Abstract
Background/Objectives
To evaluate the ability of swept-source optical coherence tomography (SS-OCT) implemented with angiography analysis (SS-OCTA) to detect neuro-retinal and vasculature changes in patients with Parkinson’s disease (PD) and essential tremor (ET), and to distinguish between both pathologies.
Subjects/Methods
A total 42 PD and 26 ET patients and 146 controls underwent retinal evaluation using SS-OCT plus OCT-Angio™. The macular (m) and peripapillary (p) retinal nerve fiber layer (RNFL) and ganglion cell layer (GCL), and macular vasculature were assessed. A Linear discriminant function (LDF) was calculated to evaluate the diagnostic ability of SS-OCTA in both PD and ET.
Results
PD patients presented a reduction in mRNFL (p < 0.005), mGCL (all sectors, p < 0.05) and pRNFL (p < 0.005) vs healthy controls, and in mRNFL and pRNFL vs ET patients (p < 0.001). ET patients showed a significant reduction in mGCL vs controls (p < 0.001). No differences were observed in the macular vasculature between groups. Predictive diagnostic variables were significant only for PD and a LDF was obtained with an area under the ROC curve of 0.796.
Conclusions
Neuro-retinal thinning is present in both diseases, being greater in PD. While SS-OCT could be useful in diagnosing ET and PD, the diagnostic potential for SS-OCTA based on an LDF applies only to PD, not ET.
Similar content being viewed by others
Introduction
Essential tremor (ET) is a common neurological disease [1] whose primary motor feature is action tremor [2]. Patients with ET may often manifest intention tremor [3], mild gait ataxia [4], and other features of subtle cerebellar dysfunction [5]. The pathophysiology of ET is unclear, though recent research suggests it is likely degenerative [6, 7].
Parkinson’s disease (PD) is a chronic progressive disease characterized by motor and non-motor symptoms. It affects millions of people worldwide and is the second commonest neurodegenerative disease after Alzheimer’s. The main cause of disability in patients with PD is the loss of dopaminergic neurons in the central nervous system [8, 9]. Dopamine, which plays a vital role in visual processing in the retina, has been reported to be deficient in the retina of patients with PD [10].
ET and PD are different pathological and clinical entities. However, some cases may share overlapping clinical features, making differential diagnosis difficult [11]. Accurate confirmatory diagnostic tests for both ET and PD are very limited, and diagnosis still depends on medical history and neurological examination [12].
Patients suffering from PD present a reduction in retinal thickness and inner retinal layers [13, 14], observable using digital imaging technologies such as spectral domain optical coherence tomography (SD-OCT) and the more recent swept-source OCT (SS-OCT). Digital imaging technologies in ophthalmology have recently improved with the implementation of OCT technology accompanied by angiographic analysis of the retinal vascular plexus. OCT angiography (OCTA) provides a new and more extensive evaluation of the retina and its vasculature in different pathological processes, including neurodegenerative disease [15, 16], thus enabling a better understanding of the pathophysiology of retinal degeneration.
Despite recent research pointing to a degenerative pathophysiology of ET [6, 7], very few studies evaluate possible neuroretinal changes in this disease, or whether retinal measurements are any different from those in the more debilitating PD [17, 18]. Current published studies are heterogeneous, and comparisons with healthy eyes showed contradictory results [17, 19] In this study, complete assessment of the neuroretina (in the macular and peripapillary areas) was performed on patients with PD and ET. In addition, the macular vasculature was evaluated in PD, ET and control subjects. Our main purpose was to evaluate the diagnostic ability of SS-OCT and SS-OCTA technology in patients with these two common degenerative tremoring diseases.
