Abstract
Plants protect their tissues from insect herbivory with specialized structures and chemicals, such as cuticles, trichomes, and metabolites contained therein. Bacteria inside the insect gut are also exposed to plant defences and can potentially modify the outcome of plant–insect interactions. To disentangle this complex multi-organism system, we used tomato mutants impaired in the production of plant defences (odorless-2 and jasmonic acid–insensitive1) and two cultivars (Ailsa Craig and Castlemart), exposed them to herbivory by the cabbage looper (Trichoplusia ni H.) and collected the insect frass for bacterial community analysis. While the epicuticular wax and terpene profiles were variable, the leaf fatty acid composition remained consistent among genotypes. Moreover, larval weight confirmed the negative association between plant defences and insect performance. The distinctive frass fatty acid profiles indicated that plant genotype also influences the lipid digestive metabolism of insects. Additionally, comparisons of leaf and insect-gut bacterial communities revealed a limited overlap in bacterial species between the two sample types. Insect bacterial community abundance and diversity were notably reduced in insects fed on the mutants, with Enterobacteriaceae being the predominant group, whereas putatively pathogenic taxa were found in wildtype genotypes. Altogether, these results indicate that plant defences can modulate insect-associated bacterial community composition.
Similar content being viewed by others
Introduction
Plants and insect herbivores are involved in an evolutionary arms race. Upon feeding, insect herbivores are subject to plant structural and chemical defences that delay their development and reproduction. To counteract the effects of plant defences, insects have developed mechanisms to avoid and/or detoxify plant defence metabolites1,2,3,4,5. This has led to an escalation of metabolic diversity in plants6,7. Notably, plant- and insect-associated bacteria also play roles in modulating the outcome of plant–insect interactions8. Examples show that insect-gut associated bacteria can suppress plant antiherbivore defences and degrade toxic plant specialized compounds; meanwhile, plant-associated bacteria can aid in plant resistance by slowing insect growth4,9,10. However, insect herbivores frequently host advantageous bacterial endosymbionts that play roles in facilitating their growth and enhancing their stress resistance11. Previous studies have shown that caterpillar gut bacterial communities can be influenced by the plant species the insect feeds on and environmental factors8,12. Yet, how different genotypes of the same plant with varying levels of defences impact the structure of bacterial communities is vastly unknown.
From the first contact with plants, insects are targeted by plant preformed specialized metabolites and structures. The aerial surface of all plants is covered by a protective epicuticular wax layer composed of several major classes of lipidic components such as alkanes, alcohols, free fatty acids, and triterpenoids. The chemical composition of this surface can alter the feeding and locomotion of insect herbivores13,14,15,16. For example, cabbage loopers, aphids (Acyrthosiphon pisum H.), and diamondback caterpillars (Plutella xylostella L.) display increased locomotion and lower falling frequencies in plants with reduced wax abundance14. Additionally, plant epidermal surfaces can be covered by hair-like structures (trichomes) that aid in resistance against insect herbivores. Non-glandular trichomes can hinder the movement of small insects and rupture their gut peritrophic matrix upon ingestion, whereas glandular trichomes produce chemical exudates that negatively impact insect growth and development17,18,19,20. Tomato leaves are abundant in glandular trichomes which accumulate terpenes, acylsugars, and anthocyanins6,21,22. These chemicals are toxic to insects and the rupture of glandular trichomes by caterpillars can induce the expression of defensive genes 23.
Once insect herbivores bypass constitutive defences, they are exposed to inducible defence mechanisms that are coordinated predominantly by the lipid-based phytohormone jasmonic acid (JA) and its derivatives. The cascade of responses is initiated by perception of damage-associated molecular patterns (DAMPs) released from insect oral secretions and damaged plant tissue, that in turn induce JA biosynthesis in plants6,24. JA is conjugated with isoleucine to form the bioactive jasmonoyl-L-isoleucine (JA-Ile), which binds to the CORONATINE-INSENSITIVE1 (COI1)-JASMONATE ZIM-domain coreceptor, and induces transcriptional changes that lead to increased production of plant chemical and structural defences against herbivores25,26.
Considering the abundance of plant defensive strategies that herbivores are exposed to, it is crucial to understand whether and how insect gut bacterial communities are affected by their presence. Plant terpenes can disturb gut chemiosmosis and enhance the crossover of plant toxins from the gut to the hemolymph, altering the composition and abundance of insect-associated bacteria27,28. Sakuranetin, a JA-induced specialized metabolite from rice, was shown to limit the growth of beneficial endosymbionts in an Hemipteran insect29. However, most Lepidopteran gut bacterial microbiomes are relatively simple and consist of a few dominant bacterial taxa, predominantly belonging to the genera Enterobacter, Pseudomonas, and Enterococcus30,31. Enterobacter spp. can produce enzymes that help Lepidopteran species to detoxify plant phenolics, as well as provide essential amino acids that insects cannot synthesize32. Members of Pseudomonas can detoxify alkaloids, which are JA-regulated specialized metabolites, in the gut of two Lepidopteran species33. Gut bacteria from the genus Bacillus, Enterococcus, and Staphylococcus can help overcome chemical induced defenses with their protease activity31. Despite the wide distribution of microbes in the environment, interspecific differences in the gut bacterial composition between two Lepidopteran species consuming the same plant host have been seen. This implies that internal selective forces that influence the gut bacterial microbiome structure are at play12. While these findings highlight the importance and roles of insect gut bacteria, the extent to which plant defences affect the insect gut bacterial microbiome is still to be determined. Therefore, comprehensive approaches that examine the tripartite relationship by assessing the chemical composition of plant hosts, insect herbivore performance, and bacterial communities are needed.
Here, we use the interaction between tomato (Solanum lycopersicum L.) and the cabbage looper (Trichopulsia ni H.: Lepidoptera) to determine if bacterial communities associated with the insect digestive tract change in response to plant defences on the host plant. To manipulate the levels of plant defences, we used two tomato cultivars (Ailsa Craig and Castlemart) and two tomato mutants, jasmonic acid-insensitive1 (jai1) and odorless-2 (od-2), with reduced levels of plant defences34,35. The largest defect in plant defences is seen in the tomato jai1, which contains a knock-out mutation on the COI1 gene. Hence, jai1 mutants are highly susceptible to insect herbivores due to the inability to induce all jasmonate-related defences, including protease inhibitors, reduced trichomes and terpenes35,36. In contrast, the tomato od-2 mutant has phenotypes associated with constitutive epidermal defences, including lower density and distorted type VI glandular trichomes, trace amounts of trichome-borne volatile compounds and flavonoids. Whereas the accumulation of acylsugars, glycoalkaloids, and protease inhibitors induced by JA are not affected. The defects make od-2 susceptible to Coleopterans and Hemipterans34,37.These mutants provided two levels of defects in plant defences for testing the impact on the associated microbiome. We used a combination of metabolite profiling of lipid-derived compounds, insect bioassays and 16S rRNA gene sequencing to ask if microbial communities inside the gut change in response to plant defences. Our results highlight the significance of including the microbiome in plant–insect studies, recognizing that the microbiome is an active participant that responds to plant defences.
