Abstract
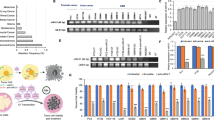
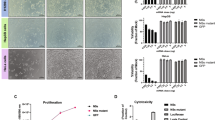
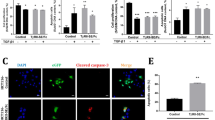
We have previously reported that recombinant adeno-associated virus serotype 3 (AAV3) vectors transduce human liver tumors more efficiently in a mouse xenograft model following systemic administration. Others have utilized AAV8 vectors expressing miR-26a and miR-122 to achieve near total inhibition of growth of mouse liver tumors. Since AAV3 vectors transduce human hepatic cells more efficiently than AAV8 vectors, in the present studies, we wished to evaluate the efficacy of AAV3-miR-26a/122 vectors in suppressing the growth of human hepatocellular carcinoma (HCC) cells in vitro, and human liver tumors in a mouse model in vivo. To this end, a human HCC cell line, Huh7, was transduced with various multiplicities of infection (MOIs) of AAV3-miR-26a or scAAV3-miR-122 vectors, or both, which also co-expressed a Gaussia luciferase (GLuc) reporter gene. Only a modest level of dose-dependent growth inhibition of Huh7 cells (~12–13%) was observed at the highest MOI (1 × 105 vgs/cell) with each vector. When Huh7 cells were co-transduced with both vectors, the extent of growth inhibition was additive (~26%). However, AAV3-miR-26a and scAAV3-miR-122 vectors led to ~70% inhibition of growth of Huh-derived human liver tumors in a mouse xenograft model in vivo. Thus, the combined use of miR-26a and scAAV3-miR-122 delivered by AAV3 vectors offers a potentially useful approach to target human liver tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol. 2014;10:153–61.
Wang X, Wang N, Cheung F, Lao L, Li C, Feng Y. Chinese medicines for prevention and treatment of human hepatocellular carcinoma: current progress on pharmacological actions and mechanisms. J Integr Med. 2015;13:142–64.
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55.
Greene CM, Varley RB, Lawless MW. MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled. World J Gastroenterol. 2013;19:5212–26.
Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, et al. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433–42.
Ling CQ, Fan J, Lin HS, Shen F, Xu ZY, Lin LZ, et al. Clinical practice guidelines for the treatment of primary liver cancer with integrative traditional Chinese and Western medicine. J Integr Med. 2018;16:236–48.
Lee RC, Feinbaum RL, Ambrost V. The C. Elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-4. Cell. 1993;75:843–54.
Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–33.
Port JD, Sucharov C. Role of microRNAs in cardiovascular disease: therapeutic challenges and potentials. J Cardiovasc Pharmacol. 2010;56:444–53.
Pager CT, Wehner KA, Fuchs G, Sarnow P. MicroRNA-mediated gene silencing. Prog Mol Biol Transl Sci. 2009;90:187–210.
Hammond SM. MicroRNA therapeutics: a new niche for antisense nucleic acids. Trends Mol Med. 2006;12:99–101.
Chen J, Zhang K, Xu YJ, Gao YP, Li C, Wang R, et al. The role of microRNA-26a in human cancer progression and clinical application. Tumor Biol. 2016;37:7095–108.
Sartorius K, Sartorius B, Cheryl W, Chuturgoon A, Makarova J. The biological and diagnostic role of mirna’s in hepatocellular carcinoma. Front Biosci. 2018;23:1701–20.
Yang X, Liang L, Zhang XF, Jia HL, Qin Y, Zhu XC, et al. MicroRNA-26a suppresses tumor growth and metastasis of human hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway. Hepatology. 2013;58:158–70.
Chai Z, Kong J, Zhu X, Zhang Y, Lu L, Zhou J, et al. MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma. PLoS ONE. 2013;8:e77957.
Yang X, Zhang XF, Lu X, Jia HL, Liang L, Dong QZ, et al. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–85.
Ji JF, Shi J, Budhu A, Yu ZP, Forgues M, Roessler S, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–47.
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–9.
Xu J, Zhu XM, Wu LJ, Yang R, Yang ZR, Wang QF, et al. microRNA-122 suppresses cell proliferation and induces cell apoptosis in hepatocellular carcinoma by directly targeting Wnt/β-catenin pathway. Liver Int. 2012;32:752–60.
Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, et al. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–9.
Wang B, Hsu SH, Wang XM, Kutay H, Bid HK, Yu JH, et al. Reciprocal regulation of microRNA-122 and c-Myc in hepatocellular cancer: role of E2F1 and TFDP2. Hepatology. 2014;59:555–66.
