Abstract
Objective
To search for and critically appraise the psychometric quality of patient-reported outcome measures (PROMs) developed or validated in optic neuritis, in order to support high-quality research and care.
Methods
We systematically searched MEDLINE(Ovid), Embase(Ovid), PsycINFO(Ovid) and CINAHLPlus(EBSCO), and additional grey literature to November 2021, to identify PROM development or validation studies applicable to optic neuritis associated with any systemic or neurologic disease in adults. We included instruments developed using classic test theory or Rasch analysis approaches. We used established quality criteria to assess content development, validity, reliability, and responsiveness, grading multiple domains from A (high quality) to C (low quality).
Results
From 3142 screened abstracts we identified five PROM instruments potentially applicable to optic neuritis: three differing versions of the National Eye Institute (NEI)-Visual Function Questionnaire (VFQ): the 51-item VFQ; the 25-item VFQ and a 10-item neuro-ophthalmology supplement; and the Impact of Visual Impairment Scale (IVIS), a constituent of the Multiple Sclerosis Quality of Life Inventory (MSQLI) handbook, derived from the Functional Assessment of Multiple Sclerosis (FAMS). Psychometric appraisal revealed the NEI-VFQ-51 and 10-item neuro module had some relevant content development but weak psychometric development, and the FAMS had stronger psychometric development using Rasch Analysis, but was only somewhat relevant to optic neuritis. We identified no content or psychometric development for IVIS.
Conclusion
There is unmet need for a PROM with strong content and psychometric development applicable to optic neuritis for use in virtual care pathways and clinical trials to support drug marketing authorisation.
摘要
目的: 寻找并严格评估在视神经炎中开发或验证的患者报告结局测量 (PROMs) 的心理测量质量, 以支持高质量的研究和临床诊疗。
方法: 我们系统地检索了MEDLINE(Ovid)、Embase(Ovid)、PsycINFO(Ovid)和CINAHLPlus(EBSCO), 以及截至2021年11月的其他文献, 以确定适用于与任何系统性或神经性疾病相关的成人视神经炎的PROM开发或验证的研究。我们将经典测试理论或Rasch分析方法开发的工具用于研究。我们使用既定的质量标准来评估内容开发、有效性、可靠性和响应性, 将多个领域从A (高质量) 到C (低质量) 分级。
结果: 从3142份筛选的摘要中, 确定了五种可能适用于视神经炎的PROM工具: 国家眼科研究所 (NEI) 视觉功能问卷 (VFQ) 的三个不同版本: 51-item VFQ;25-item VFQ和10-item神经眼科补充问卷;以及视觉障碍影响量表 (IVIS), 这是多发性硬化症生活质量清单 (MSQLI) 手册的组成部分, 来源于多发性硬化症功能评估 (FAMS) 。心理测量评估显示, NEI-VFQ-51和10 item神经模块有些相关的内容, 但心理测量发展较慢, 而FAMS使用Rasch分析法有较快的心理测量开发, 但只与视神经炎有一定的关联。我们没有发现IVIS的内容或心理测量开发。
结论: 目前对适用于视神经炎且具有强大内容和心理测量开发的PROM的虚拟诊疗路径和临床试验尚不多, 不足以支持药物上市授权。
Similar content being viewed by others
Introduction
Finding more effective treatments for rare diseases and inflammatory conditions, including optic neuritis (ON), is a research priority highlighted by stakeholders internationally [1, 2]. Whilst in the UK, optic neuritis is most strongly and frequently associated with Multiple Sclerosis (MS), a similar number of patients develop optic neuritis in association with other infectious and immune-mediated inflammatory diseases (IMIDs) combined [3]. The acutely sight-threatening and potentially irreversible nature of untreated non-MS optic neuritis, makes vital the early differentiation from MS-optic neuritis, for consideration of high dose corticosteroids. A substantial proportion of patients then follow a relapsing course, including those with Neuromyelitis Optica Spectrum Disorder (NMOSD), Myelin Oligodendrocyte Glycoprotein antibody-associated disease and neurosarcoidosis. These patients often need chronic steroid-sparing systemic immunosuppressives to reduce the risk of flares and progressive disability [4]. Beyond the vision impacts and side effects of treatment, ON has important psychological and social impacts. The unpredictable nature of ‘attacks’ makes it difficult for patients to gain a sense of control over their illness [5, 6]. Both ON and MS-ON disproportionately affect young adults, limiting daily activities in their most socioeconomically productive years [7]. Since there is currently no cure for MS, or the majority of other rare diseases associated with ON, treatments are directed at symptom alleviation, or reduction of relapse frequency [8]. Furthermore, active optic neuritis, although visually limiting, may not be readily apparent to others, thereby contributing to the sense of isolation experienced by many people with ON and MS-ON [9].
Visual acuity remains the most established outcome parameter used by regulatory agencies, including the Food and Drug Administration (FDA) when considering therapeutic efficacy of diseases involving the visual pathway. However, this metric’s limitations are recognised. There has been growing focus on patient-centred definitions of efficacy which capture the extent of an individual’s lived experience of their condition [10]. Better integration of the patient voice is advancing research priority setting, outcomes design, and routine clinical practice in medicine, and in neurology and ophthalmology specifically [11,12,13,14]. Patient-reported outcome measures (PROMs) facilitate quantitative capture of the subjectively experienced impacts of disease and its treatment (Table 1 Glossary) [15]. Vision-related PROMs focus on the symptoms and impacts generic to many different eye diseases and conditions, whilst health-related PROMs focus on symptoms and impacts on a person’s health more generally, and some disease-specific PROMs have been developed. PROMs are particularly useful when interventions reveal otherwise similar efficacy using traditional outcome measures, or when an intervention provides only a small clinical improvement, yet patients experience other benefits or harms [16]. To be useful, for drug marketing authorization [13, 17], or for integration in remote care pathways emerging from the COVID-19 pandemic, PROMs need to be targeted to the constructs of interest, possess sound psychometric performance properties (e.g. as assessed using Rasch Analysis or item response theory (IRT) models), and be valid, reliable, responsive and acceptable to users [17, 18]. Well-designed PROMs yield a precise, interval-scaled measure for each quality of life domain, which is amenable to quantitative statistical analysis, and thus of tremendous value to a variety of stakeholders within and beyond the clinical trial space, including patients and clinicians [19].