Methods
Plant growth conditions
Seeds of tomato (S. lycopersicum) cv. Castlemart (CT), cv. Ailsa Craig (AC), the mutants od-2 and jai1 (in the CT background) were kindly provided by Dr. Gregg Howe (Michigan State University, USA), as per the seed import guidelines provided by the Canadian Food Inspection Agency. Seeds were surface sterilized with chlorine gas (97% bleach and 3% HCl) and germinated under sterile conditions in the dark. The jai1 homozygous seedlings were selected by application of 1 mM methyl-jasmonate (MeJA, PhytoTechnology Laboratories), as previously described35. The jai1 homozygotes were identified based on their longer roots and the absence of purple hypocotyls, and further confirmed by PCR. Five days post germination, seedlings were transferred to autoclaved substrate (1:1 Pro-Mix PGX: HP MYCORRHIZAE) and grown in a growth chamber set at 26°C (12h with light) and 22°C (12h darkness). Four-week-old plants were moved into the greenhouse at the University of Toronto—Scarborough with a daily average temperature of 25.1°C and 32.5% humidity. This study complies with local and national guidelines. As S. lycopersicum is a commonly grown vegetable, no permission is required to collect or grow it.
Insect rearing
T. ni eggs were obtained from the Insect Production Services (Great Lakes Forestry Centre, Sault Ste. Marie, ON, Canada) and hatched at room temperature38. Caterpillars were reared on five-week-old plants of four different genotypes (AC wildtype, CT wildtype, jai1, od-2), from neonate stage until they reached the last instar stage (approximately two weeks). Two butterfly cages were set up per genotype, each containing eight plants and thirty newly hatched caterpillars. Caterpillar weight was recorded at several time points throughout the experiment and insects were returned to the same cage after measurements.
Foliar volatile terpene analysis
To better control the amount of insect damage, plants used for metabolite analysis were treated by caging one second- or third-instar insect to the fifth youngest leaf. The leaf immediately above (fourth youngest) was used for all metabolite analysis. From this leaf, different leaflets were used for terpene and cuticular wax analysis. Three to four replicates per genotype were used. Analysis of volatile terpenes was conducted as previously described with some modifications39,40. One leaflet was dipped in 1 mL of hexane containing 10 μg/mL of tetradecane (Alfa Aesar) as internal standard and gently shaken for 5 min at room temperature. The solvent was transferred to a 2mL-glass vial and leaflets were dried overnight in an oven before measuring dry weight. Samples were run on the Agilent 6890N Series GC-FID (gas chromatography-flame ionization detector) System (Agilent Technologies). Separation was achieved by injecting 1μl of hexane extract into an HP-1 column (30 m × 0.32 mm × 1.00 μm; Agilent) using the following temperature profile: 50 °C for 2 min; 5 °C/min to 230 °C; 45 °C/min to 300 °C with 5 min hold time. For peak identification, a representative sample was run on a GC–MS. Mono- and sesquiterpene compounds were identified by comparing their mass spectra to a volatile library41. Peaks areas were normalized to the internal standard and dry leaf weight.
Cuticular wax profiling
For wax extraction, a second leaflet from the same leaf used for terpene analysis was collected. Leaflet areas were scanned prior to being stored at −80 °C. Cuticular wax extraction was performed as per previous protocols42. Leaflets were dipped in 10 mL of chloroform containing 1 μg/μL of the internal standard tetracosane (Agilent Technologies). Separation was achieved by injecting 1 μL into a GC-FID equipped with a HP-1 column (30 m × 0.32 mm × 1.00 µm; Agilent) using the following temperature profile: 50 °C for 2 min; 45 °C/min to 200 °C with 1 min hold time; 4 °C/min to 300 °C with 10 min hold time. Peaks were identified as described above and peak areas were normalized to the internal standard and leaflet surface area.
Fatty acid methyl ester analysis
Fatty acid methyl esters (FAME) were extracted as per previous protocols43, with minor modifications. Briefly, frass and leaf samples were ground to powder with liquid nitrogen and 40 mg weighed in glass vials. Each frass and leaf sample had three technical replicates. To each vial, 2 mL of a 1.5 M H2SO4 solution containing 5 μg/mL nonadecanoic acid (internal standard; Agilent Technologies) was added, then heated to 85°C for 1.5 h with periodic mixing. After cooling, pentane and 0.9% NaCl (1:1) was added to separate methyl esters. The organic phase was analyzed by an Agilent 5977A Series GC-MSD (GC-mass spectrophotometer detector) fitted with a 30 m × 0.25 mm × 0.25 μm HP-5 column using the following temperature program: 8 °C/min from 100 to 250 °C with 10 min hold time. Compounds were identified by comparing their spectra against the NIST library, and peak areas were integrated using MassHunter Quantitative Analysis (Agilent Technologies). Areas were normalized to the internal standard and ground weight.
Leaf- and insect-associated bacteria amplicon sequencing
Frass (insect feces) was used as a proxy for insect gut-associated bacteria. This choice was supported by previous studies on cabbage-fed T. ni that showed similar bacterial communities across different organs (i.e., the alimentary canal, Malpighian tubules, and mandibular glands)44. Similarly, in Lymantria dispar grown in the wild and the lab, small differences were found in bacterial abundance in frass and gut45. To avoid environmental contamination of the samples during frass collection, insects from each cage were transferred to individual sterile containers for 4–6 h. The third leaf from the top was collected from three randomly selected tomatoes from each cage. Frass and leaf samples were collected after approximately two weeks of herbivory. All samples were frozen in liquid nitrogen and stored at −80°C until processing. 16S rRNA gene sequencing and analysis was performed by Microbiome Insights (Vancouver, BC, Canada), using primers targeting the V4 region on an Illumina MiSeq, as per previous protocols46. Peptic nucleic acid (PNA) PCR clamps were included in all samples to limit the amplification of host chloroplast- and mitochondria-derived 16S rRNA genes.
Fastq files were quality-filtered and clustered into 97% similarity Operational Taxonomic Units (OTUs), using the mothur software package and its standard operational protocol47. Briefly, sequence pairs were concatenated and resulting sequences with lengths higher than 275 bases or ambiguous bases in them were removed. Sequences were then aligned against the Silva v132 master alignment, and trimmed to the region delimited by 13,862 and 23,444 positions. Chimeras were removed with uchime. Sequences were then classified with the Silva database using the RDP Naïve Bayesian classifier, and those classified as mitochondria, chloroplast, archaea, Eukaryota, or unknown were removed to account for non-specific amplification.