Materials/subjects and methods
The study was performed at Miguel Servet University Hospital, in Zaragoza (Spain). Participants were recruited from June 2020 to December 2020. Patients with confirmed idiopathic PD diagnosis were included in an observational cross-sectional study. In addition, 26 patients with confirmed ET diagnosis were recruited. Diagnosis of ET was based on the Consensus Statement on the classification of tremors of the International Parkinson and Movement Disorder Society (Bathia et al. 2018), and cases of other Axis 1 isolated tremor syndromes, isolated focal tremors (such as head or voice tremor), orthostatic tremor and tremor + dystonia were excluded. A power calculation was performed, based in the results of our previous studies and assuming an alpha error of 5% and a beta error of 10%. A standard sample size equation was used to calculate the required number of subjects (23 in each group). To increase the power of the study, we included a higher number of participants. A total of 42 eyes of 42 patients with PD, 26 eyes of 26 patients with ET, and 146 eyes of 146 healthy individuals were evaluated. All procedures conformed with the tenets of the Declaration of Helsinki. The experimental protocol was approved by the Miguel Servet Hospital Ethics Committee (Comité Ético de Investigaciones Científicas de Aragón). All study participants provided informed consent in writing.
The diagnoses of PD and ET were made by a trained neurologist (RA) based on the United Kingdom Brain Bank Criteria [20] and the Consensus Statement on the classification of tremors of the International Parkinson and Movement Disorder Society (for PD and ET, respectively). Information was recorded regarding disease severity (Hoehn Yahr scale), duration, and treatment. All subjects received a full ophthalmological evaluation. The optic disc of all participants was thoroughly examined, excluding patients with significant refractive errors (>5 diopters of spherical equivalent refraction or 3 diopters of astigmatism). Further exclusion criteria included observable ocular changes during ophthalmological evaluation (e.g., retinal changes associated with a shallow chamber or high myopia), intraocular pressure ≥21 mmHg, concomitant ocular disease (including history of glaucoma or retinal pathology), media opacification, systemic conditions potentially affecting the visual system—including diabetes and neurological diseases (e.g., dementia and multiple sclerosis) and peripheral nerve disease attributable to processes other than PD)—and eyes with suspected glaucomatous damage. Healthy individuals were examined thoroughly and screened to the same exclusion criteria as patients. The control subjects presented neither signs nor history of ocular, neurological, or systemic disease. Finally, a best-corrected visual acuity threshold was set at >20/30 (Snellen scale) to exclude amblyopic eyes.
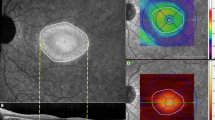
SS-OCT (Triton plus; Topcon Corporation, Tokyo, Japan), coupled with non-invasive OCTA technology (SS-OCT-Angio™), was used to obtain the retinal images. This device uses a tunable laser as a light source to provide a 1050 nm centered wavelength and allows for visualization of the deepest structures of the retina, detecting even low microvascular density with high sensitivity. For retinal structural measurements, the 3D Wide protocol for SS-OCT Triton was used on all subjects. This protocol offers a wide scanning range that includes both the macular (ETDRS scan/TSNIT scan) and peripapillary areas (TSNIT scan). A TSNIT scan takes separate automated measurements of 6 sectors (superonasal, superotemporal, superior, inferonasal, inferotemporal, and inferior) of the retinal layers in the peripapillary and macular areas. A TSNIT scan also takes measurements of 4 quadrants (superior, nasal, inferior, and temporal) of the peripapillary area and of 12 clock sectors. All study subjects were evaluated as per the TSNIT (macular and peripapillary) protocol, which included measurement of RNFL and GCL thicknesses in the 6 macular and peripapillary sectors. Vasculature analysis was performed using Triton SS-OCT-Angio™ images (6 × 6 mm cube centered on the fovea, 320 × 320 resolution). Surface area was measured with proprietary software (Topcon IMAGEnet®, version 1.19) following automated segmentation of the macular area into superficial vascular plexuses (SVP, SVP-foveal avascular zone). Large vessels were included, and vessel density was calculated between the internal limiting membrane and the inner plexiform layer and was interpreted as the percentage of scanned area (6 × 6 mm) occupied by the vessel’s lumens (Fig. 1).
The vascular plexus can be displayed throughout different ocular levels: superficial (A), deep (B), outer retina (C) and choriocapilaris (D). Objective measurements are automatically calculated for the superficial plexus (from the inner limiting membrane to the inner plexiform layer –marked with an arrow–) as the percentage of the scanned area (in four quadrants and central area) occupied by the lumens of the vessels (E).