Fan CG, Wang CM, Tian C, Wang Y, Li L, Sun WS, et al. miR-122 inhibits viral replication and cell proliferation in hepatitis B virus-related hepatocellular carcinoma and targets NDRG3. Oncol Rep. 2011;26:1281–6.
Sun J, Lu HQ, Wang X, Jin HC. MicroRNAs in hepatocellular carcinoma: regulation, function, and clinical implications. Sci World J. 2013;2013:1–14.
Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–82.
Li MX, Jayandharan GR, Li BZ, Ling C, Ma WQ, Srivastava A, et al. High-Efficiency transduction of fibroblasts and mesenchymal stem cells by Tyrosine-Mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21:1527–43.
Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17.
Hsu SH, Wang B, Kota J, Yu JH, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Investig. 2012;122:2871–83.
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9.
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8.
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno–associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–90.
Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–7.
Gaudet D, Méthot J, Déry S, Brisson D, Essiembre C, Tremblay G, et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–9.
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in Hemophilia B. N Engl J Med. 2011;365:2357–65.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of Factor IX gene therapy in Hemophilia B. N Engl J Med. 2014;371:1994–2004.
Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno-associated virus vector 5-human Factor IX in adults with Hemophilia B. Blood. 2018;131:1022–31.
George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity Factor IX variant. N Engl J Med. 2017;377:2215–27.
Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-Factor VIII gene transfer in severe Hemophilia A. N Engl J Med. 2017;377:2519–30.
Pasi KJ, Rangarajan S, Mitchell N, Lester W, Symington E, Madan B, et al. Multiyear follow-up of AAV5-hFVIII-SQ gene therapy for Hemophilia A. N Engl J Med. 2020;382:29–40.
Hwu WL, Muramatsu S, Tseng SH, Tzen KY, Lee NC, Chien YH, et al. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci Transl Med. 2012;4:134ra61.
MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37.
Guy J, Feuer WJ, Davis JL, Porciatti V, Gonzalez PJ, Koilkonda RD, et al. Gene therapy for Leber hereditary optic neuropathy: low- and medium-dose visual results. Ophthalmology. 2017;124:1621–34.
Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–22.
Ling C, Lu Y, Cheng B, McGoogan KE, Gee SWY, Ma WQ, et al. High-Efficiency transduction of liver cancer cells by recombinant adeno-associated virus serotype 3 vectors. J Vis Exp. 2011;49:2538.
Cheng BB, Ling C, Dai Y, Lu Y, Glushakova LG, Gee SWY, et al. Development of optimized AAV3 serotype vectors: mechanism of high-efficiency transduction of human liver cancer cells. Gene Ther. 2012;19:375–84.
Ling C, Wang Y, Zhang YH, Ejjigani A, Yin ZF, Lu Y, et al. Selective in vivo targeting of human liver tumors by optimized AAV3 vectors in a murine xenograft model. Hum Gene Ther. 2014;25:1023–34.
Liu Y, Joo KI, Wang P. Endocytic processing of adeno-associated virus type 8 vectors for transduction of target cells. Gene Ther. 2013;20:308–17.
Kube DM, Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic Acids Res. 1997;25:3375–6.
Ling C, Wang Y, Lu Y, Wang LN, Jayandharan GR, Aslanidi GV, et al. Enhanced transgene expression from recombinant single-stranded D-sequence-substituted adeno-associated virus vectors in human cell lines in vitro and in murine hepatocytes in vivo. J Virol. 2015;89:952–61.
Aslanidi GV, Rivers AE, Ortiz L, Song L, Ling C, Govindasamy L, et al. Optimization of the capsid of recombinant adeno-associated virus 2 (AAV2) vectors: the final threshold? PLoS ONE. 2013;8:e59142.
Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–63.
Nowrouzi A, Penaud-Budloo M, Kaeppel C, Appelt U, Le Guiner C, Moullier P, et al. Integration frequency and intermolecular recombination of rAAV vectors in non-human primate skeletal muscle and liver. Mol Ther. 2012;20:1177–86.
Guillouzo A, Corlu A, Aninat C, Glaise D, Morel F, Guguen-Guillouzo C. The human hepatoma HepaRG cells: a highly differentiated model for studies of liver metabolism and toxicity of xenobiotics. Chem Biol Interact. 2007;168:66–73.
Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, Liu C, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Mol Genet Metab. 2009;98:289–99.