Statistical analyses
Data analysis for terpenes, epicuticular waxes, and larval weight was performed on MVApp40. Briefly, data normality and variance were assessed using either the Bartlett’s or Levene’s tests. If these conditions were not satisfied, data was log-transformed to achieve normality. Further, we performed one-way and two-way ANOVA analyses to examine variation. Principal component analysis was performed in R using the vegan package48. OTUs were plotted in R to visualize the relative abundances of the bacteria at the phylum and genus level, and alpha- and beta-diversities49. We tested for difference among diets with PERMANOVA using vegan48. Negative binomial tests (DESEq2)50 were performed for differential OTU abundance analysis relative to plant host. Graphical representations were performed on R version 4.1.151 with ggplot252.
Results
Volatile terpene profiles varied with tomato genotype
To assess the effect of leaf specialized metabolites on the insect microbiome, we first compared the leaf volatile terpene composition of the jai1 and od-2 mutants, their corresponding wildtype background (CT), and an additional tomato cultivar (AC). Under our experimental conditions, five monoterpenes (⍺-pinene, ∂-carene and ⍺-phellandrene, ⍺-terpinene, and β-phellandrene) and lower levels of three sesquiterpenes (∂-elemene, β-caryophyllene, and ⍺-humulene) were detected. Consistent with previous reports34,35, the volatile terpene levels of jai1 and od-2 were significantly affected, with a reduction of 75% observed in jai1 and undetectable levels in od-2 (Fig. 1). The two wildtype lines also differed in terpene accumulation, with AC accumulating approximately 40% less terpenes compared to CT. Despite their independent biosynthetic origins, mono- and sesquiterpene levels followed similar patterns. This reduction is expected given the trichome alterations observed in both mutants (Supplementary Fig. S1).
Basal volatile terpene levels in leaves of AC, CT, jai1, and od-2. Mono- (left panel) and sesqui- (right panel) terpenes were collected in hexane and run on GC-FID (method conditions led to coelution of δ-carene with α-phellandrene). Inset figures represent significant differences in the total average of monoterpenes (one-way ANOVA, p = 0.0336) and sesquiterpenes (one-way ANOVA, p = 0.0221) in each genotype. Three pairwise comparisons were done using a Tukey HSD test, with a Bonferroni adjustment for post-hoc comparisons. The od-2 mutant was excluded from the comparison due to undetectable levels. The different letters indicate significant differences. Each data point represents the mean ± SE of four biological replicates. nd, not detected.
To assess potential changes in the leaf terpene accumulation upon insect feeding in the mutants, mono- and sesquiterpene levels were compared in systemic leaves over the course of 48 h of herbivory (Supplementary Fig. S2). Unexpectedly, the amount of terpenes did not increase in preformed leaves. Overall, the genotype and time had a significant impact on both monoterpene (two-way ANOVA, log10-transformed data, F(3)time = 5.02, ptime = 0.0059; F(2)genotype = 23.62, pgenotype = 5.85e-07) and sesquiterpene levels (two-way ANOVA, log10-transformed data, F(3)time = 5.21, ptime = 0.0075; F(2)genotype = 7.16, pgenotype = 0.0043). Upon insect feeding, jai1 plants maintained their lower levels of accumulation compared to CT, and terpenes were still below detection in od-2 plants despite having a functional jasmonate signaling pathway. These results indicate that, although there are dynamic changes in volatile terpenes with herbivory, the basal levels of preformed leaves are a good representation of the differences across genotypes.
Additional surface compounds affected in tomato defence mutants
To determine if the tomato genotypes also differ in their cuticular wax composition, larger non-polar lipids were collected. The leaf epicuticular waxes consisted of very-long-chain (VLC) alkanes (C27-C33) and methyl-branched alkanes (C30-C32), fatty acids (C16 and C18), and triterpenoids (⍺- and β- amyrin) (Fig. 2). Hentriacontane (C31) was the predominant component of foliar waxes in all genotypes, followed by 2-methyltriacontane (C30). Overall, the total load of alkanes and methyl-branched alkanes were lower in AC than CT (Fig. 2). The jai1 mutation did not significantly affect cuticular wax accumulation, although lower levels of alkanes were present in jai1 relative to CT. Interestingly, the od-2 mutant seemed to accumulate higher amounts of alkanes and branched alkanes, as well as triterpenoids, although not significantly. The accumulation of triterpenoids in od-2 contrasts with the severe defect in the accumulation of mono- and sesquiterpenes.
Basal foliar wax profiles of AC, CT, jai1, and od-2. Leaf wax compounds were grouped into four major classes: (1) alkanes, (2) branched alkanes, (3) fatty acids, and (4) triterpenoids. Inset graph indicates total wax amount for each chemical class. No significant differences were observed among genotypes in either of the classes (one-way ANOVA, palkanes = 0.276, pbranched alkanes = 0.332, pfatty acids = 0.247, ptriterpenoids = 0.294). Each data point represents mean values ± SE (nAC,CT = 3, nja1,od-2 = 4).
Upon herbivory, similar trends were observed for different compound classes regardless of whether they were derived from very-long-chain fatty acids (alkanes, branched alkanes and fatty acids) or from isoprenyl diphosphates (triterpenoids) (Supplementary Fig. S2). No significant differences were observed over time when compounds were combined into classes, except for free fatty acids. Overall, time had a significant impact on the VLC fatty acids levels for both wildtypes (two-way ANOVA, log10-transformed data, F(3) = 6.53, p = 0.0029). Whereas genotype played a more significant role in the VLC fatty acids trends observed in the mutants relative to their background cultivar (two-way ANOVA, log10-transformed data, F(2) = 3.08, p = 0.017). The od-2 mutant generally maintained higher levels of all compound classes relative to CT (Supplementary Fig. S3), whereas the jai1 showed consistently, but not significantly, lower levels than CT.
Insect performance is influenced by plant host
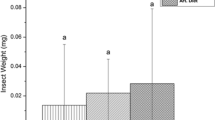
To test if the mutations in jai1 and od-2 translate to differences in insect performance under our experimental conditions, neonate larvae were allowed to feed ad libitum and weighed. The interaction between plant host and feeding time had a significant impact on the insect performance (two-way ANOVA, F(6, 313) = 22.095, p < 2.2e−16) (Fig. 3a). Despite the differences in volatile terpene and wax content, insects reared on both wildtype cultivars gained similar weight. Seven days after feeding, caterpillars reared on jai1 and od-2 were significantly heavier than their wildtype counterparts. However, at later time points, the caterpillars reared on od-2 performed similarly to those reared on the wildtype cultivars, whereas the ones on jai1 consistently gained more weight.
