosaccharides and is the major constituent of fungal cell walls, insect exoskeletons and egg shells of nematodes. Earlier studies demonstrated that chitin or fungal cell wall extracts, applied to plants, induced plant cell physiological changes, including lignification, phytoalexin production, membrane depolarization, and extracellular alkalization. These observations indicate there is a chitin perception system in plants which activates defenses against fungal pathogens65,66,67,68. Further research revealed that both monocot and dicot plants can perceive chitin through a membrane-located receptor and kinase protein complex69, leading to signal transduction and activation of defense-related genes70,71. In this study, chitin treatment of Sun Chu Sha mandarin induced differential expression of citrus defense-related genes, including WRKY22, GST1, RAR1, EDS1, PAL1, NPR2, and ICS1, at 1 h after the treatment (Fig. 1a), and this effect was not observed at 18 h after treatment (Fig. 1b). The quick activation of defense genes suggests citrus has a similar chitin perception system that can rapidly respond to this elicitor. In the responses of Arabidopsis to PAMPs, chitin-triggered transcriptional reprogramming was the strongest between 0.5 and 3 h after exposure70, and flg22-induced defense gene activation from 0.5 to 5 h after exposure53. Citrus gene induction at 1 h but not at 18 h after treatment indicates HC induced a transient defense in Sun Chu Sha mandarin and the timing appears to be consistent with those in model plants.
In a previous study, flg22 from the citrus canker causing bacterium Xanthomonas citri ssp. citri upregulated the expression of WRKY22, GST1, EDS1, NDR1, RAR1, SGT1, PAL1, and NPR3 in Sun Chu Sha mandarin50, a pattern highly similar to that induced by HC (Fig. 1a), indicating shared defense pathways between flg22- and chitin-induced defenses in citrus. Among the HC-induced genes, WRKY22 and GST1 are considered early markers for flg22-initiated PTI53 and WRKY22 was also shown to be induced by chitin46; RAR1 and EDS1 are important genes for R gene-mediated pathogen resistance (ETI)56,72, basal and nonhost resistance related to PTI73,74,75; PAL1 and ICS1 encode key enzymes for two different pathways for biosynthesis of the defense hormone SA61,62, and plants respond to chitin in a SA-dependent manner70. In addition, trans‐cinnamic acid, the production of which is catalyzed by PAL1, is a precursor for biosynthesis of lignin and antimicrobial secondary metabolites that contribute to pathogen resistance61,76,77. Application of chitin to leaves increases activity of the enzyme PAL and enhances total phenolic content78. Taken together, these results suggest that chitin activates defense pathways in citrus that is similar to other plant species, and may have a role in citrus resistance to pathogens and possibly to insect pests.
Studies on chitin-triggered defenses have focused on the plant-pathogen interactions79, where evidence has shown that perception of chitin can confer resistance to biotrophic and necrotrophic fungal pathogens46. Only a few investigations have addressed chitin-induced insect resistance as an insect-derived elicitor, although crude insect extracts that may contain chitin were shown to induce PTI-like responses in Arabidopsis and leaf infiltration resulted in higher insect mortality48. In this study, EPG was employed to investigate ACP feeding activities as influenced by chitin treatment. Results from 18 h recordings indicated ACP had reduced feeding activity on HC-treated leaves, including lower counts of intercellular probing (C), and count and duration of xylem feeding (G) (Table 1). HC treatment led to reduced duration of xylem ingestion (G), phloem ingestion (E2), and intercellular probing (C) and greatly increased non-probing (Np) time (Fig. 2). This suggests HC-induced defense in Sun Chu Sha mandarin has an inhibiting effect on ACP feeding. This effect may result from rapid defense initiation upon chitin perception, SA-mediated defense and/or production of inhibitory secondary metabolites80, which were reflected as transcriptional activation of genes in the related pathways in this study (Fig. 1a). It is likely that additional plant defense mechanisms, that are dependent or independent to what were investigated in this study, may have contributed to the alteration of ACP feeding as a result of HC treatment, which is worthy of analysis through systematic approaches such as transcriptomics and proteomics. However, in a previous study it was discovered that formic acid, a carboxylic acid produced as a breakdown product of citrus volatiles81, induced the same set of defense-associated genes in citrus within several hours of gaseous exposure, and EPG showed reduced ACP feeding on the treated plants82. These results suggest a good correlation between expression activation of these genes and citrus resistance to ACP feeding, and hence supports potential for using transcriptional markers for resistance assessment in citrus.
