Abstract
Background/Aims
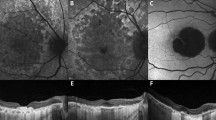
To demonstrate two distinct pathways to geographic atrophy (GA) that originate from soft drusen/ pigment epithelial detachments (PEDs) and subretinal drusenoid deposits (SDDs), respectively, and are characterized by their final quantitative autofluorescence (qAF) levels.
Methods
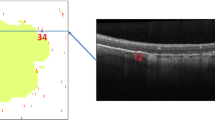
23 eyes of 18 patients with GA underwent spectral-domain optical coherence tomography (SD-OCT) and qAF imaging on the qAF-ready Heidelberg Spectralis. 52 GA Regions-of-interest (ROIs), or clusters of adjacent lesions, were selected, and the ROIs were divided into groups by the dominant iAMD precursors on prior serial tracked SD-OCT scans. Mean qAF values and structural SD-OCT findings of groups were compared.
Results
Group 1 lesions (soft drusen/PED precursors, 18/52) were isolated, with lower mean qAF (35.88 ± 12.75 units); group 3 lesions (SDD precursors, 12/52) were multilobular, with significantly higher mean qAF (71.62 ± 12.12 units, p < 0.05). Group 2 lesions, (mixed precursors, 22/52) had intermediate mean qAF (58.13 ± 67.92 units). Significantly greater prevalence of split RPE/ Bruch’s membrane complex in SDD-associated GA, suggesting basal laminar deposit (BLamD), than in drusen-associated lesions was the major structural difference.
Conclusion
Quantitative autofluorescence (qAF) of GA lesions may reflect two distinct pathogenic pathways and structural outcomes, originating from soft drusen/PED and subretinal drusenoid deposits (SDDs), with the final qAF values lower or higher, respectively. Basal laminar deposit specifically in and adjacent to SDD-associated lesions may account for their greater autofluorescence. The potential importance of this paradigm is that it could direct, simplify and facilitate research on geographic atrophy by dividing the disease into two components that may be studied separately.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
$259.00 per year
only $14.39 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sadda SR, Guymer R, Holz FG, Schmitz-Valckenberg S, Curcio CA, Bird AC, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125:537–48.
Oak AS, Messinger JD, Curcio CA. Subretinal drusenoid deposits: further characterization by lipid histochemistry. Retina. 2014;34:825–6.
Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117:303–12.e1.
Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145:317–26.
Pumariega NM, Smith RT, Sohrab MA, LeTien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118:1619–25.
Xu L, Blonska AM, Pumariega NM, Bearelly S, Sohrab MA, Hageman GS, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33:1850–62.
Nagiel A, Sarraf D, Sadda SR, Spaide RF, Jung JJ, Bhavsar KV, et al. Type 3 neovascularization: evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina. 2015;35:638–47.
Smith RT, Merriam JE, Sohrab MA, Pumariega NM, Barile G, Blonska AM, et al. Complement factor H 402H variant and reticular macular disease. Arch Ophthalmol. 2011;129:1061–6.
Tong YAT, Curcio C, Smith RT. Hyperspectral autofluorescence characterization of drusen and sub-RPE deposits in age-related macular degeneration. Ann Eye Sci. 2021;6:4.
Schmitz-Valckenberg S, Sahel J-A, Danis R, Fleckenstein M, Jaffe GJ, Wolf S, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study). Ophthalmology. 2016;123:361–8.
Biarnes M, Arias L, Alonso J, Garcia M, Hijano M, Rodriguez A, et al. Increased fundus autofluorescence and progression of geographic atrophy secondary to age-related macular degeneration: the GAIN study. Am J Ophthalmol. 2015;160:345–53.e5.
Fleckenstein M, Schmitz-Valckenberg S, Martens C, Kosanetzky S, Brinkmann CK, Hageman GS, et al. Fundus autofluorescence and spectral-domain optical coherence tomography characteristics in a rapidly progressing form of geographic atrophy. Investigative Ophthalmol Vis Sci. 2011;52:3761–6.
Fleckenstein M, Schmitz-Valckenberg S, Lindner M, Bezatis A, Becker E, Fimmers R, et al. The “diffuse-trickling” fundus autofluorescence phenotype in geographic atrophy. Invest Ophthalmol Vis Sci. 2014;55:2911–20.
Monés J, Biarnés M. Geographic atrophy phenotype identification by cluster analysis. Br J Ophthalmol. 2017;102:388–92.
Biarnes M, Colijn JM, Sousa J, Ferraro LL, Garcia M, Verzijden T, et al. Genotype and phenotype-based subgroups in geographic atrophy secondary to age-related macular degeneration. The EYE-RISK Consortium. Ophthalmol Retina. 2020;4:1129–37.