Performance of cabbage loopers on four tomato genotypes. (a) Insect performance timeline relative to diet source at 7, 10, and 14 days of herbivory. Larval weight was significantly impacted by the diet source (two-way ANOVA, F = 115.795, p < 2.2e−16) and time (two-way ANOVA, F = 46.887, p < 2.2e−16). Six pairwise comparisons of the log10-transformed data were done using a Tukey HSD test, with a Bonferroni adjustment for post-hoc comparisons. The stars represent significant differences between the defence mutants relative to the background cultivar, CT. No significance was observed between caterpillars fed on AC and CT. (b) Representative damage after 48 h of feeding by two last-instar caterpillars. Caterpillars were grown on wheat germ diet until the fourth instar before placing on a detached leaf from each genotype.
Within the cage setting, insects reached the last instar at different time points depending on their host plant, complicating comparisons of tissue damage. Hence fourth instar caterpillars were placed on a detached leaf from each genotype to compare tissue consumption. After 48 h, damage is evident on all leaves, yet the jai1 showed more defoliation relative to the other genotypes (Fig. 3b). This indicates that the increased weight sustained by the mutant plants is accompanied by more leaf damage by last instar caterpillars.
Fatty acid composition altered during insect digestion
In this study, we focused on measuring lipidic specialized metabolites (terpenes and waxes); however, we did not know if primary metabolites were also affected in the mutants. To tackle this issue, we used fatty acids, an important component of the insect diet, to survey the changes that occur during digestion by comparing the fatty acid composition of the food (leaves) and end-product of digestion (frass). The leaf fatty acid composition consisted of three saturated, eight unsaturated, and three branched long-chain fatty acids (Fig. 4). Unlike the differences in specialized metabolites, no significant differences were found across plant genotypes, indicating that the mutations in plant defences present in jai1 and od-2 are not likely affecting lipid primary metabolism.
Variation in relative abundance of fatty acids in leaf and frass samples. Fatty acids measured as fatty acid methyl esters were grouped into four classes: (a) medium chain saturated, (b) long chain saturated, (c) long chain unsaturated, and (d) branched long chain saturated. Each bar represents the relative mean of four biological replicates. nd, Not detected.
Frass contains a mixture of fatty acids produced by the plant, the insect, and also by microbes. Most compounds were shared between leaf and frass samples, except for three saturated medium-chain fatty acids, pentadecanoic acid, and pentadecenoic acid that were unique to frass samples. Meanwhile, two isomers of octadecadienoic acid were identified exclusively in the leaf. Palmitic acid (C16) and stearic acid (C18) were the predominant compounds in leaves and frass but did not differ in quantity (Fig. 4b). Overall, there seems to be a tendency towards shorter fatty acids in the frass as expected from catabolic processes. However, the leaves had a higher relative abundance of saturated long chain fatty acids than frass (Supplementary Fig. S4). Differences in the relative abundance of unsaturated and branched long chain fatty acids were also observed between frass and leaves (Fig. 4c, d; Supplementary Fig. S4a). Overall, leaf samples cluster closer to each other and separately from frass samples (Fig. 5). This indicates that although food retention time for Lepidopterans is short, there are large changes in fatty acid composition as plant material is digested.
Principal component analysis (PCA) of fatty acids from leaf and frass samples. Two-dimensional PCA score plots reveal separation driven by sample type. Ellipses represent the 95% confidence interval. Based on the 1.5 IQR rule, one outlier was identified and removed in the frass samples (nleaf = 12 and nfrass = 11).
Interestingly, insects fed on CT had higher quantity of fatty acids in their frass (Supplementary Fig. S4b). These insects were exposed to an active jasmonate signalling pathway and to the largest amount of terpenes and waxes in their diet. The detected amounts of medium-chain saturated fatty acids were similar in frass samples from caterpillars fed on AC and CT (Fig. 4a). However, insects fed on jai1 and od-2 had a 56% and 61% reduction, respectively, in undecanoic acid (C11) levels relative to the insects fed on the background cultivar. In contrast, pentadecanoic (C15) and 14-methyl-pentadecanoic acid (iC15:0) acid relative abundance levels were elevated 50% in jai1 and od-2 relative to the amounts detected in CT (Fig. 4b, d). This indicates that the genotype of the host plant, not only affects larval weight, but also disturbs insect digestive metabolism of lipids.
Insect bacterial communities did not mirror leaf bacterial communities
The short food retention time and relatively simple insect digestive tract of Lepidopterans has been used to question the functionality of the insect gut bacterial microbiome53. If this is true, we hypothesized that frass bacterial communities would be a close reflection of the bacterial communities present on the leaf. To test this, leaf and frass bacterial communities were compared to determine the extent of the contribution of the leaf microbiome to the frass. 16S rRNA genes were amplified from frass and the insect’s corresponding diet source, generating a total of 823,749 reads. Leaf samples represent only approximately 37% of the total reads indicating possible under sampling; however, strong ecological drivers in bacterial populations can still be identified54.
Non-metric multidimensional scaling (NMDS) was used to visualize similarity between samples. The analysis showed that leaf and frass bacterial communities are clearly separated (Fig. 6a). A Permutation Multivariate Analysis of Variance (PERMANOVA) indicated that both sample type (frass or leaf) and genotype (AC, CT, jai1 and od-2) had a significant impact on segregation of bacterial communities. To investigate the diversity within the communities, alpha diversity was estimated by comparing the Chao1, ACE, Shannon, and Simpson indexes (Supplementary Table S1). Leaf bacterial communities had many diverse groups, with few dominating bacterial genus (Supplementary Fig. S5). In contrast, frass samples had reduced bacterial diversity as their communities were dominated by members of Enterobactericeae, Enterobacterales, Yersinaceae, and Chrysoeobacterium.
Comparison of bacterial community composition. (a) A Bray–Curtis dissimilarity index was used to compare frass and leaf samples. Non-metric multidimensional scaling (NMDS) analysis separates samples based on sample type and genotype. The separation between the communities was significantly impacted by both genotype and sample type (PERMANOVA, n = 4–7, p = 2e−04). (b) Shared and unique OTUs found in AC and CT leaves and frass from insects reared on those genotypes.