Ling C, Li BZ, Ma WQ, Srivastava A. Development of optimized AAV serotype vectors for high-efficiency transduction at further reduced doses. Hum Gene Ther Method. 2016;27:143–9.
Zhang YH, Wang Y, Yusufali AH, Ashby F, Zhang D, Yin ZF, et al. Cytotoxic genes from traditional Chinese medicine inhibit tumor growth both in vitro and in vivo. J Integr Med. 2014;12:483–94.
Qadir MI, Rizvi SZ. MiRNA in hepatocellular carcinoma: Pathogenesis and therapeutic approaches. Crit Rev Eukaryot Gene Expr. 2017;27:355–61.
Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122—a key factor and therapeutic target in liver disease. J Hepatol. 2015;62:448–57.
Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94.
Chang L, Li K, Guo T. miR-26a-5p suppresses tumor metastasis by regulating EMT and is associated with prognosis in HCC. Clin Transl Oncol. 2017;19:695–703.
Fukuhara T, Kambara H, Shiokawa M, Ono C, Katoh H, Morita E, et al. Expression of microRNA miR-122 facilitates an efficient replication in nonhepatic cells upon infection with hepatitis C virus. J Virol. 2012;86:7918–33.
Mizuguchi Y, Takizawa T, Yoshida H, Uchida E. Dysregulated miRNA in progression of hepatocellular carcinoma: a systematic review. Hepatol Res. 2016;46:391–406.
Yin J, Tang HF, Xiang Q, Yu J, Yang XY, Hu N, et al. MiR-122 increases sensitivity of drug-resistant BEL-7402/5-FU cells to 5-fluorouracil via down-regulation of bcl-2 family proteins. Pharmazie. 2011;66:975–81.
Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA. 2003;100:6081–6.
Sarkar R, Tetreault R, Gao G, Wang L, Bell P, Chandler R, et al. Total correction of Hemophilia A mice with canine FVIII using an AAV 8 serotype. Blood. 2004;103:1253–60.
Li S, Ling C, Zhong L, Li M, Su Q, He R, et al. Efficient and targeted transduction of nonhuman primate liver with systemically delivered optimized AAV3B vectors. Mol Ther. 2015;23:1867–76.
Wang L, Bell P, Somanathan S, Wang Q, He Z, Yu H, et al. Comparative study of liver gene transfer with AAV vectors based on natural and engineered AAV capsids. Mol Ther. 2015;23:1877–87.
Vercauteren K, Hoffman BE, Zolotukhin I, Keeler GD, Xiao JW, Basner-Tschakarjan E, et al. Superior in vivo transduction of human hepatocytes using engineered AAV3 capsid. Mol Ther. 2016;24:1042–9.
El-Serag HB. Current status of Sorafenib use for treatment of hepatocellular carcinoma. Gastroenterol Hepatol. 2017;13:623–5.
Acknowledgements
This research was supported in part by Public Health Service grants R01 HL-097088, R41 AI-122735, and R21 EB-015684 from the National Institutes of Health; a grant from the Children’s Miracle Network; and support from the Kitzman Foundation (to AS). LY was supported by the International Postdoctoral Exchange Fellowship Program 2018 by the Office of China Postdoctoral Council.
Author information
Authors and Affiliations
Contributions
LY, GDK, YZ, KQ performed the experiments. LY, GDK, YZ, BEH, CL, KQ, and AS analyzed the data. CL and AS conceived of the idea. LY and AS wrote the paper, and all authors read and approved the final version.
Corresponding authors
Ethics declarations
Conflict of interest
AS is a cofounder of, and holds equity in, Lacerta Therapeutics, aaVective, KASHX Bio, and Nirvana Therapeutics, and is an inventor on several issued patents on rAAV vectors that have been licensed to various gene therapy companies. All other authors declare no conflict of interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, L., Keeler, G.D., Zhang, Y. et al. AAV3-miRNA vectors for growth suppression of human hepatocellular carcinoma cells in vitro and human liver tumors in a murine xenograft model in vivo. Gene Ther 28, 422–434 (2021). https://doi.org/10.1038/s41434-020-0140-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0140-1
This article is cited by
-
Recent advances in various adeno-associated viruses (AAVs) as gene therapy agents in hepatocellular carcinoma
Virology Journal (2024)
-
MicroRNA-122 in human cancers: from mechanistic to clinical perspectives
Cancer Cell International (2023)
-
Hepatic microRNA modulation might be an early event to non-alcoholic fatty liver disease development driven by high-fat diet in male mice
Molecular Biology Reports (2022)