The landmark Optic Neuritis Treatment trial, 30 years ago, explored a specific subgroup of ON patients aged 18 to 45 years with acute unilateral ON, with no known systemic disease (besides MS) [20]. This evidence base is predominantly applicable to MS-ON, and not to non-MS ON, which is responsible for over half of all incident ON in the UK [3]. Furthermore, a new corticosteroid treatment trial for optic neuritis has been proposed, addressing multiple limitations of the earlier trial [20, 21]. These include aspects of trial design, exploring the role of hyperacute steroid treatment, and use of more robust outcome measures aligned with contemporary clinical practice [21]. There is explicit need for a PROM able to capture treatment benefits and side effects across multiple quality of life domains [21]. This could shed important new insights for patient management in ON through the disease course. This systematic review aimed to identify and psychometrically evaluate the quality of PROMs developed for, or validated in, adults with optic neuritis, to consider whether any existing instruments meet the needs of a new trial. This review is part of a wider project informing the development of robust PROMs and item banks for use in ophthalmology [22, 23].
Methods
The methodology followed our published PROSPERO protocol (CRD42019151652) [24]. The systematic review is reported in line with PRISMA guidance [24,25,26].
Searches
We searched the following electronic databases on 11 November 2019, and updated the searches to 5 November 2021: MEDLINE (Ovid), Embase (Ovid), PsycINFO (Ovid) and CINAHL Plus (EBSCO). The search strategy combined index and free text terms for optic neuritis (and also, separately, scleritis and uveitis), and terms relating to quality of life, health status indicators or patient-reported outcomes, with no restrictions on the language or year of publication (see Supplementary Panel 1). The MEDLINE search strategy was adapted for use on all databases. We screened references of included studies, to identify additional instruments. Where multiple studies referenced the same PROM, we searched citations to obtain the study reporting the original PROM’s development and any subsequent revisions and reports relating to instrument quality appraisal or validation. Two reviewers (TB and JP) also independently searched a database maintained by the United States National MS Society to identify potentially relevant PROMs for optic neuritis [27].
Study selection
We included studies reporting PROM content identification, development, psychometric assessment, or validation to assess the impact of optic neuritis in adult patients. We included optic neuritis of any cause, at any time from first presentation, and did not limit our search to demyelinating optic neuritis. We included broad search terms for patient-reported outcomes and ‘quality of life’, considering ‘quality of life’ as an umbrella term including multiple domains (see Table 1) [28]. We sought studies using disease-relevant content development methods such as structured/semi-structured interviews, focus groups and/or literature reviews, but did not exclude validation studies with weaker content development (e.g. based on expert opinion). We excluded editorials, reviews, conference abstracts and studies reporting instruments developed solely for use in children. We excluded studies reporting PROM use without development or validation, but searched the references of such studies to ensure capture of the original instrument’s development.
Main outcomes
For each included study, we extracted study characteristics (publication year, citation, country/region, sample size) and characteristics of patients on whom the instrument was developed/assessed/validated. This included disease type(s) and subtypes, age, sex, ethnicity, and, if reported, the proportion of patients on systemic antimicrobial or anti-inflammatory therapy. We extracted the name of the PROM, the QoL domains covered, the number of items in each domain, and any subtypes of optic neuritis covered by the PROM.
Data extraction, synthesis and analysis
Search results were uploaded to Endnote 20 (Clarivate Analytics). All titles and abstracts were screened by two independent reviewers (CO/TB and TB/XL), to remove irrelevant articles. Full text articles were obtained for studies that potentially met eligibility criteria. Abstracts that did not provide the reviewers with sufficient information to make a decision were taken forward for full-text screening, to minimise the risk of missing a potentially relevant article. At any stage, if the reviewers were unable to reach consensus, an additional reviewer was consulted (KP). Two reviewers (TB and OLA/JP/CO) independently extracted data from studies meeting the inclusion criteria, using a standardised form. We attempted to contact investigators for clarification where we were unable to grade elements not reported.
PROM quality assessment
Two reviewers (TB and OLA/CO), with adjudication by a third (KP), considered the overall extent to which the instrument’s items were relevant to optic neuritis, based on the patient samples used for item identification and development, and for instrument validation. We graded relevance as very relevant, somewhat relevant, or not very relevant.
We assessed the quality of each PROM using established quality criteria (see Supplementary Table 1 definitions), adapted from the US Food and Drug Administration framework and guidelines [29], and COSMIN Standards for the selection of health status Measurement Instruments [30], grading each of multiple domains from A (high quality) to C (low quality) [31]. The framework has been used previously to appraise the quality of PROMs in ophthalmology [17, 32], including retinal disease [31], cataract [33], refractive surgery [34], refractive error [35], amblyopia and strabismus [36], and keratoconus [37]. We reviewed instrument content development, and appraised item identification and item selection. For item identification we assigned a grade ‘A’ for, “comprehensive consultation with patients,” if a sufficient number (i.e. more than 30) of relevant patients were included to achieve content saturation [38]. For item selection, we assigned a grade ‘A’, based on the COSMIN guidelines, if the pilot instrument contained more than seven times the number of patients than items in the instrument (or in the case of multidimensional instrument, seven times the number of items in the largest domain representing a unidimensional construct); if the patient sample was fewer than five times the number of items we graded this domain ‘inadequate’ (grade ‘C’) [39].
For instruments developed using classic test theory-based psychometric approaches, we assessed acceptability, item targeting and internal consistency, but we highlighted as a limitation that more modern psychometric approaches had not been considered (highlighting Table 2 cells in dark red to emphasise ‘not done’) [40]. For instruments developed using the more rigorous Rasch Analysis approach, we assessed response categories, dimensionality, measurement precision, item fit statistics, differential item functioning and targeting [19].
In both study types, we assessed validity (concurrent, convergent, discriminant and known group validity), reliability (test-retest) and responsiveness (see Supplementary Table 1 for definitions). Where the patient sample used to validate the instrument was not independent from the sample used to develop it (across one or more published papers) we highlighted this as a limitation of the instrument.
Results
The systematic search of bibliographic databases and cited references identified 3876 records, reducing to 3412 after removal of duplicates. We identified three studies reporting differing versions of a vision disorder specific instrument, the National Eye Institute Visual Function Questionnaire, containing 25 items (NEI-VFQ-25), 51 items (NEI-VFQ-51), and a 10-item add-on module, validated for neuro-ophthalmic conditions (including MS-associated optic neuritis) [41,42,43].