The frass and leaf bacterial communities of the two wildtype cultivars (AC and CT) were further compared for common taxa. Only 5% of the OTUs were shared between the four samples (Fig. 6b). Within the 5%, we found bacterial members of Enterobacteriaceae OTU0001, Pseudomonas OTU0002, and Acinetobacter OTU0003. Similar pairwise comparisons between the insect-associated bacteria and their corresponding diet source for each of the four genotypes indicated that on average only 13.75 ± 1.97% OTUs are shared (Supplementary Table S2. Altogether, this indicates that although the insects are feeding entirely on tomato, their gut bacterial microbiome is not an exact reflection of the bacterial communities present on the leaf.
Larval gut bacterial microbiomes varied across tomato genotypes
In addition to differences in plant specialized metabolite composition and insect growth among genotypes, we also observed differences in the fatty acid profiles and bacterial communities of plants and insect guts. Next, we tested if gut bacterial communities change relative to genotype insects were reared on. The insect-associated OTUs were classified into three main phyla: Bacteroidota, Firmicutes, and Proteobacteria. Proteobacteria was predominant in all insects regardless of their diet source, accounting for over 90% of the abundance across all frass samples. However, at the genus level, differences in bacterial abundance between the two wildtype cultivars as well as between the mutants and their background cultivar were observed (Fig. 7a). Bacterial groups belonging to unclassified genera in the Enterobacteriaceae family predominated across the samples. The unclassified Enterobacteriaceae group made up 48% of the bacterial abundance in insects reared on CT, and over 85% in insects reared on AC (87%), jai1 (90%), and od-2 (99%). Significant differences were observed across frass samples in unclassified Chryseobacterium, Pseudomonas, Yersiniaceae, Acinetobacter, and unclassified Enterobacterales (p < 0.001 for each) (Supplementary Table S3). Based on OTU counts, Chryseobacterium OTU005 and Pseudomonas OTU002 were more abundant in CT relative to the mutants and the other wildtype cultivar (Fig. 7b, c).
Frass bacterial community composition as function of host plant genotype. (a) The bar plot displays the average bacterial genus abundance of five replicates. Depicted bacterial genera accounted for at least 2% of the total, otherwise they were categorized as “Other”. (b, c) Relative abundance of the two most predominant bacteria genera was compared across samples relative to their diet source using DESeq (p < 0.05; log10-transformed data).
We further tested for differences in alpha and beta diversity in the frass bacterial communities across plant genotypes. The Simpson diversity index was highest in the samples of insects fed CT relative to all the others (Fig. 8a). The insects reared on od-2 had a Simpson index of 0.023 ± 0.002, suggesting that their community structure was dominated by few bacterial groups (Supplementary Table S4). Based on Bray–Curtis dissimilarity indexes, the genotype of the plant on which the insects fed on had a significant impact on driving the segregation between the frass bacterial communities (Fig. 6a). Insects fed on CT had scattered clustering compared to the ones fed on either the mutants or AC. However, clear clustering patterns relative to diet source were observed, supporting that the plant genotype is a key modulator of the insect gut bacterial microbiome composition. Interestingly, insects fed on genotypes not impacted in defences had more unique OTUs relative to the mutants (23% unique OTUs in CT and 16% in AC compared to 6% in jai1 and 4% in od-2) (Fig. 8b). Bacterial members of Cyanobacteriia OTU0020, Streptococcus OTU0045, and Lachnospiraceae OTU0054, along 16 other OTUs, were shared among insects fed on the two wildtypes, but not found in the mutant-fed insects. Moreover, only 2% of the OTUs were shared among insects fed on CT and the two defence-deficient mutants. Overall, this suggests that the presence of plant defences is associated with increased diversity of taxa found in the frass.
Discussion
Tomato defences affect insect growth
The damage and increased weight on the mutants were proportional to the defect in plant defences in jai1 and od-2. JAs signaling coordinates the biosynthesis of many specialized metabolites, one class being terpenes23,34,55. In contrast, despite the severe reduction in terpenes in od-2, other defensive metabolites and proteins still accumulate. After seven days of feeding, insects reared on od-2 gained more weight than those on wildtype, but the difference in growth was not maintained at later time points. This suggests that metabolites that are impaired in od-2 are particularly important in defending plants at early stages of infestation and/or when larvae have recently hatched. This is consistent with the defects of od-2 on several constitutive surface-related structures and chemicals34. Interestingly, although the insects reared on the od-2 mutant caught up in growth with the ones on WT after 10 days, we could still detect differences in the bacterial communities present in larvae from the later instars. This suggests that the effects of plant defences on bacterial communities are not transient and emphasize the need to include additional measurements in plant–insect interactions studies, since insect weight only provides a partial picture. In contrast, insects reared on the jai1 mutant showed increased weight across 14 days, expected from the larger defects observed upon disruption of JA signaling. Structures and specialized metabolites present in the epidermis are likely to be more relevant in early stages of plant–insect interactions, before induction of jasmonate defences.
The jai1 and od-2 mutants have been previously reported to have defects in the accumulation of defensive metabolites and trichomes, without major effects on growth or development34,35. As expected, the defence-deficient mutants, had lower terpene accumulation than their background cultivar (CT). In od-2 and jai1, the levels of mono- and sesquiterpenes are reduced; however, similar levels of triterpenes were detected in their epicuticular waxes relative to the background CT. This could indicate that the biosynthesis of triterpenes is under independent control in epicuticular waxes, and/or the supply of isoprenoid substrates is not the limiting factor for the emission of volatile terpenes in either od-2 or jai1. In addition to the major defects in trichomes, od-2 has irregularly-shaped and raised epidermal pavement cells34. The data presented here indicates that this phenotype is accompanied by an increase in cuticular waxes, particularly linear and branched alkanes. It is worth noting that to be emitted into the atmosphere, terpenes produced in trichomes and epidermal cells must cross the cuticle; hence alterations in the cuticle can have downstream effects on emission of terpenes56. It is possible that the odorless phenotype is a consequence of a larger developmental defect in epidermal cells, including trichomes and cuticular waxes coating epidermal cells. As the first line of defence against the environment, disentangling the multiple strategies present in epidermal cells and their impact on insect herbivory requires further exploration.
Because the phenotypes in the mutants could go beyond the specialized metabolites screened here and previously reported 34,35, we were interested in testing if the total fatty acid profile was affected as a proxy of differences in primary metabolism. Dietary fatty acids are essential in insect metabolism, playing roles in fat body, oocyte (eggs), and hormone synthesis57,58. Overall, there were no significant differences in the leaf fatty acid composition, unlike the differences observed in specialized metabolites, suggesting that the nutritional value was similar among cultivars and genotypes. As plant lipids are digested, they are hydrolyzed by lipases into free fatty acids, absorbed in the midgut, and further oxidized in the mitochondria for energy production57. Interestingly, even though leaf samples had similar fatty acid composition, frass samples showed some differences depending on the genotype the insects were fed on. Compared to leaf samples, frass samples had an approximate 10% and 50% reduction in long-chain saturated and branched fatty acids, respectively. These observations suggest that insect digestion of fatty acids can be modulated by plant defences.