Additional studies of HC-induced defense on citrus–ACP interactions were conducted using host-selection behavior and mortality assays. Results indicated no significant differences in host selection by ACPs between HC-treated and control leaves, and no host preference was observed in experiments conducted under either light or dark conditions (Fig. 3). Plants activate a wide range of defenses in response to herbivore attack or pathogen infection, resulting in release of volatile organic compounds (VOCs)83,84,85. It was reported that citrus plants infected with HLB were more attractive to D. citri than healthy plants, possibly due to higher amounts of methyl salicylate released from infected plants, suggesting an interplay between citrus defense and insect host-selection behavior86. In this study, leaves with HC injection did not show change in attractiveness to ACP, suggesting HC-induced local defenses may not be associated with the release of VOCs that modulate host selection. It is possible that the effect from HC treatment of single leaves may not lead to sufficient production of olfactory cues to influence ACP host selection. In addition, the activation of SA-associated defense genes by HC (Fig. 1) implies the initiation of systemic defenses in citrus87 which can lead to protection of distant tissues and even neighboring plants from herbivores85. Hence it will be interesting to conduct tests at the whole plant scale for effect of chitin on the odorant-mediated ACP host preference and on systemic induction of defense against insects.
To determine if HC-induced feeding inhibition affected ACP fitness, mortality was analyzed on detached leaves within 4 days of treatment, and leaves from intact plants within 10 days of treatment. Results showed no difference in ACP mortality between HC and control treatments at the time points studied (Fig. 4). In the gene expression studies, the effect of the HC treatment occurred at 1 h but not at 18 h after the treatment, suggesting the antifeedant effect is also a short-term effect which may not be strong or long-lasting enough to impact ACP mortality. In the study of flg22-triggered PTI in citrus, leaves infiltrated with flg22 showed reduced pathogenic bacterial growth at 2 and 4 days post inoculation (DPI) but the effect disappeared by 6 DPI50, similar to the transient effect HC induced in citrus against ACP and suggesting that ACP may have resumed normal feeding when the effect ceased. In tobacco–green peach aphid interactions, insects fitness was analyzed through nymph feeding of tobacco leaves transiently expressing the effector protein Mp1088. Nymphs were fed with Agro-infiltrated leaves for 6 days and moved to newly Agro-infiltrated leaves until the emergence of adults and next generation of nymphs, where reduced fecundity (number of nymphs produced per adult) was observed due to plant defense induced by Mp1088. Hence, a similar assay may be useful to investigate if HC-induced defense in citrus may lead to a meaningful insect resistance phenotype.
In conclusion, this study showed that HC is an herbivore-associated molecular pattern in citrus that triggered transient induction of defense-associated genes, including WRKY22, GST1, RAR1, EDS1, PAL1, NPR2, and ICS1. The HC treatment resulted in an inhibitory effect on ACP feeding behavior, including reduced intercellular probing and xylem feeding and increased duration of non-probing activity, in an 18-h EPG recording. Moreover, HC-treated citrus leaves did not have altered host attractiveness regardless of presence or absence of light. Finally we showed that a HC-induced, transient antifeedant effect did not lead to increased ACP mortality.
Materials and methods
Plant and insect materials
Sun Chu Sha mandarin (Citrus reticulata Blanco) plants were grown from seeds in cone containers under greenhouse conditions. Seedling plants <1 year old with multiple young fully expanded leaves were selected for experiments. The plants were watered and acclimated in a walk-in chamber (26 °C, 65% relative humidity, 14-h light and 10-h dark cycle) 1 day prior to experiments. The colony of Asian citrus psyllids (Diaphorina citri) were maintained at the insectary of USDA-ARS US Horticultural Research Laboratory using Citrus macrophylla as host plants. The sex ratio of the colony was about 1:1. Adult psyllids at 8–10 days old were collected from the cage via vacuum and used for experiments.