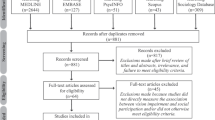
Searching the National Multiple Sclerosis Society PROM database identified eight other instruments validated for use in MS, and two further included studies. We included one subscale from the Multiple Sclerosis Quality of Life Inventory, the Impact of Visual Impairment Scale (IVIS), which we understand was derived from the Functional Capacity Assessment, more commonly called the Functional Assessment of Multiple Sclerosis (FAMS) and referred to as FAMS from here on in [5, 44]. We excluded the remaining six instruments (see Supplementary Table 2 reporting MS PROMs) because of very limited coverage of items relevant to optic neuritis. The study selection process is presented in Fig. 1. Table 2 summarises the characteristics of the included studies. Table 3 summarises the findings comparing the psychometric quality appraisal of included studies against our predefined criteria (Supplementary Table 1). A justification of each grading assigned is available (Supplementary Table 2).
We excluded a study reporting preliminary development of a 46-item instrument in 15 patients with neuromyelitis optica (a cause of optic neuritis), as whilst a protocol for further instrument development was outlined, we could not find a manuscript reporting instrument completion, and did not hear from the authors following email enquiry [45, 46].
National Eye Institute Visual Function Questionnaire (NEI VFQ-25)
The original NEI VFQ was developed between 1994 and 1998 for English-speaking adults aged ≥21 years with vision impairment from age-related macular degeneration, cataract, diabetic neuropathy, glaucoma or cytomegalovirus retinitis, following initial content development with multi-condition focus groups [47, 48]. A total of 262 patients were recruited from five academic centres, then a further 597 people were recruited in 1996 from multi-condition focus groups. The original 51-item instrument was developed from a 96-item pilot instrument, and took 15 minutes to administer. The shorter 25-item NEI VFQ-25 was developed in 2001 [42]. This included 11 vision-related subscales (general vision, near vision, distance vision, driving, peripheral vision, colour vision, ocular pain, vision-specific role difficulties, vision-specific dependency, vision-specific social functioning, and vision-specific mental health) and one general health item, with a few items per quality of life domain. Each subscale was scored by adding up ordinal values assigned to response categories (summary scoring) so that 0 represented the lowest and 100 the best possible score.
We graded the original NEI VFQ-25 development with ‘not done’ for item selection with respect to specific application to ON, though for its intended purpose as an eye disease-generic vision-specific tool it could be graded ‘A’. We scored NEI VFQ-25 ‘A’ for internal consistency based on classic test theory, but ‘B’ for acceptability and ‘C’ for targeting. However, with a single scale containing so many quality of life domains, with few items per domain, there is multidimensionality. This has been shown in Rasch analysis of NEI-VFQ data in other diseases [49]. Moreover, the purported 11 domains have been repeatedly shown to not be valid when tested using the Rasch model in other eye diseases. All four types of validity were assessed, but only concurrent and known group validity were graded ‘A’ in this tool’s capacity as an eye disease-generic vision-specific instrument, with convergent validity graded ‘B’ and discriminant validity graded ‘C’.
NEI-VFQ 10-item Neuro-Ophthalmic Supplement (NOS-10)
Cleary et al. reported, “a questionnaire designed to assess the impact of an episode of optic neuritis on their quality of life,” six months after entry to the landmark 1991 Optic Neuritis Treatment Trial, which recruited patients with monocular acute ON [4]. The question set was completed by 87% (n = 382/438) patients [20]. We could not identify further detail in the literature on selection/development of question items. We reviewed the original 1991 ONTT case report form, which included questions on the earliest visual symptom, positive visual phenomena, presence, type and severity of pain in the affected eye, and a free text question on other ocular symptoms, but could not verify if these were the ‘questionnaire’ items [50]. Using the same questionnaire, Ma et al. later developed a 7-item MS-specific vision questionnaire (MSVQ) [20], for co-administration with the NEI VFQ-25 [51]. The instrument included six questions on vision (whether blurry, difficulty in bright sunlight, difficulty when eyes tired, two eyes see differently, trouble focusing on moving objects, binocular double vision), and one on vision-related functioning (difficulty using computer). No psychometric evaluation was used in the development of these two questionnaires, and it is possible the item content was selected by neurology or neuro-ophthalmology experts.
Raphael et al. subsequently reported validation of a 10-item Neuro-Ophthalmic supplement to the NEI-VFQ among 145 patients with MS including 47 patients who had a history of acute optic neuritis [41]. This supplement used the same content from the MSVQ (7-items) [20], along with three additional questions, selected from those items (including open questions and content from a questionnaire designed for patients following corneal surgery) [52], most frequently reported by a group of 80 MS patients to cause ‘slight difficulty’ (or worse) [41]. The three extra items included one additional question on vision-related functioning (difficulty parking car), and two on whether the eye/lid appearance was unusual, or ptosis was present, aiming to extend relevance of the instrument to patients affected by additional conditions such as myasthenia gravis.
Like the main NEI VFQ-25 instrument, in the validation study, items were presented using a categorical scale format, scored on a 0 to 100 scale. A composite score was calculated as the unweighted average of the 10 items. As there was no psychometric evaluation of items included in this instrument’s development, we graded this instrument ‘B’ for item identification and ‘C’ for item selection (no statistical justification provided), and considered it ‘somewhat relevant’ to optic neuritis. Content limitations aside, we graded ‘A’ for targeting, internal consistency, known group validity and concurrent validity. Other psychometric domains, including acceptability, responsiveness, repeatability and 2 other forms of validity were not reported.
NEI-VFQ-51 validated in optic neuritis
Whilst the NEI-VFQ-51 was not developed for optic neuritis, Cole et al. reported validation of the original 51-item NEI–VFQ among 244 patients with acute unilateral MS-optic neuritis [43]. The questionnaire was administered as part of testing during an annual eye examination. The NEI-VFQ-51 included 14 subscales which were scored using a categorical scale, on a 0 to 100 scale (with 100 indicating highest function) [43]. The 14 subscales included overall health, overall vision, difficulty with near vision activities, difficulty with distance vision activities, limitations in social functioning due to vision, role limitations due to vision, dependency due to vision, mental health symptoms due to vision, future expectations for vision, driving difficulties, limitations with peripheral and colour vision, and pain or discomfort in or around eyes [43].