Frass and leaves share limited bacterial taxa
Leaves are not homogenous surfaces, they are covered by a waxy cuticle, trichomes and a diversity of defensive compounds contained therein. These conditions can shape the structure of the phyllo- and endosphere bacterial communities59,60. Similarly, the insect gut provides a challenging habitat for microbial colonization, especially considering the alkaline conditions and that the midgut’s peritrophic matrix changes with every molt61. Overall, little is known about the mechanisms involved in bacterial colonization in the Lepidopteran gut. As insects consume leaves, their guts are exposed to the plant microbiome, which made us hypothesize that this would be the major source of bacterial communities53. However, our findings suggest that only a small percentage of bacteria are shared between plant and caterpillar gut bacterial microbiomes. These observations are consistent with previous reports in other Lepidopterans fed on tomato and Brassica spp.62,63, and hint at additional mechanisms for colonization. We used insects that were provided as surface-sterilized eggs, hence maternal transmission of bacteria is expected to be minimal. Moreover, seeds were also surface-sterilized before being planted in autoclaved growing media and grown in the same greenhouse environment, providing a homogenous environment for bacterial communities to colonize; rather than an environment with well-established resident bacteria from the egg, seed and soil. It is worth noting that, despite the range of conditions under which similar studies have been conducted, there is a core set of taxa that are consistently found associated with insect guts, including Enterobacteriaceae, Pseudomonas, Acinetobacter, and Enterococcus31. Even under these homogenizing conditions, insect gut bacterial communities were not a mere reflection of the leaf microbiome, supporting that physicochemical conditions of the gut are a crucial component in shaping the bacterial communities. How those conditions are changed by plant structural and chemical defences, and whether the effect is direct or indirect by affecting insect physiology requires further investigation44,61,64.
The cabbage looper gut bacterial community was predominantly composed of bacteria belonging to the Enterobacteriaceae family. This is consistent with previous findings in cabbage loopers reared on wheat germ diet, Arabidopsis thaliana, S. lycopersicum, and Brassica oleracea28,44,62, suggesting that Enterobacteriaceae spp. may be a constituent group. Some Enterobacteriaceae spp. have been shown to respond to terpene exposure28. In our studies, we noticed that the more terpenes a plant contains, the lower relative abundance of Enterobacteriaceae spp. Moreover, several Enterobacteriaceae isolates from Spodoptera exigua (Lepidoptera) have been shown to modulate JA-mediated defences in tomato by downregulating polyphenol oxidase and trypsin protease inhibitor activity, hence benefitting insect digestion65. In silkworm larvae (Lepidoptera), Enterobacter spp. (Enterobacteriaceae) contribute to the prevention of pathogen colonization11. In addition, Enterobacteriaceae OTUs were also found in leaves from AC and CT. In the case of shared OTUS, it would be interesting to follow up the colonization. The shared OTUs could be introduced from the plant to the insect by feeding, or introduced from the insect to the plant by regurgitation. This has been previously observed in Enterobacter isolates from Helicoverpa zea (Lepidoptera), where inoculation by regurgitation promotes tomato plant growth and yield without compromising anti-herbivore defenses66. In the particular case of the large Enterobacteriaceae spp. group, it would be important to improve the taxonomic resolution within this taxon before speculating on the function to the insect host. Amplicon sequencing is a valuable tool, yet it can underestimate the full diversity present in the samples67.
Insects feeding on plants with more defences have increased gut bacterial diversity
Despite having predominant groups, the bacterial communities in the cabbage looper gut were sensitive to the changes in plant defences presented in the mutants. For example, CT plants contained more trichomes and specialized metabolites, and as expected, insects reared on CT had poor growth. But this was also accompanied by an increased number of unique OTUs identified in their frass and a larger amount of total fatty acids. This could indicate that insect digestion has been negatively affected by the defences present in the plant, whether directly by impairing insect metabolism, or by shaping the gut bacterial microbiome remains to be determined. Additionally, we observed a pattern between the foliar terpene quantity and frass bacterial alpha diversity. Overall, alpha diversity was lower in insects that fed on plants with reduced terpenes. Although they varied in their bacterial community census, insects reared on AC and jai1 had similar diversity indexes. Interestingly, the terpene and wax loads of AC and jai1 were also similar. With minimal exposure to terpenes, the bacterial community of insects fed on od-2 was almost entirely composed of Enterobacteriaceae.
Additionally, our results show that Chryseobacterium spp. and Pseudomonas spp. were significantly more abundant in caterpillars with poor performance that had been reared on tomatoes with intact defences. Chryseobacterium spp. are known to be overrepresented in terpene exposed caterpillars and induce higher mortality in Protaetia brevitarsis seulensis (Coleoptera) due to their pathogenic nature68,69. Bacteria of the genus Pseudomonas inhabit cabbage looper midguts and have been linked to weaker immune systems in other insects44,70,71. However, the presence of Pseudomonas spp. may also indicate symbiotic relationships between insects and bacteria. For instance, the Colorado potato beetle (Coleoptera) uses Pseudomonas symbionts to supress tomato defences10. Our results suggest that plant defences may promote the colonization of the gut with a more diverse microbiome, including bacteria with known negative effects on insect growth. It is worth noting that mutations in the biosynthesis of specialized metabolites can often result in pleiotropic effects in other pathways72. Therefore, it is not clear if the affected bacterial communities are a direct consequence of structural and chemical defences affected in the mutants, or other non-described physiological defects. Future studies are needed to establish the relation between terpenes and certain OTUs; for example, by overexpression or knock-out of a terpene synthase. While we observed an interaction between plant genotype and insect microbiome, we cannot yet decouple the effects that the plant genotype has on bacterial communities.
In summary, our findings demonstrate that plant defences can have an influence on the insect gut bacterial microbiome. This study brings a thorough and unique perspective into the plant–insect-microbiome system by incorporating plant-specialized metabolite profiling, genotypic variation, frass lipid profiling and microbial community analyses. Insect gut bacterial communities are an integral component of plant–insect interactions, and a better mechanistic understanding of their roles is needed to utilize them in pest control practices.
Data availability
The 16S rRNA gene dataset generated during this study is deposited at NCBI SRA repository, under accession number PRJNA1006157 https://dataview.ncbi.nlm.nih.gov/object/PRJNA1006157?reviewer=ucffsjlcbebg142qc66bg47vbl.
References
Huber, M. et al. A beta-glucosidase of an insect herbivore determines both toxicity and deterrence of a dandelion defense metabolite. eLife 10, e68642 (2021).