All scans were performed by the same experienced operator. Only images obtained with a quality score above 50 (for OCT structural measurements) and 40 (OCTA) were included (ranging from 0 “poor quality” to 100 “excellent quality”). Images with movement artefacts were excluded prior to data analysis. One eye per patient was chosen at random for the study.
The variables were entered in a database created using commercially available software (FileMaker Pro 8.5; File-Maker, Inc., Santa Clara, CA), with intraocular pressure and age as modifier variables. Commercially available predictive analytics software (SPSS, version 20.0; SPSS, Inc., Chicago, IL) was used for statistical analysis. Sample distribution normality was confirmed via the Kolmogorov–Smirnov test. Comparisons between both groups of patients (PD and ET) and controls were made using the ANOVA test, and a P value ≤ 0.05 was considered of statistical significance for all calculations. Bonferroni correction for multiple comparisons was applied (see Tables).
Logistic regression analysis was performed to ascertain whether any of the structural parameters evaluated in the study had the ability to differentiate between healthy eyes and those with ET or PD. Our dataset was used for 2 binary logistic regression analyses, where the dependent variables were PD (yes or no) or ET (yes or no), respectively, and the predictive variables were the RNFL, GCL and vascular density measurements. Independent variables’ relative importance was assessed using stepwise binary logistic regression analysis (forward Wald method). A linear discriminant function (LDF) was obtained from regression analysis as a score calculated by taking the weighted sum of the predictor variables. Receiver Operating Characteristic (ROC) curves were plotted for all parameters and compared against the proposed LDF.
Results
A total of 214 subjects (214 eyes) were included in the study: 42 patients with PD, 26 patients with ET and 146 healthy individuals. There were no differences in age (p = 0.237) or intraocular pressure (p = 0.591) between the groups. Disease duration in the PD group was 8.95 ± 6.58 years; disease duration in the ET group was 17.77 ± 15.64 years. The median Hoehn Yahr stage was 2.67 ± 0.68 in the PD cohort. All demographic variables, including treatments, can be seen in Table 1.
Macular measurements
Measurements of the macular RNFL revealed significant differences between healthy eyes and PD eyes affecting the superior (p = 0.006) and superonasal (p < 0.001) sectors. PD eyes also showed significant differences when compared with ET patients in the superonasal (p < 0.001), inferonasal (p = 0.001) and inferotemporal (p = 0.037) sectors. No differences in RNFL thickness were observed between ET eyes and healthy eyes (Table 2).
PD eyes presented reduced GCL thickness in all macular sectors vs healthy controls (p < 0.05), especially in the superior (p < 0.001), superonasal (p < 0.001), inferonasal (p < 0.001) and inferior (p = 0.003) sectors. No significant differences in GCL thickness were observed between PD eyes and ET eyes. However, the GCL in ET eyes was significantly thinner than it was in controls in the superonasal (p < 0.001) and inferonasal (p < 0.001) sectors (See Table 2 and Fig. 2).
Peripapillary measurements
The RNFL was reduced in PD patients vs controls in the temporal (p < 0.001), superotemporal (p = 0.002) and inferotemporal (p = 0.022) sectors. PD measurements were also lower than in ET patients in the temporal sector (p < 0.001). ET patients presented a reduction of the RNFL in the nasal sector vs healthy controls (p = 0.013).
The peripapillary GCL did not show significant differences between the groups, except for a slight reduction in the superotemporal sector in PD eyes vs ET eyes (p = 0.012).
All peripapillary measurements can be seen in Table 3.
OCTA measurements
No significant differences in macular vascular density were observed between the groups (Supplementary table 1).
Logistic regression analysis
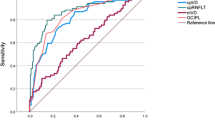
Logistic regression analysis showed 2 significant parameters with ability to discriminate between healthy eyes and those with PD: the superior macular sector of the GCL (p = 0.020) and the temporal sector of the OCTA (p = 0.023). The LDF obtained was LDF = 92.935 − (0.955 * temporal density of the OCTA) − (0.734 * upper macular sector of the GCL). Once this LDF was applied to our sample, we obtained an area under the ROC curve of 0.796 (Fig. 1).