Item identification and selection were both graded as ‘not relevant’ as this study was seeking to validate a previously developed instrument. We graded acceptability as ‘B’ as the percentage of missing data in all subscales was below 40% (highest in difficulty with near vision activities at 10%). We graded internal consistency as ‘A’ as the average internal consistency over the 10 multi-item subscales (omitting the visual expectation subscale) was 0.86. We graded known group validity ‘A’, as there was a significant difference (p < 0.01) in NEI–VFQ Subscale Scores for distance activities, mental health, role difficulties, driving and peripheral vision in an independent subgroup. We graded construct validity ‘C’ as rank correlations between the NEI–VFQ subscales and the clinical vision tests ranged from small to modest. Other psychometric domains, including responsiveness, repeatability and discriminant validity were not reported.
Impact of Visual Impairment Scale (IVIS)
The Impact of Visual Impairment Scale (IVIS) was reportedly derived from the Functional Assessment of Multiple Sclerosis (FAMS), developed by the Michigan Commission for the Blind. This instrument was only briefly outlined in the MSQLI handbook, without reference to a development or validation study. Therefore, we tried snowballing citations and searched PubMed between 1998 and 2006 for, ‘Impact of Visual Impairment Scale’ and ‘Functional Assessment’ and for all first authors who had published in MSQLI, but we were unable to identify further evidence of IVIS development or validation. We retained this instrument in our review but were unable to conduct a quality appraisal beyond the limited domains reported (without citation) in the MSQLI handbook.
The IVIS is a self-reported five-item instrument administered as a questionnaire or interview to provide an assessment of difficulties with simple visual tasks such as reading, watching television and recognising house numbers [44]. The MSQLI handbook reports the IVIS to have a reported Cronbach’s alpha of 0.86 (grade A), without detailing a study from which this derived [44]. In the original field testing of the MSQLI, the IVIS was reported to ‘significantly correlate’ with Visual item of the Kurtzke Functional Systems and with visual acuity (convergent and concurrent grade A, but we could not further verify this correlation) [44].
Functional Assessment of Multiple Sclerosis (FAMS)
It was unclear where the five items in IVIS originated but we considered if possible they were informed by FAMS, as mentioned in the 1997 MSQLI handbook. We therefore reviewed the development and validation of FAMS published by Cella et al. [5]. The original 59-item FAMS instrument was developed from an 88-item pilot instrument. This included six subscales (mobility, symptoms, emotional wellbeing, general contentment, thinking/fatigue, and family/social well-being). Each subscale was scored on a five-category scale so that ‘0’ represented not affected and ‘4’ was very affected.
Initial content development for FAMS included a semi-structured interview with 20 MS patients and five MS specialists (yielding 135 new items), literature review and inclusion of 28 items from the Functional Assessment of Cancer Therapy, General version (FACT-G) and from the Fatigue Severity Scale developed by the Department of Neurology at the University of Chicago. Items were winnowed down to the 88-item pilot instrument. In the development and validation study, a total of 433 patients with MS were recruited from two hospitals in Chicago, USA. Of these 377 (74% completion rate) participated via postal survey, and the remaining 56 (81.2% completion rate) patients participated during a clinic visit, with the latter group completing additional validation tests (completing the Kurtzke Extended Disability Status Scale (EDSS) and the Scripps Neurological Rating Scale (NRS)), and test-retest reliability 3-7 days later [53, 54].
We graded the original FAMS ‘A’ for item identification and item selection in its intended purpose, as an MS-specific tool. We graded this tool as ‘somewhat relevant’ to optic neuritis, as although the domains addressed included mobility and emotional well-being which are important in ophthalmic quality of life, the study mentioned no information on the history or time course of optic neuritis in the 20 MS patients interviewed during item generation. Treatments and vision levels were also not reported. The content of FAMS may be driven by the quality of life impacts of MS, which are many and varied, only a subset of which are likely relevant to ON.
Instrument development was fairly strong, using responses from 377 MS postal survey patients and principal component analysis (PCA), to identify 63 items in five distinct ‘factors’ or subscales with identifiable conceptual meaning (accounting for 47.7% of the total variance) [55]. Prior to Rasch analysis, one of the factors (emotional wellbeing) was divided into two subscales for conceptual and practical reasons. Rasch analysis using BIGSTEPS further refined and developed the instrument into 44 items in six unidimensional sub-scales. The full data and outputs from the Rasch analysis were not reported in the manuscript, limiting complete quality appraisal. An additional 15 unscored items were also retained in the final 59-item instrument, ‘based on their potential clinical and empirical value’, which was a quality limitation.
Both CTT and limited Rasch metrics were reported for FAMS. We scored FAMS ‘A’ for internal consistency, with Cronbach’s alpha coefficients reported as universally high (range 0.82 to 0.961), and ‘A’ for measurement precision, response categories and dimensionality. We graded test-retest repeatability ‘A’, as the reliability coefficients ranged from 0.85 to 0.91. Independent validation data was available for 56 patients in whom the instrument was not developed, and was of excellent quality with many different instruments included to explore three aspects of validity. Whilst the investigators reported that concurrent validity was assessed, we did not find a clinical measure (defined in the quality appraisal criteria used in our review) against which the instrument was assessed and so graded this ‘not reported’. We were unable to assess external generalisability as the patients affected by MS-optic neuritis differ from optic neuritis generally.
Discussion
To our knowledge, this is the first systematic review to appraise the psychometric quality of PROMs developed for and/or validated in optic neuritis. The review highlights a relative paucity, especially of tools developed or validated for application in non-MS ON. Psychometric appraisal revealed the 10-item neuro module to supplement the NEI-VFQ-25 had some relevant content development, some validation, but slightly limited psychometric development by contemporary standards. The FAMS had stronger psychometric development and stronger validation and reliability assessment, but content development may have been only somewhat relevant to MS-ON. In addition, this study did not report subgroup analysis exploring whether clinical differences between patients with and without optic neuritis (e.g. visual acuity, contrast sensitivity, visual field loss and/or scotomas) influenced responses (i.e. differential item functioning). We identified no published content or psychometric development or validation for IVIS.