HC treatment
Chitin-derived HC (Megazyme Inc., Chicago, IL, USA) was used as an elicitor. HC was dissolved at 10 mg per 1 mL of deionized water, autoclaved for 10 min and centrifuged for 5 min at maximum speed with a benchtop centrifuge. The supernatant was collected and lyophilized into powder. A 50 µg/mL water-based solution was prepared for experiments. Leaf infiltration of the HC solution was used and water infiltration was used as the controls.
EPG recordings
EPG was performed using a DC-monitor, GIGA-8 system (EPG-Systems, Wageningen, the Netherlands) to record the feeding activities of adult ACP on HC or water injected citrus leaves. For each setup, a single psyllid was tethered to recording equipment using a gold wire at 1.5 cm long and 25 µm diameter (Sigmund Cohn Corp., Mt. Vernon, NY) attached with silver conducting glue (Ladd Research Industries, Burlington, VT), and then settled on a leaf at the area of treatment (at 1 h after treatment). To complete the circuit, a second electrode (ground electrode) was inserted into soil at the base of the plant. EPG recordings were conducted in a climate-controlled chamber (26 °C and 60–65% humidity) for 18 h under lighted conditions. For each treatment, a total of 10 setups were subject to EPG (n = 10). Waveforms were classified by visual inspection according to previous reports into six feeding states89: intercellular probing (C), phloem penetration (D), salivation at phloem (E1), phloem ingestion (E2), xylem ingestion (G), and non-probing (NP). The waveforms were annotated in the Dataq Waveform Browser (Dataq Instruments Inc., Akron, OH). The number of waveform bouts (count), mean duration and total duration (count × mean duration) for individual waveforms were analyzed by Kruskal–Wallis test (α = 0.05) using JMP without differentiating insect sex (v. 10, SAS Inc, Cary, NC, USA).
Expression analysis of citrus defense-associated genes
Multiple young fully expanded leaves from each Sun Chu Sha mandarin plant were infiltrated with HC solution or water. The treatment used a 1-mL insulin syringe with a needle to inject solution into the abaxial surface of leaves until the leaf was saturated. Three plants per treatment were used as the biological replicates. Leaf samples were collected at 1 h and 18 h after infiltration for RNA extraction. Samples were put in liquid nitrogen and stored in a −80 °C freezer. Total RNA was isolated by TriZol reagent (Invitrogen, Carlsbad, CA, USA) combined with on-column DNase treatment and purification with an RNeasy Plant Mini Kit (Qiagen, Gaithersburg, MD, USA). The cDNA was synthesized using a QuantiTect Reverse Transcription kit (Qiagen) and diluted to 5 ng/µL concentration. Quantitative real time PCR using SYBR green reagent (Thermo Fisher Scientific, Waltham, MA) was performed amplifying from 10 ng of cDNA template. Primers of citrus defense-associated genes were referenced from a previous study50. The comparative Ct (ΔΔCt) method was used to calculate the relative quantification of each gene and the citrus glyceraldehyde-3-phosphate dehydrogenase C2 (GAPC2) was used as the endogenous gene for normalization90. Statistical significance was determined by nonparametric method (α = 0.05) using R (The R Foundation for Statistical Computing, Vienna, Austria).
Insect mortality assays
For the detached leaf assay, HC or water treated leaves were removed from plants and petioles were placed in 0.5-mL centrifuge tubes filled with water and sealed by Parafilm. Each leaf was put in a 50-mL plastic centrifuge tube with a mesh top, and 10 psyllids were released to feed on the leaf under constant light. The number of dead psyllids was recorded at 1, 3, and 4 days after the setup. For the intact plant assay, HC or water treated leaves were enclosed by a transparent Petri Dish with the edge sealed by Parafilm. Each leaf was fed on by 10 psyllids under constant light. The mortality was recorded at 2, 6, and 10 days after the setup.
Insect host-selection assay
For each setup, one HC infiltrated and one water infiltrated citrus leaf were removed from the plant immediately after treatment. The petioles were placed in water in 0.5-mL centrifuge tubes sealed with Parafilm. Each leaf was enclosed in a non-transparent vial with a hole on the cap (diameter of 3.8 mm). The two vials were placed in a small cage where 50 adult psyllids were released subsequently. The number of psyllids entering each vial was recorded after 24 h. The assay was repeated 14 times under constant light and 20 times in dark in a controlled environment chamber (26 °C, 65% relative humidity).