No significant parameters were obtained to differentiate ET eyes from those of healthy subjects.
Discussion
In this study we evaluated structural and vascular changes in the retina of PD and ET patients using SS-OCT. Retinal thinning has been widely demonstrated in PD in the past decade, with affectation of the RNFL and GCL being the most common finding [13, 14, 21, 22]. However, literature on retinal changes in ET is scarce, and few published articles compare both diseases within the same study [17, 18]. In addition, to the best of our knowledge, this is the first study assessing retinal vascular density both in PD (using SS-OCT technology) and ET.
PD patients in our sample presented a reduction in GCL in all macular sectors vs controls, as well as a reduction in RNFL in different areas. Such observations support previous published results on retinal thinning in PD [13, 14, 22, 23], which point to a loss of the inner retinal layers being a manifestation of dopamine depletion and neurodegeneration in these patients.
In ET eyes, we observed reduced GCL thickness vs controls in the nasal sectors of the macular area. In addition, the pRNFL was reduced in the nasal sector in ET (although it did not reach Bonferroni significance levels). Few studies have focused on evaluation of retinal changes in ET [17,18,19, 24], and only one previous study analyzed the GCL in these patients [24]. In 2010, Cubo et al. [18]. published a small comparative pilot study on PD and ET, showing decreased foveal thickness in PD. However, no data on the comparison of ET with controls was mentioned and it was assumed no differences were observed. Turkel et al. [19] evaluated RNFL thickness in ET patients and found thinner RNFL only in the nasal quadrant vs controls. A recent study by Tak et al. showed for the first time decreased GCL thickness in ET patients vs controls using SD-OCT, as well as increased choroidal thickness [24]. Tugcu et al. [17] however, did not find significant differences between ET and healthy eyes. Our results support the findings by Tak and those by Turkel, providing a more extensive analysis of the macula and the peripapillary area not described in previous reports.
Comparison between PD and ET has also been poorly documented in the past and evaluations were far from uniform and complete [17, 18]. In a very recent study, Tugcu et al. evaluated the peripapillary RNFL of PD and ET patients, finding a reduction in PD vs ET patients [17]. Total macular thickness also showed a significant reduction in PD, but other inner retinal layers were not analyzed. Different OCT devices were used in these past studies and sample sizes also differed. Using the Triton OCT device, we observed a reduction in the superonasal and nasal sectors of the mRNFL and in the temporal sector of the pRNFL in PD vs ET, supporting previous findings on differences between these 2 processes.
The diagnostic ability of OCT analysis of the inner retinal layers has been well established in recent years for different neurodegenerative processes [13, 14, 25,26,27]. However, its utility in the diagnosis of ET vs PD is controversial. Given the scarce current literature and the complexity of ET and other tremor disorders, the possible application of OCT measurements in differential diagnosis between ET and PD should be analyzed with caution. Tugcu et al. [17] did not find significant differences between control and ET eyes, nor between more complex cases of ET (such as ET + PD), suggesting OCT measurements were not useful as an indirect biomarker of these diseases. Our results, however, along with the findings of Tak et al. [24]. may point to the possible applicability of OCT as a diagnostic tool for ET when patients meet current clinical criteria; the retinal GCL and RNFL measurements could serve to reinforce ET diagnosis, also supporting the notion that ET has a neurodegenerative basis. Moreover, we observed macular GCL damage affecting the nasal sectors of ET patients, but this damage is not observed in the topographic corresponding areas of the RNFL. This suggests a primary affectation of the macular ganglion cells in ET, as seems to be the case in PD. The pathogenesis in PD has long pointed to a dopamine depletion in the macular layers. The fact that different regions of the macula are affected in ET does not per se clarify the neurological basis for the disease, but it might suggest that ET and PD are not so dissimilar and a common mechanism might underlie GCL depletion.