There is need for a robust PROM applicable to both MS-optic neuritis and non-MS optic neuritis and their treatments, to inform future care, and to support virtual patient monitoring and new trials [12]. Our quality appraisal highlighted multiple weaknesses. The primary limitation of most available PROMs for ON (NEI-VFQ and IVIS) is that they were developed prior to the now widespread use of psychometric development approaches based on Item Response Theory. Petrillo and colleagues have outlined multiple issues with using classic test theory for psychometric evaluation [56]. Specifically, analysis is not based on interval-level measurement but on counts (summary scores of items), findings are dependent on the scale and sample, missing data cannot be handled easily, and the standard error of measurement around individual patient scores are assumed to have a constant value. Contemporary psychometric tools, such as the Rasch model, permit more robust examination of validity and interpretability. For example, multiple studies have psychometrically evaluated the NEI-VFQ-25 in patients with different ocular conditions and the general population, and have identified major shortcomings with respect to reliability, validity and dimensional structure [42,43,44,45,46]. Exploring data from 2487 patients with retinal disease, Petrillo et al. reported that the NEI-VFQ-25 contained disordered response thresholds (15/25 items) and mis-fitting items (8/25 items) [47, 57]. The psychometric performance has been similarly critiqued in low vision and cataract populations, with studies identifying only two unidimensional scales individually fitting the Rasch model [44, 45]. A Rasch re-engineered NEI-VFQ with two domains and fewer items has been developed [45, 47], but has not been validated in ON. The NEI-VFQ-25 remains widely used as a secondary outcome measure in ophthalmic clinical trials, and in ON trials specifically, including the 10- and 15-year follow-up studies of the 1991 Optic Neuritis Treatment Trial (n = 319) [58], and the more recent RENEW trial of Opicinumab (n = 82) [59, 60].
The FDA have noted the lack of validated PROMs in ophthalmology, and indicated that PROMs developed and validated using Rasch Analysis approaches would be required for the high-stakes situation of a pharmaceutical labelling claim [61]. The PROMs identified in our review were also developed and validated many years before the widespread application of COSMIN guidelines and Rasch Analysis-based quality appraisal tools. For example, only one included study reported on differential item functioning (DIF) [5]. Even with stronger psychometric analysis approaches able to explore DIF than were available decades ago, there is still unmet need for detailed and transparent reporting on item measures in relation to the specific sample of persons chosen to participate in a given study, as the different effects of different types of visual impairments (near and distance visual acuity, colour, contrast sensitivity and field of vision), or comorbidities, on item responses may violate the Rasch requirement of homogeneity of variance in measurement uncertainty. Clear reporting on missing data is also needed to permit appraisal of any potential risk of bias resulting from model artefact. For example, under the assumptions of Classic Test Theory or Lickert scoring, missing data in the raw scores (for example where the participant selects “not applicable” or “don’t do for reasons other than my vision”) cause distortion in the summary variable. Similarly, missing data may lead to disordered response thresholds, generated as an artefact of the model employed (eg the partial credit model or Andrich rating scale model).
A further general theme emerging from this review was very limited content development for ON. We could not identify how many patients with optic neuritis were consulted in the development and selection of the items which went on to be included in the PROMs. Typically, the COSMIN guidelines suggest that in order to develop a structurally valid PROM, at least seven times the number of relevant patients as the number of unidimensional items being assessed for inclusion are needed to develop ‘very good’ content; whereas if the patient sample is fewer than five times the number of items in the instrument, this is ‘inadequate’ [39]. To aim for a disease-specific PROM for every medical condition would be both unachievable and undesirable. Whilst there is likely to be very major overlap in the vision-related impacts of different eye diseases, if using the NEI-VFQ-25 in a clinical stakes trial, it may be useful to first validate the assumption that content from the original patients, who had one of just six eye diseases (age-related macular degeneration, cataract, diabetic neuropathy, glaucoma or cytomegalovirus retinitis) yields necessary and sufficient vision-related quality of life insights for the eye disease under investigation. PROMs developed without comprehensive content identification (saturation) are unlikely to have adequate external generalisability to other settings (different countries, demographics, disease subtypes and treatments), limiting translation into clinical practice. Of note, the quality appraisal criteria (Supplementary Table 1) themselves do not account for whether the patients included in content development were relevant to the outcome of interest for which their quality is being appraised. We therefore added an additional item pertaining to ‘relevance to ON’ in our appraisal process.
Also evident was a historic desire for short instruments with completion times around five minutes to minimise participant burden, in the context of clinical trial examination protocols. Quality appraisal indicates that this focus on speed may have come at the cost of psychometric instrument performance. Evidence suggests there are at least 10 domains of quality of life relevant to people with ophthalmic disease, extending beyond, but including symptoms of disease (see Table 1) [23]. Each domain of interest needs to be measured with a sufficient number of items, spread out on an interval scale, to yield a precise measure for that domain. This is impossible when only one item is included per scale, and the measure is likely to have low precision and reliability when only a few items are included per domain. Fortunately, the advent of computer adaptive testing offers a solution to the ‘time burden’ problem [62].
These multiple limitations may explain the historically poor uptake of PROMs in MS and ON clinical trials, in spite of the FDA encouraging incorporation of PROMs into clinical trials for over a decade [29]. For example, FAMS (developed 1996) was not included as a primary or secondary outcome measure in key phase III clinical trials for drugs which gained subsequent FDA approval for MS, including the AFFIRM trial of natalizumab, CARE-MS trial of alemtuzumab, INFORMS trial of fingolimod or DEFINE trial of dimethyl fumarate [63,64,65,66]. There have been some exceptions. However, where older non disease specific PROMs, developed using CTT, have been included in trials they have methodological limitations and have failed to find any significant differences. For example, Jacobs et al. included the Sickness Impact Profile (SIP) as a secondary outcome measure in the phase 3 study of recombinant interferon beta-la as treatment for relapsing-remitting MS [67]. Whilst the physical component summary score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) was included as a secondary outcome in the ORATARIO phase 3 trial assessing the impact of intravenous ocrelizumab in primary progressive MS [68]. We hypothesise that the lack of differences detected at the person level may be due to the lack of disease-specific content, or the lack of precision of the summary scoring approach or both.
There has been variable uptake of PROMs into clinical practice, despite enthusiastic support from stakeholders and patient advocacy organizations [69]. A common theme has been historic emphasis on ‘hard’ outcomes, such as relapse rates or radiological features as surrogate markers of disease progression, particularly in MS trials. Reliance on such objective outcomes has been understandable but they may miss important aspects of morbidity [70]. In addition, clinical trial results reporting often does not provide PROM interpretation guidelines, which may exacerbate a sense of mystery around PROMs that does not exist for other outcomes [70].
Strengths and limitations
We adhered to sound systematic review methodology including a comprehensive search for published PROMs and robust quality appraisal of identified instruments. We did not extensively search the grey literature or conference abstracts and may have overlooked reports of unpublished PROMs. We consider it unlikely that this would have resulted in the identification and inclusion of any high-quality PROMs not identified through the main search. Our search strategy included optic neuritis of any cause, but we did not conduct a separate search for all immune-mediated inflammatory diseases with which ON may be associated, and may therefore have overlooked some sets of relevant questions.