Howe, G. & Schaller, A. direct defenses in plants and their induction by wounding and insect herbivores. In Induced Plant Resistance to Herbivory 7–29 (2008). https://doi.org/10.1007/978-1-4020-8182-8_1.
Felton, G. W. & Tumlinson, J. H. Plant–insect dialogs: complex interactions at the plant–insect interface. Curr. Opin. Plant Biol. 11, 457–463 (2008).
Mason, C. J., Peiffer, M., Hoover, K. & Felton, G. Tomato chemical defenses intensify corn earworm (Helicoverpa zea) mortality from opportunistic bacterial pathogens. J. Chem. Ecol. https://doi.org/10.1007/s10886-023-01420-7 (2023).
Tian, D., Tooker, J., Peiffer, M., Chung, S. H. & Felton, G. W. Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236, 1053–1066 (2012).
Howe, G. & Jander, G. Plant Immunity to insect herbivores. Annu. Rev. Plant Biol. 59, 41–66 (2008).
Lewandowska, M., Keyl, A. & Feussner, I. Wax biosynthesis in response to danger: its regulation upon abiotic and biotic stress. New Phytol. 227, 698–713 (2020).
Noman, A., Aqeel, M., Qasim, M., Haider, I. & Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci. Total Environ. 699, 134181 (2020).
Badri, D. V., Zolla, G., Bakker, M. G., Manter, D. K. & Vivanco, J. M. Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol. 198, 264–273 (2013).
Chung, S. H. et al. Host plant species determines symbiotic bacterial community mediating suppression of plant defenses. Sci. Rep. 7, (2017).
Gibson, C. M. & Hunter, M. S. Extraordinarily widespread and fantastically complex: Comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecol. Lett. 13, 223–234 (2010).
Jones, A., Mason, C. J., Felton, G. W. & Hoover, K. Host plant and population source drive diversity of microbial gut communities in two polyphagous insects. Sci. Rep. 9, 2792 (2019).
Adati, T. & Matsuda, K. Feeding stimulants for various leaf beetles (Coleoptera:Chrysomelidae)in the leaf surface wax of their host plants. Appl. Entomol. Zool. 28, 319–324 (1993).
Eigenbrode, S. D. The effects of plant epicuticular waxy blooms on attachment and effectiveness of predatory insects. Arthropod Struct. Dev. 33, 91–102 (2004).
Eigenbrode, S. & Espelie, K. Effects of plant epicuticular lipids on insect herbivores. Annu. Rev. Entomol. 40, 171–194 (2003).
Lin, S., Binder, B. F. & Hart, E. R. Insect feeding stimulants from the leaf surface of populus. J. Chem. Ecol. 24, 1781–1790 (1998).
Eisner, T., Eisner, M. & Hoebeke, E. R. When defense backfires: Detrimental effect of a plant’s protective trichomes on an insect beneficial to the plant. Proc. Natl. Acad. Sci. 95, 4410–4414 (1998).
Kennedy, G. G. Tomato, pests, parasitoids, and predators: Tritrophic interactions involving the genus Lycopersicon. Annu. Rev. Entomol. 48, 51–72 (2003).
Weinhold, A. & Baldwin, I. T. Trichome-derived O-acyl sugars are a first meal for caterpillars that tags them for predation. Proc. Natl. Acad. Sci. 108, 7855–7859 (2011).
Kaur, I. & Kariyat, R. R. Eating barbed wire: Direct and indirect defensive roles of non-glandular trichomes. Plant Cell Environ. 43, 2015–2018 (2020).
Bleeker, P. M. et al. Tomato-produced 7-epizingiberene and R-curcumene act as repellents to whiteflies. Phytochemistry 72, 68–73 (2011).
Gonzales-Vigil, E., Hufnagel, D. E., Kim, J., Last, R. L. & Barry, C. S. Evolution of TPS20-related terpene synthases influences chemical diversity in the glandular trichomes of the wild tomato relative Solanum habrochaites. Plant J. 71, 921–935 (2012).
Peiffer, M., Tooker, J. F., Luthe, D. S. & Felton, G. W. Plants on early alert: Glandular trichomes as sensors for insect herbivores. New Phytol. 184, 644–656 (2009).
Erb, M., Meldau, S. & Howe, G. A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17, 250–259 (2012).
Zhang, X., Yang, Y., Wu, Z. & Weng, P. The modulatory effect of anthocyanins from purple sweet potato on human intestinal microbiota in vitro. J. Agric. Food Chem. 64, 2582–2590 (2016).
Guo, Q. et al. JAZ repressors of metabolic defense promote growth and reproductive fitness in Arabidopsis. Proc. Natl. Acad. Sci. 115, E10768–E10777 (2018).
Gershenzon, J. & Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 3, 408–414 (2007).
Nuñez-Mejía, G., Valadez-Lira, J. A., Gomez-Flores, R., Rodríguez-Padilla, C. & Tamez-Guerra, P. Trichoplusia ni (Lepidoptera: Noctuidae) survival, immune response, and gut bacteria changes after exposure to Azadirachta indica (Sapindales: Meliaceae) volatiles. Fla. Entomol. 99, 12–20 (2016).
Liu, M. et al. Sakuranetin protects rice from brown planthopper attack by depleting its beneficial endosymbionts. Proc. Natl. Acad. Sci. 120, e2305007120 (2023).
Mason, C. J. Complex relationships at the intersection of insect gut microbiomes and plant defenses. J. Chem. Ecol. 46, 793–807 (2020).
Paniagua Voirol, L. R., Frago, E., Kaltenpoth, M., Hilker, M. & Fatouros, N. E. Bacterial symbionts in lepidoptera: Their diversity, transmission, and impact on the host. Front. Microbiol. 9, (2018).
Xia, X. et al. Metagenomic sequencing of diamondback moth gut microbiome unveils key holobiont adaptations for herbivory. Front. Microbiol. 8, (2017).
Vilanova, C., Baixeras, J., Latorre, A. & Porcar, M. The generalist inside the specialist: Gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front. Microbiol. 7, 1005 (2016).
Kang, J.-H. et al. The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 154, 262–272 (2010).
Li, L. et al. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, Jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16, 126–143 (2004).
Chen, H., Wilkerson, C. G., Kuchar, J. A., Phinney, B. S. & Howe, G. A. Jasmonate-inducible plant enzymes degrade essential amino acids in the herbivore midgut. Proc. Natl. Acad. Sci. 102, 19237–19242 (2005).
Wang, F., Park, Y.-L. & Gutensohn, M. Glandular trichome-derived mono- and sesquiterpenes of tomato have contrasting roles in the interaction with the potato aphid Macrosiphum euphorbiae. J. Chem. Ecol. 47, 204–214 (2021).