References
da Graca, J. V. Citrus greening disease. Annu Rev. Phytopathol. 29, 109–136 (1991).
Bove, J. M. Huanglongbing: a destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 88, 7–37 (2006).
Gottwald, T. R. Current epidemiological understanding of citrus Huanglongbing. Ann. Rev. Phytopathol 48, 119–139 (2010).
Halbert, S. E. & Manjunath, K. L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: a literature review and assessment of risk in Florida. Fla Entomol. 87, 330–353 (2004).
Folimonova, S. Y., Robertson, C. J., Garnsey, S. M., Gowda, S. & Dawson, W. O. Examination of the responses of different genotypes of citrus to huanglongbing (citrus greening) under different conditions. Phytopathology 99, 1346–1354 (2009).
Stover, E. & McCollum, G. Incidence and severity of huanglongbing and Candidatus liberibacter asiaticus titer among field-infected citrus cultivars. Hortscience 46, 1344–1348 (2011).
Stover, E., Inch, S., Richardson, M. L. & Hall, D. G. Conventional citrus of some scion/rootstock combinations show field tolerance under high huanglongbing disease pressure. Hortscience 51, 127–132 (2016).
Stover, E., Hall, D. G., Shatters, R. G. & Moore, G. A. Influence of citrus source and test genotypes on inoculations with Candidatus Liberibacter asiaticus. Hortscience 51, 805–809 (2016).
Miles, G. P., Stover, E., Ramadugu, C., Keremane, M. L. & Lee, R. F. Apparent tolerance to huanglongbing in citrus and citrus-related germplasm. Hortscience 52, 31–39 (2017).
Qureshi, J. A. & Stansly, P. A. Dormant season foliar sprays of broad-spectrum insecticides: An effective component of integrated management for Diaphorina citri (Hemiptera: Psyllidae) in citrus orchards. Crop Prot. 29, 860–866 (2010).
Qureshi, J. A., Kostyk, B. C. & Stansly, P. A. Effectiveness of selective insecticides to control Asian citrus psyllid and citrus leafminer during leaf flushing. Proc. Fla. State Hortic. Soc. 124, 85–89 (2011).
Qureshi, J. A., Kostyk, B. C. & Stansly, P. A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. Plos ONE 9, e112331 (2014).
Michaud, J. P., Olsen, L. E. & Olsen, L. E. Suitability of Asian citrus psyllid, Diaphorina citri, as prey for ladybeetles. BioControl 49, 417–431 (2004).
Michaud, J. P. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol. Control 29, 260–269 (2004).
Juan-Blasco, M., Qureshi, J. A., Urbaneja, A. & Stansly, P. A. Predatory mite, Amblyseius swirskii (Acari: Phytoseiidae), for biological control of asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla Entomol. 95, 543–551 (2012).
Kistner, E. J., Melhem, N., Carpenter, E., Castillo, M. & Hoddle, M. S. Abiotic and biotic mortality factors affecting Asian citrus psyllid (Hemiptera: Liviidae) demographics in southern California. Ann. Èntomol. Soc. Am. 109, 860–871 (2016).
Qureshi, J. A., Rogers, M. E., Hall, D. G. & Stansly, P. A. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol. 102, 247–256 (2009).
Kistner, E. J., Amrich, R., Castillo, M., Strode, V. & Hoddle, M. S. Phenology of Asian citrus psyllid (Hemiptera: Liviidae), with special reference to biological control by Tamarixia radiata, in the residential landscape of southern California. J. Econ. Entomol. 103, 1047–1057 (2016).
Richardson, M. L. & Hall, D. G. Resistance of Poncirus and Citrus × Poncirus germplasm to the Asian citrus psyllid. Crop Sci 53, 183–188 (2013).
Hall, D. G., George, J. & Lapointe, S. L. Further investigations on colonization of Poncirus trifoliata by the Asian citrus psyllid. Crop Prot. 72, 112–118 (2015).r
Hall, D. G., Hentz, M. G. & Stover, E. Field survey of Asian citrus psyllid (Hemiptera: Liviidae) infestations associated with six cultivars of Poncirus trifoliata (Rutaceae). Fla Entomol. 100, 667–668 (2017).