We felt the quality criteria we used (Supplementary Table 1), were limited in not holding studies utilising older and more simple classic test theory approaches to the same level of account in the grading scheme as studies developed using more modern Rasch Analysis approaches with principal components analysis [29,30,31, 71]. It is worth noting that not all the quality assessment criteria in Supplementary Table 1 are of equal value and importance. The possession of interval scaling and Rasch validity (especially precision and uni-dimensionality) is more important than assessments of validity, reliability, or acceptability. The criteria also did not require assessment of whether or not the patient samples used to develop and to validate a PROM were independent, which is important. We added these and recommend them as a modification to the grading criteria.
Implications
The lack of methodologically robust PROMs in optic neuritis is a significant problem for multiple reasons. The recent coronavirus global pandemic has ushered in a period of accelerated service transformation in health systems internationally. This is driving major shifts towards virtual review and remote monitoring and in this context, PROMs could have an important role to play. PROMs improve patient satisfaction with care, symptom management, quality of life and survival rates [72]. The integration of PROM data through technological infrastructure has progressed rapidly leading to the incorporation of internet-based applications, touchscreen tablets and electronic health records [73]. For clinicians, PROM collection has been shown to enhance shared decision making by allowing the clinicians to better understand the patient’s symptoms and the impact on their quality of life. Furthermore, it can enhance workflow efficiency and save time when used regularly, e.g. by using the limited clinic time to explore a particular symptom burden highlighted from the instrument [74].
The potential value of using a PROM with strong psychometric performance as a trial endpoint cannot be understated. Not only do these permit alignment with the outcomes that most matter to patients, but there are major resource implications. Narrow standard errors around an outcome measure permit recruitment of smaller samples, with major cost saving for trial funders. Based on our quality appraisal, we are not able to recommend any of the currently available PROMs for therapeutic trials in optic neuritis.
Future research
Further research to develop robust PROMs for optic neuritis is needed. Adherence to best practice in PROMs development (as described in guidance from the FDA) will support development of more robust, sensitive PROMs [75]. Larger samples of patients are generally needed for content identification and instrument development than have been used in the PROMs reported here. Future studies could aim for independence of development and validation samples, and recruit a sufficient sample size (>7x patients than number of items in largest unidimensional scale) for robust psychometric development using the Rasch Analysis approach. Transparent reporting on any differential item functioning by potentially relevant clinical characteristics or condition or disease co-morbidities is also needed. Investigators may find the PROTEUS, SPIRIT-PRO and CONSORT-PRO guidelines on the selection and reporting of PROMs for clinical trials helpful [75,76,77,78].
Conclusion
This systematic review highlights an important, unmet need for the development and validation of PROMs that are able to measure the impact of optic neuritis, and its treatment, on multiple domains of quality of life. Demand for robust PROMs is anticipated to rise as not only patients and clinicians [74], but regulators, payers, accreditors, and professional organisations recognise their potential value [73]. Given the time and cost taken to develop a new PROM, and the increasingly important role for PROMs both in clinical trials and the modern health service, further research is needed to identify novel ways to reduce the multiple barriers to their development and wider generalisability. This will be essential to capture the quality of life outcomes that really matter to people.
References
Alliance JL. Sight loss and vision priority setting partnership. London, UK: The College of Optometrists, Fight for Sight and the James Lind Alliance; 2013.
Post AEM, Klockgether T, Landwehrmeyer GB, Pandolfo M, Arneson A, Reinard C, et al. Research priorities for rare neurological diseases: a representative view of patient representatives and healthcare professionals from the European Reference Network for Rare Neurological Diseases. Orphanet J Rare Dis. 2021;16:135.
Braithwaite T, Subramanian A, Petzold A, Galloway J, Adderly NJ, Mollan SP, et al. Trends in Optic Neuritis Incidence and Prevalence in the UK and Association With Systemic and Neurologic Disease. JAMA Neurol. 2020;77:1514–23.
Cleary PA, Beck RW, Anderson MM Jr, Kenny DJ, Backlund JY, Gilbert PR. Design, methods, and conduct of the Optic Neuritis Treatment Trial. Controlled Clin Trials. 1993;14:123–42.
Cella DF, Dineen K, Arnason B, Webster KA, Karabatsos G, Chang C, et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129–39.
Braithwaite T, Wiegerinck N, Petzold A, Denniston A. Vision Loss from Atypical Optic Neuritis: Patient and Physician Perspectives. Ophthalmol Ther. 2020;9:215–20.
Patterson MB, Foliart R. Multiple sclerosis: understanding the psychologic implications. Gen Hosp Psychiatry. 1985;7:234–8.
Reder AT, Antel JP. Clinical spectrum of multiple sclerosis. Neurol Clin. 1983;1:573–99.
Murray TJ. The psychosocial aspects of multiple sclerosis. Neurol Clin. 1995;13:197–223.
Chen BS, Galus T, Archer S, Tadic V, Horton M, Pesuodvs K, et al. Capturing the experiences of patients with inherited optic neuropathies: a systematic review of patient-reported outcome measures (PROMs) and qualitative studies. Graefes Arch Clin Exp Ophthalmol. 2022;260:2045–55.
Dean S, Mathers JM, Calvert M, Kyte DG, Conroy D, Folkard A, et al. “The patient is speaking”: discovering the patient voice in ophthalmology. Br J Ophthalmol. 2017;101:700–8.
Braithwaite T, Calvert M, Gray A, Pesudovs K, Denniston AK. The use of patient-reported outcome research in modern ophthalmology: impact on clinical trials and routine clinical practice. Patient Relat Outcome Measures. 2019;10:9–24.
Administration USFaD. FDA Patient-focused drug development guidance series for enhancing the incorporation of the patient’s voice in medical product development and regulatory decision making. 2020. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical. Accessed 1 Oct 2020.
The Lancet N. Patient-reported outcomes in the spotlight. Lancet Neurol. 2019;18:981. https://www.thelancet.com/journals/laneur/article/PIIS1474-4422(19)30357-6/fulltext Accessed 1 Oct 2020.
FaDAF UDoHaHS. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims. 2009. Accessed 25 Sep 2018.
Frost MH, Reeve BB, Liepa AM, Stauffer JW, Hays RD. Mayo FDAP-ROCMG. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health. 2007;10:S94–105.