Roe, A. D., Demidovich, M. & Dedes, J. Origins and history of laboratory insect stocks in a multispecies insect production facility, with the proposal of standardized nomenclature and designation of formal standard names. J. Insect Sci. 18, 1 (2018).
Schilmiller, A. et al. Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J. 62, 391–403 (2010).
Julkowska, M. M. et al. MVApp: Multivariate analysis application for streamlined data analysis and curation. Plant Physiol. 180, 1261–1276 (2019).
Adams, R. P. Identification of essential oil components by gas chromatography/mass spectrometry. (Allured Publishing Corporation, 2007).
Gonzales-Vigil, E., Hefer, C. A., von Loessl, M. E., La Mantia, J. & Mansfield, S. D. Exploiting natural variation to uncover an alkene biosynthetic enzyme in poplar. Plant Cell 29, 2000–2015 (2017).
Chen, J. Y., Mumtaz, A. & Gonzales-Vigil, E. Evolution and molecular basis of substrate specificity in a 3-ketoacyl-CoA synthase gene cluster from Populus trichocarpa. J. Biol. Chem. 102496 (2022). https://doi.org/10.1016/j.jbc.2022.102496.
Lawrence, S. D., Novak, N. G., Shao, J., Ghosh, S. K. B. & Blackburn, M. B. Cabbage looper (Trichoplusia ni Hübner) labial glands contain unique bacterial flora in contrast with their alimentary canal, mandibular glands, and Malpighian tubules. MicrobiologyOpen 9, e994 (2020).
Mason, C. & Raffa, K. Acquisition and structuring of midgut bacterial communities in gypsy moth (Lepidoptera: Erebidae) Larvae. Environ. Entomol. 43 (2014).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing Amplicon sequence data on the MiSeq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Oksanen, J. et al. Vegan: Community ecology package. R package version 2.6–4. (2022).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
R Core Team. R: The R project for statistical computing. https://www.r-project.org/.
Wickham, H. ggplot2: Elegant graphics for data analysis (Springer-Verlag, 2016).
Hammer, T. J., Janzen, D. H., Hallwachs, W., Jaffe, S. P. & Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. 114, 9641–9646 (2017).
Bullington, L. S., Lekberg, Y. & Larkin, B. G. Insufficient sampling constrains our characterization of plant microbiomes. Sci. Rep. 11, 3645 (2021).
Chen, X., Wang, D.-D., Fang, X., Chen, X.-Y. & Mao, Y.-B. Plant Specialized Metabolism Regulated by Jasmonate Signaling. Plant Cell Physiol. 60, 2638–2647 (2019).
Ray, S., Savoie, B. M., Dudareva, N. & Morgan, J. A. Diffusion of volatile organics and water in the epicuticular waxes of petunia petal epidermal cells. Plant J. 110, 658–672 (2022).
Toprak, U., Hegedus, D., Doğan, C. & Güney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 104, e21682 (2020).
Turunen, S. Digestion and absorption of lipids in insects. Comp. Biochem. Physiol. A Physiol. 63, 455–460 (1979).
Vorholt, J. A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840 (2012).
Bodenhausen, N., Bortfeld-Miller, M., Ackermann, M. & Vorholt, J. A. A Synthetic community approach reveals plant genotypes affecting the phyllosphere microbiota. PLOS Genet. 10, e1004283 (2014).
Engel, P. & Moran, N. A. The gut microbiota of insects: Diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735 (2013).
Leite-Mondin, M. et al. The gut microbiota composition of Trichoplusia ni is altered by diet and may influence its polyphagous behavior. Sci. Rep. 11, 5786 (2021).
Hannula, S. E., Zhu, F., Heinen, R. & Bezemer, T. M. Foliar-feeding insects acquire microbiomes from the soil rather than the host plant. Nat. Commun. 10, 1254 (2019).
Mason, C. J., Jones, A. G. & Felton, G. W. Co-option of microbial associates by insects and their impact on plant-folivore interactions: Herbivore co-option of microbes. Plant Cell Environ. 42, 1078–1086 (2019).
Mason, C. J. et al. Plant defenses interact with insect enteric bacteria by initiating a leaky gut syndrome. Proc. Natl. Acad. Sci. 116, 15991–15996 (2019).
Pan, Q., Shikano, I., Hoover, K., Liu, T.-X. & Felton, G. W. Enterobacter ludwigii, isolated from the gut microbiota of Helicoverpa zea, promotes tomato plant growth and yield without compromising anti-herbivore defenses. Arthropod-Plant Interact. 13, 271–278 (2019).
Adeolu, M., Alnajar, S., Naushad, S., & S. Gupta, R. 2016. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 66, 5575–5599.
Gasmi, L. et al. Can herbivore-induced volatiles protect plants by increasing the herbivores’ susceptibility to natural pathogens?. Appl. Environ. Microbiol. 85, e01468-e1518 (2018).
Lee, J., Hwang, S. & Cho, S. Immune tolerance to an intestine-adapted bacteria, Chryseobacterium sp., injected into the hemocoel of Protaetia brevitarsis seulensis. Sci. Rep. 6, 31722 (2016).
Wang, J. et al. Parasitoid causes cascading effects on plant-induced defenses mediated through the gut bacteria of host caterpillars. Front. Microbiol. 12, (2021).
Vacheron, J. et al. T6SS contributes to gut microbiome invasion and killing of an herbivorous pest insect by plant-beneficial Pseudomonas protegens. ISME J. 13, 1318–1329 (2019).
Sugimoto, K., Zager, J. J., Aubin, B. S., Lange, B. M. & Howe, G. A. Flavonoid deficiency disrupts redox homeostasis and terpenoid biosynthesis in glandular trichomes of tomato. Plant Physiol. 188, 1450–1468 (2022).
Acknowledgements
We thank Dr. Gregg Howe and Dr. Cornelius Barry from Michigan State University for providing access to the tomato accessions used in this publication. We would also like to thank Osmond Hui and Syed Turab Hasan for their technical support. This work was supported by the Connaught Fund New Researcher Award to E.G.V. A.B. was supported by the Sustainable Food and Farming Futures Clusters at the University of Toronto in Scarborough. Research in the E.G.V. laboratory is funded by an NSERC Discovery Grant (RGPIN-201904772).
Author information
Authors and Affiliations
Contributions
A.B. and E.G.V. conceived the project. A.B. and E.G.V. designed and performed the experiments. A.B. and E.C.P. analyzed the microbiome data. A.B. and E.G.V. prepared the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bosorogan, A., Cardenas-Poire, E. & Gonzales-Vigil, E. Tomato defences modulate not only insect performance but also their gut microbial composition. Sci Rep 13, 18139 (2023). https://doi.org/10.1038/s41598-023-44938-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44938-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.