George, J. & Lapointe, S. L. Host-plant resistance associated with Poncirus trifoliata influence oviposition, development and adult emergence of Diaphorina citri (Hemiptera: Liviidae). Pest Manag Sci. 75, 279–285 (2018).
Hanley, M. E., Lamont, B. B., Fairbanks, M. M. & Rafferty, C. M. Plant structural traits and their role in anti-herbivore defence. Perspect. Plant Ecol., Evol. Syst. 8, 157–178 (2007).
Agrawal, A. A. et al. Phylogenetic ecology of leaf surface traits in the milkweeds (Asclepias spp.): chemistry, ecophysiology, and insect behavior. New Phytol. 183, 848–867 (2009).
He, J. et al. Chrysanthemum leaf epidermal surface morphology and antioxidant and defense enzyme activity in response to aphid infestation. J. Plant Physiol. 168, 687–693 (2011).
Howe, G. A. & Jander, G. Plant immunity to insect herbivores. Ann. Rev. Plant Biol. 59, 41–66 (2008).
Usha Rani, P. & Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant 32, 695–701 (2010).
War, A. R., Paulraj, M. G., War, M. Y. & Ignacimuthu, S. Jasmonic acid-mediated-induced resistance in groundnut (Arachis hypogaea L.) Against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Growth Regul. 30, 512–523 (2011).
War, A. R., Paulraj, M. G., War, M. Y. & Ignacimuthu, S. Herbivore- and elicitor-induced resistance in groundnut to Asian armyworm, Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Plant Signal. Behav. 6, 1769–1777 (2011).
Treutter, D. Significance of flavonoids in plant resistance: a review. Environ. Chem. Lett. 4, 147 (2006).
Barbehenn, R. V. & Peter Constabel, C. Tannins in plant–herbivore interactions. Phytochemistry 72, 1551–1565 (2011).
Vandenborre, G., Smagghe, G. & Van Damme, E. J. M. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 72, 1538–1550 (2011).
Broadway, R. M. & Duffey, S. S. Plant proteinase inhibitors: mechanism of action and effect on the growth and digestive physiology of larval Heliothis zea and Spodoptera exiqua. J. Insect Physiol. 32, 827–833 (1986).
Edmonds, H. S., Gatehouse, L. N., Hilder, V. A. & Gatehouse, J. A. The inhibitory effects of the cysteine protease inhibitor, oryzacystatin, on digestive proteases and on larval survival and development of the southern corn rootworm (Diabrotica undecimpunctata howardi). Èntomol. Exp. et. Appl. 78, 83–94 (1996).
Ortego, F., Farinós, G. P., Ruíz, M., Marco, V. & Castañera, P. Characterization of digestive proteases in the weevil Aubeonymus mariaefranciscae and effects of proteinase inhibitors on larval development and survival. E`ntomol. Exp. et. Appl. 88, 265–274 (1998).
Gatehouse, A. M. R. et al. Digestive proteolytic activity in larvae of tomato moth, Lacanobia oleracea; effects of plant protease inhibitors in vitro and in vivo. J. Insect Physiol. 45, 545–558 (1999).
Annadana, S. et al. Effects of cysteine protease inhibitors on oviposition rate of the western flower thrips, Frankliniella occidentalis. J. Insect Physiol. 48, 701–706 (2002).
War, A. R. et al. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7, 1306–1320 (2012).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Walling, L. L. The myriad plant responses to herbivores. J. Plant Growth Regul. 19, 195–216 (2000).
Taylor, J. E., Hatcher, P. E. & Paul, N. D. Crosstalk between plant responses to pathogens and herbivores: a view from the outside in. J. Exp. Bot. 55, 159–168 (2004).
Garcia-Brugger, A. et al. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Inter. 19, 711–724 (2006).
Maffei, M. E., Mithöfer, A. & Boland, W. Before gene expression: early events in plant–insect interaction. Trends Plant Sci. 12, 310–316 (2007).