Khadka J, McAlinden C, Pesudovs K. Quality assessment of ophthalmic questionnaires: review and recommendations. Optom Vis Sci. 2013;90:720–44.
Pallant JF, Tennant A. An introduction to the Rasch measurement model: an example using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol. 2007;46:1–18.
Stover AM, McLeod LD, Langer MM, Chen WH, Reeve BB. State of the psychometric methods: patient-reported outcome measure development and refinement using item response theory. J Patient Rep Outcomes. 2019;3:50.
Cleary PA, Beck RW, Bourque LB, Backlund JC, Miskala PH. Visual symptoms after optic neuritis. Results from the Optic Neuritis Treatment Trial. J Neuro-Ophthalmol. 1997;17:18–23.
Petzold A, Braithwaite T, van Oosten BW, Balk L, Martinez-Lapiscina EH, Wheeler R, et al. Case for a new corticosteroid treatment trial in optic neuritis: review of updated evidence. J Neurol Neurosurg Psychiatry. 2020;91:9–14.
O’Donovan C, Panthagani J, Aiyegbusi OL, Liu X, Bayliss S, Calvert M, et al. Evaluating patient-reported outcome measures (PROMs) for clinical trials and clinical practice in adult patients with uveitis or scleritis: a systematic review. J Ophthalmic Inflamm Infect. 2022;12:29.
Khadka J, Fenwick E, Lamoureux E, Pesudovs K. Methods to Develop the Eye-tem Bank to Measure Ophthalmic Quality of Life. Optom Vis Sci. 2016;93:1485–94.
Braithwaite TL, Panthagani X, Aiyegbusi OL, Bayliss S, Calvert M, Pesudovs K, et al. Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with ocular immune-mediated inflammatory diseases (uveitis, scleritis or optic neuritis): a systematic review. 2019. https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019151652. Accessed 1 Oct 2020.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Society NMS. Clinical Study Measures: All Clinical Study Measures. 2020. https://www.nationalmssociety.org/For-Professionals/Researchers/Resources-for-Researchers/Clinical-Study-Measures/. Accessed 18 Sep 2020.
Fairclough DL. Design and analysis of quality of life studies in clinical trials 2nd edition. New York: Chapman and Hall/CRC; 2010.
US Department of Health and Human Services FaDAF. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims. 2009. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed 25 Sept 2018.
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol D, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63:737–45.
Prem Senthil M, Khadka J, Pesudovs K. Assessment of patient-reported outcomes in retinal diseases: a systematic review. Surv Ophthalmol. 2017;62:546–82.
Pesudovs K, Burr JM, Harley C, Elliott DB. The development, assessment, and selection of questionnaires. Optom Vis Sci. 2007;84:663–74.
Lundstrom M, Pesudovs K. Questionnaires for measuring cataract surgery outcomes. J Cataract Refractive Surg. 2011;37:945–59.
Kandel H, Khadka J, Goggin M, Pesudovs K. Patient-reported Outcomes for Assessment of Quality of Life in Refractive Error: A Systematic Review. Optom Vis Sci. 2017;94:1102–19.
Kandel H, Khadka J, Lundstrom M, Goggin M, Pesudovs K. Questionnaires for Measuring Refractive Surgery Outcomes. J Refract Surg. 2017;33:416–24.
Kumaran SE, Khadka J, Baker R, Pesudovs K. Patient-reported outcome measures in amblyopia and strabismus: a systematic review. Clin Exp Optom. 2018;101:460–84.
Kandel H, Pesudovs K, Watson SL. Measurement of Quality of Life in Keratoconus. Cornea. 2020;39:386–93.
Hennink MM, Kaiser BN, Weber MB. What Influences Saturation? Estimating Sample Sizes in Focus Group Research. Qual Health Res. 2019;29:1483–96.
Mokkink LB, de Vet HCW, Prinsen CAC, Patrick DL, Alonso J, Bouter LM, et al. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual Life Res. 2018;27:1171–9.
Streiner DL NG. Health measurement scales. A practical guide to their development and use 4th edition ed. Oxford: University Press; 2008.
Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–35.
Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD, et al. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119:1050–8.
Cole SR, Beck RW, Moke PS, Gal RL, Long DT. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Optic Neuritis Treatment Trial. Investig Ophthalmol Vis Sci. 2000;41:1017–21.
MSQLI. Impact of Visual Impairment Scale (IVIS). 1997. https://www.nationalmssociety.org/For-Professionals/Researchers/Resources-for-MS-Researchers/Research-Tools/Clinical-Study-Measures/Impact-of-Visual-Impairment-Scale-(IVIS)
Moore P, Jackson C, Mutch K, Methley A, Pollard C, Hamid S, et al. Patient-reported outcome measure for neuromyelitis optica: pretesting of preliminary instrument and protocol for further development in accordance with international guidelines. BMJ Open. 2016;6:e011142.
Methley AM, Mutch K, Moore P, Jacob A. Development of a patient-centred conceptual framework of health-related quality of life in neuromyelitis optica: a qualitative study. Health Expect. 2017;20:47–58.
Mangione CM, Berry S, Spritzer K, Janz NK, Klein R, Owsley C, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–33.
Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). NEI-VFQ Field Test Investigators. Arch Ophthalmol. 1998;116:1496–504.
Pesudovs K, Gothwal VK, Wright T, Lamoureux EL. Remediating serious flaws in the National Eye Institute Visual Function Questionnaire. J Cataract Refract Surg. 2010;36:718–32.
Group ONTTS. Optic Neuritis Treatment Trial 1991 Datasets & Documents. 1991. https://public.jaeb.org/datasets/othereyediseases. Accessed 9 Dec 2022.
Ma SL, Shea JA, Galetta SL, Jacobs DA, Markowitz CE, Maguire MG, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133:686–92.
Bourque LB, Cosand BB, Drews C, Waring GO 3rd, Lynn M, Cartwright C. Reported satisfaction, fluctuation of vision, and glare among patients one year after surgery in the Prospective Evaluation of Radial Keratotomy (PERK) Study. Arch Ophthalmol. 1986;104:356–63.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–52.
Sipe JC, Knobler RL, Braheny SL, Rice GP, Panitch HS, Oldstone MB. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984;34:1368–72.
Nunnally JC and Bernstein IH. Psychometric theory 3rd edition. New York: McGraw-Hill; 1994.