Delori F, Greenberg JP, Woods RL, Fischer J, Duncker T, Sparrow J, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2011;52:9379–90.
Armenti ST, Greenberg JP, Smith RT. Quantitative fundus autofluorescence for the evaluation of retinal diseases. J Vis Exp. 2016;11:53577.
Demirkaya N, van Dijk HW, van Schuppen SM, Abràmoff MD, Garvin MK, Sonka M, et al. Effect of age on individual retinal layer thickness in normal eyes as measured with spectral-domain optical coherence tomography. Investigative Opthalmology Vis Sci. 2013;54:4934.
Sura AA, Chen L, Messinger JD, Swain TA, McGwin G Jr., Freund KB, et al. Measuring the contributions of basal laminar deposit and Bruch’s membrane in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2020;61:19.
Orellana-Rios J, Yokoyama S, Agee JM, Challa N, Freund KB, Yannuzzi LA, et al. Quantitative fundus autofluorescence in non-neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retin. 2018;49:S34–42.
Ach T, Tolstik E, Messinger JD, Zarubina AV, Heintzmann R, Curcio CA. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3242–52.
Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE Jr., Freund KB, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7362–9.
Thomson RJ, Chazaro J, Otero-Marquez O, Ledesma-Gil G, Tong Y, Coughlin AC, et al. Subretinal drusenoid deposits and soft drusen: are they markers for distinct retinal diseases? Retina. 2022;42:1311–8.
Guymer RH, Wu Z, Hodgson LAB, Caruso E, Brassington KH, Tindill N, et al. Subthreshold nanosecond laser intervention in age-related macular degeneration: the LEAD randomized controlled clinical trial. Ophthalmology. 2019;126:829–38.
Theodore Smith R. Sub-threshold nanosecond laser (SNL) treatment in intermediate AMD (IAMD). Ann Eye Sci. 2019;4:2.
Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523.
Cheng H, Kaszubski PA, Hao H, Saade C, Cunningham C, Freund KB, et al. The relationship between reticular macular disease and choroidal thickness. Curr eye Res. 2016;41:1492–7.
Garg A, Oll M, Yzer S, Chang S, Barile GR, Merriam JC, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013;54:7075–81.
Lains I, Wang J, Providencia J, Mach S, Gil P, Gil J, et al. Choroidal changes associated with subretinal drusenoid deposits in age-related macular degeneration using swept-source optical coherence tomography. Am J Ophthalmol. 2017;180:55–63.
Nesper PL, Soetikno BT, Fawzi AA. Choriocapillaris nonperfusion is associated with poor visual acuity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2017;174:42–55.
Switzer DW Jr., Mendonca LS, Saito M, Zweifel SA, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012;32:1265–71.
Tong Y, Ami TB, Hong S, Heintzmann R, Gerig G, Ablonczy Z. et al. Hyperspectral autofluorescence imaging of drusen and retinal pigment epithelium in donor eyes with age-related macular degeneration. Retina (Philadelphia. Pa). 2016;36:S127–36.
Tan ACS, Astroz P, Dansingani KK, Slakter JS, Yannuzzi LA, Curcio CA, et al. The evolution of the plateau, an optical coherence tomography signature seen in geographic atrophy. Investigative Ophthalmol Vis Sci. 2017;58:2349–58.
Querques G, Capuano V, Frascio P, Zweifel S, Georges A, Souied EH. Wedge-shaped subretinal hyporeflectivity in geographic atrophy. Retina. 2015;35:1735–42.
Fragiotta S, Parravano M, Sacconi R, Costanzo E, Viggiano P, Prascina F, et al. A Common Finding in Foveal-Sparing Extensive Macular Atrophy with Pseudodrusen Implicates Basal Laminar Deposits. Retina. 2022;42:1319–29.
Funding
NIH R01 EY015520 (RTS), Macula Foundation (KBF). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
WW: acquisition of data, analysis and interpretation of data, manuscript preparation MM: acquisition of data, analysis and interpretation of data, manuscript preparation. OOM: analysis and interpretation of data, manuscript preparation. YT: analysis and interpretation of data, manuscript preparation. ES: analysis and interpretation of data, manuscript preparation. GQ: analysis and interpretation of data, manuscript preparation. KBF: design, acquisition of data, analysis and interpretation of data, manuscript preparation. RTS: design, acquisition of data, analysis and interpretation of data, manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, W., Mazzola, M., Otero-Marquez, O. et al. Two potentially distinct pathways to geographic atrophy in age-related macular degeneration characterized by quantitative fundus autofluorescence. Eye 37, 2281–2288 (2023). https://doi.org/10.1038/s41433-022-02332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02332-8