Kunze, G. et al. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16, 3496–3507 (2004).
Zipfel, C. et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428, 764 (2004).
Wan, J. et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20, 471–481 (2008).
Mithöfer, A. & Boland, W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 146, 825–831 (2008).
Prince, D. C., Drurey, C., Zipfel, C. & Hogenhout, S. A. The leucine-rich repeat receptor-like kinase BRASSINOSTEROID INSENSITIVE1-ASSOCIATED KINASE1 and the cytochrome P450 PHYTOALEXIN DEFICIENT3 contribute to innate immunity to aphids in Arabidopsis. Plant Physiol. 164, 2207–2219 (2014).
De Vos, M. & Jander, G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ 32, 1548–1560 (2009).
Shi, Q., Febres, V. J., Jones, J. B. & Moore, G. A. Responsiveness of different citrus genotypes to the Xanthomonas citri ssp. citri-derived pathogen-associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker. Mol. Plant Pathol. 16, 507–520 (2015).
Roby, D., Gadelle, A. & Toppan, A. Chitin oligosaccharides as elicitors of chitinase activity in melon plants. Biochem. Bioph. Res. Co. 143, 885–892 (1987).
Ishikawa, K. et al. Bacterial effector modulation of host E3 ligase activity suppresses PAMP-triggered immunity in rice. Nat. Commun. 5, 5430 (2014).
Asai, T. et al. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002).
Shirasu, K. et al. A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in. C. elegans. Cell 99, 355–366 (1999).
Parker, J. E. et al. Characterization of eds 1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8, 2033–2046 (1996).
Azevedo, C. et al. The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076 (2002).
Century, K. S., Holub, E. B. & Staskawicz, B. J. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc. Natl Acad. Sci. USA 92, 6597–6601 (1995).
Zhang, Y. L. et al. Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J. 48, 647–656 (2006).
Fu, Z. Q. et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228 (2012).
Jung, H. W., Tschaplinski, T. J., Wang, L., Glazebrook, J. & Greenberg, J. T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
Mauch-Mani, B. & Slusarenko, A. J. Production of salicylic acid precursors is a major function of phenylalanine ammonia-lyase in the resistance of arabidopsis to Peronospora parasitica. Plant Cell 8, 203–212 (1996).
Wildermuth, M. C., Dewdney, J., Wu, G. & Ausubel, F. M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565 (2001).
Staswick, P. E. & Tiryaki, I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16, 2117–2127 (2004).
Yan, J. B. et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21, 2220–2236 (2009).
Barber, M. S., Bertram, R. E. & Ride, J. P. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 34, 3–12 (1989).
Ren, Y. Y. & West, C. A. Elicitation of diterpene biosynthesis in rice (Oryza sativa L.) by chitin. Plant Physiol. 99, 1169–1178 (1992).
Kuchitsu, K., Kikuyama, M. & Shibuya, N. N-Acetylchitooligosaccharides, biotic elicitor for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma 174, 79–81 (1993).
Felix, G., Regenass, M. & Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 4, 307–316 (1993).
Sanchez-Vallet, A., Mesters, J. R. & Thomma, B. P. The battle for chitin recognition in plant-microbe interactions. FEMS Microbiol Rev. 39, 171–183 (2015).
Zhang, B., Ramonell, K., Somerville, S. & Stacey, G. Characterization of early, chitin-induced gene expression in Arabidopsis. Mol. Plant Microbe 15, 963–970 (2002).
Libault, M., Wan, J., Czechowski, T., Udvardi, M. & Stacey, G. Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol. Plant Microbe 20, 900–911 (2007).
Aarts, N. et al. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl Acad. Sci. USA 95, 10306–10311 (1998).
Fu, D. Q., Ghabrial, S. & Kachroo, A. GmRAR1 and GmSGT1 are required for basal, R gene-mediated and systemic acquired resistance in soybean. Mol. Plant-Microbe Interact. 22, 86–95 (2009).
Yun, B. W. et al. Loss of actin cytoskeletal function and EDS1 activity, in combination, severely compromises non-host resistance in Arabidopsis against wheat powdery mildew. Plant J. 34, 768–777 (2003).