Petrillo J, Cano SJ, McLeod LD, Coon CD. Using classical test theory, item response theory, and Rasch measurement theory to evaluate patient-reported outcome measures: a comparison of worked examples. Value Health. 2015;18:25–34.
Petrillo J, Bressler NM, Lamoureux E, Ferreira A, Cano S. Development of a new Rasch-based scoring algorithm for the National Eye Institute Visual Functioning Questionnaire to improve its interpretability. Health Qual Life Outcomes. 2017;15:157.
Beck RW, Gal RL, Bhatti MT, Brodsky MC, Buckley EG, Chrousos GA, et al. Visual function more than 10 years after optic neuritis: experience of the optic neuritis treatment trial. Am J Ophthalmol. 2004;137:77–83.
Petrillo J, Balcer L, Galetta S, Chai Y, Xu L, Cadavid D. Initial Impairment and Recovery of Vision-Related Functioning in Participants With Acute Optic Neuritis From the RENEW Trial of Opicinumab. J Neuro-Ophthalmol. 2019;39:153–60.
Cadavid D, Balcer L, Galetta S, Aktas O, Ziemssen T, Vanopdenbosch L, et al. Safety and efficacy of opicinumab in acute optic neuritis (RENEW): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:189–99.
Braithwaite T, Davis N, Galloway J. Cochrane corner: why we still don’t know whether anti-TNF biologic therapies impact uveitic macular oedema. Eye. 2019;33:1830–32.
Fenwick EK, Barnard J, Gan A, Loe BS, Khadka J, Pesudovs K, et al. Computerized Adaptive Tests: Efficient and Precise Assessment of the Patient-Centered Impact of Diabetic Retinopathy. Transl Vis Sci Technol. 2020;9:3.
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl J Med. 2006;354:899–910.
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–28.
Lublin F, Miller DH, Freedman MS, Cree BAC, Wolinsky JS, Weiner H, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:1075–84.
Gold R, Kappos L, Arnold DL, Bar-Or A, Giovannoni G, Selmaj K, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N. Engl J Med. 2012;367:1098–107.
Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating-remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Multiple Sclerosis Collaborative Research Group (MSCRG). Mult Scler. 1995;1:118–35.
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N. Engl J Med. 2017;376:209–20.
Boyce MB, Browne JP. The effectiveness of providing peer benchmarked feedback to hip replacement surgeons based on patient-reported outcome measures—results from the PROFILE (Patient-Reported Outcomes: Feedback Interpretation and Learning Experiment) trial: a cluster randomised controlled study. BMJ Open. 2015;5:e008325.
Coles TM, Hernandez AF, Reeve BB, Cook K, Edwards MC, Boutin M, et al. Enabling patient-reported outcome measures in clinical trials, exemplified by cardiovascular trials. Health Qual Life Outcomes. 2021;19:164.
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539–49.
Slade A, Isa F, Kyte D, Pankhurst T, Kerecuk L, Ferguson J, et al. Patient reported outcome measures in rare diseases: a narrative review. Orphanet J Rare Dis. 2018;13:61.
Jensen RE, Rothrock NE, DeWitt EM, Spiegel B, Tucker CA, Crane HM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care. 2015;53:153–9.
Rotenstein LS, Huckman RS, Wagle NW. Making Patients and Doctors Happier—The Potential of Patient-Reported Outcomes. N. Engl J Med. 2017;377:1309–12.
Administration FD. Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. 2020. https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical. Accessed 1 Oct 2020.
Crossnohere NL, Brundage M, Calvert MJ, King M, Reeve BB, Thorner E, et al. International guidance on the selection of patient-reported outcome measures in clinical trials: a review. Qual Life Res. 2021;30:21–40.
Calvert M, Kyte D, Mercieca-Bebber R, Slade A, Chan AW, King MT, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA. 2018;319:483–94.
Calvert M, Brundage M, Jacobsen PB, Schunemann HJ, Efficace F. The CONSORT Patient-Reported Outcome (PRO) extension: implications for clinical trials and practice. Health Qual Life Outcomes. 2013;11:184.
Author information
Authors and Affiliations
Contributions
DM, TB, KP, MC and AD were involved in the initial study concept and design. SP led the search strategy and searches were run by SP and CO. All titles and abstracts were screened by two independent reviewers (TB and XL/CO/JP), to remove irrelevant articles. At any stage, if the reviewers were unable to reach consensus, an additional reviewer was consulted (KP). Two reviewers (TB and OLA/CO), with adjudication by a third (KP), independently extracted data from studies meeting the inclusion criteria, using a standardised form. All authors were involved in writing & critically reviewing the manuscript.
Corresponding author
Ethics declarations
Competing interests
MC is Director of the Birmingham Health Partners Centre for Regulatory Science and Innovation, Director of the Centre for the Centre for Patient Reported Outcomes Research and is a National Institute for Health and Care Research (NIHR) Senior Investigator. MC receives funding from the NIHR Birmingham Biomedical Research Centre, NIHR Surgical Reconstruction and Microbiology Research Centre, NIHR Birmingham-Oxford Blood and Transplant Research Unit (BTRU) in Precision Transplant and Cellular Therapeutics, and NIHR ARC West Midlands at the University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Health Data Research UK, Innovate UK (part of UK Research and Innovation), Macmillan Cancer Support, SPINE UK,UKRI, UCB Pharma, Janssen, GSK and Gilead. MC has received personal fees from Astellas, Aparito Ltd, CIS Oncology, Takeda, Merck, Daiichi Sankyo, Glaukos, GSK and the Patient-Centered Outcomes Research Institute (PCORI) outside the submitted work. OLA receives funding from the NIHR Birmingham Biomedical Research Centre (BRC), NIHR Applied Research Collaboration (ARC), West Midlands, NIHR Birmingham-Oxford Blood and Transplant Research Unit (BTRU) in Precision Transplant and Cellular Therapeutics at the University of Birmingham and University Hospitals Birmingham NHS Foundation, Innovate UK (part of UK Research and Innovation), Gilead Sciences Ltd, Janssen pharmaceuticals, Inc, and Sarcoma UK. OLA declares personal fees from Gilead Sciences Ltd, GlaxoSmithKline (GSK) and Merck outside the submitted work. The authors have no competing interests
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panthagani, J., O’Donovan, C., Aiyegbusi, O.L. et al. Evaluating patient-reported outcome measures (PROMs) for future clinical trials in adult patients with optic neuritis. Eye 37, 3097–3107 (2023). https://doi.org/10.1038/s41433-023-02478-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02478-z