Zhang, J. et al. Effector-triggered and pathogen-associated molecular pattern-triggered immunity differentially contribute to basal resistance to Pseudomonas syringae. Mol. Plant Microbe Inter. 23, 940–948 (2010).
Bate, N. J. et al. Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc. Natl Acad. Sci. USA 91, 7608–7612 (1994).
Mishina, T. E. & Zeier, J. Bacterial non-host resistance: interactions of Arabidopsis with non-adapted Pseudomonas syringae strains. Physiol. Plant. 131, 448–461 (2007).
Khan, W., Prithiviraj, B. & Smith, D. L. Chitosan and chitin oligomers increase phenylalanine ammonia-lyase and tyrosine ammonia-lyase activities in soybean leaves. J. Plant Physiol. 160, 859–863 (2003).
Wan, J., Zhang, X.-C. & Stacey, G. Chitin signaling and plant disease resistance. Plant Signal. Behav. 3, 831–833 (2008).
Wu, J. & Baldwin, I. T. New insights into plant responses to the attack from insect herbivores. Ann. Rev. Genet 44, 1–24 (2010).
George, J., Robbins, P. S., Alessandro, R. T., Stelinski, L. L. & Lapointe, S. L. Formic and acetic acids in degradation products of plant volatiles elicit olfactory and behavioral responses from an insect vector. Chem. Senses 41, 325–338 (2016).
George, J., Shi, Q., Stelenski, L. L., Stover, E. & Lapointe, S. L. Host selection, oviposition and feeding by a phytopathogen vector, Diaphorina citri (Hemiptera: Liviidae), modulated by plant exposure to formic acid. Front. Ecol. Evol. 29, https://doi.org/10.3389/fevo.2019.00078 (2019).
Paré, P. W. & Tumlinson, J. H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 121, 325–332 (1999).
Mumm, R., Posthumus, M. A. & Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 31, 575–585 (2008).
Erb, M. et al. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 6, 6273 (2015).
Mann, R. S. et al. Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLOS Pathog. 8, e1002610 (2012).
Fu, Z. Q. & Dong, X. Systemic acquired resistance: turning local infection into global defense. Ann. Rev. Plant Biol 64, 839–863 (2013).
Bos, J. I. et al. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). Plos Genet 6, e1001216 (2010).
Bonani, J. P. et al. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Èntomol. Exp. et. Appl. 134, 35–49 (2010).
Mafra, V. et al. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. Plos ONE 7, e3126310 (2012).
Acknowledgements
We thank Ellen Cochrane, Yolanda Avila, Chelsea Veith, Shelby Durden, Larry Markle, and Itze Cabral for their technical assistance. Special thanks to Matt Hentz for the guidance on the design of ACP host choice assay and use of the controlled environment chamber. We thank Anna Sarah Hill for maintaining and providing ACP for the tests. Funding was provided by the Citrus Research and Development Foundation (CRDF), Inc., Lake Alfred, FL, USA.
Author information
Authors and Affiliations
Contributions
Q.S., J.G., S.L., and E.S. conceived the project idea. Q.S. and J.G. designed and conducted the experiments. Q.S., J.G., J.K., and S.Z. analyzed the data. Q.S., J.G., J.K., and E.S. wrote the paper. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. USDA is an equal opportunity provider and employer. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the United States Department of Agriculture.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, Q., George, J., Krystel, J. et al. Hexaacetyl-chitohexaose, a chitin-derived oligosaccharide, transiently activates citrus defenses and alters the feeding behavior of Asian citrus psyllid. Hortic Res 6, 76 (2019). https://doi.org/10.1038/s41438-019-0158-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41438-019-0158-y
This article is cited by
-
De novo transcriptome assembly and global analysis of differential gene expression of aphid tolerant wild mustard Rorippa indica (L.) Hiern infested by mustard aphid Lipaphis Erysimi (L.) Kaltenbach
Functional & Integrative Genomics (2024)
-
Eco-friendly and safe alternatives for the valorization of shrimp farming waste
Environmental Science and Pollution Research (2023)
-
Increased probing activities of green peach aphid (GPA), Myzus persicae, on chitosan-treated caisim (Brassica juncea) monitored by electrical penetration graph (EPG)
International Journal of Tropical Insect Science (2021)