Further utility of OCT to discern between complex cases (such as ET + PD cases) was not tested in our study. due to the poorly understood pathophysiology of these processes. In addition, analyzing retinal changes between different subtypes of ET (ET and ET plus) was not considered in our study due to the high subjective variability in the classification of these patients by trained neurologists. ET is considered a syndrome with multiple possible etiologies. Cerebellar degeneration is the pathophysiological alteration most commonly found in ET patients, but other mechanisms for this disease are possible and likely [28]. In addition, when ET features overlap with those of PD (tremor-dominant PD), diagnosis based on clinical findings becomes difficult and inclusion of these patients might lead to inaccurate grouping and misdiagnosis. More studies are needed in the future, with larger sample sizes, comparing different ET phenotypes and other tremor diseases, to corroborate the utility of OCT devices in the diagnosis of complex cases of ET.
Evaluation of vascular alterations in neurodegenerative diseases using OCTA technology is relatively new. Previous studies have found reduced blood flow to the parafoveal areas in multiple sclerosis patients [15, 16], and reduced density of the superficial and deep vascular plexus in Alzheimer’s [15]. Retinal vascularization in PD, however, has barely been evaluated. Only one previous study assessed the macular microvasculature in these patients, finding a significant decrease vs healthy eyes [29]. Retinal vasculature in ET has not been previously studied. In our patients, we did not find any significant differences in vascular density values between PD and ET and controls. Contrary to the data reported by Kwapong et al., our results suggest that retinal hypoperfusion is unlikely playing a role in retinal degeneration in these diseases.
Few studies have evaluated the diagnostic ability of OCT in PD by calculating a LDF [30]. Garcia-Martin et al. found that the Nsite Axonal Analytics for Spectralis OCT (spectral domain technology) was useful for distinguishing between healthy subjects and PD patients (with an area under the ROC curve of 0.845). Our calculations in this study demonstrate that the ability of Triton OCT (SS technology) to distinguish between PD, ET, and healthy subjects is lower, with an area under the curve of 0.796. It seems that the distinction made between these three groups is less precise, perhaps because ET causes mild axonal damage in the RNFL, though less than PD, and because in this study we only included a small sample of ET eyes. Our LDF using OCT(A) measurements could be useful as a supplementary tool in diagnosing PD. However, our regression analysis did not yield any results for ET patients, making the diagnostic potential of OCT in patients with early-stage suspected tremor (ET vs early PD) uncertain at this time. Thus, more studies with a larger sample size are needed to evaluate the ability of the OCT LDF to detect PD (in the early stages of onset) and ET. In addition, further studies should analyze the possibility of improving this algorithm by including other ophthalmological parameters such as clinical symptoms, neuroimaging or blood test biomarkers.
In conclusion, our results suggest that ET patients present significant differences in retinal measurements vs healthy subjects and PD patients when analyzed using SS-OCT technology, a technique that is fast, non-invasive and cost-effective. PD patients showed loss of retinal ganglion cells vs both controls and ET patients, and ET subjects presented a reduction of the GCL vs controls. Retinal hypoperfusion was not observed in our patients (PD nor ET). Current diagnostic criteria for ET and PD are clinical; the lack of disease-specific diagnostic markers for both processes may lead to delayed diagnosis and, especially when features overlap, to misdiagnoses. To the best of our knowledge, this is the first study to provide a complete evaluation of the neuroretina of ET and PD patients—along with an analysis of the retinal vasculature—using an SS-OCT device. OCT technology can be a useful diagnostic tool in distinguishing between ET and PD based on neuroretinal measurements. However, the diagnostic potential for OCT based on an LDF only currently applies to PD, and not ET. Moreover, its utility in the diagnosis of overlapping ET/PD is still uncertain. More studies with larger sample sizes and longitudinal design are needed to corroborate these findings.
Summary
What was known before
-
Parkinson disease patients present with reduction of the neuro-retina.
-
There are fewer studies on the neuroretina in essential tremor patients and results are contradictory.
-
Differences in the retinal vascular plexus between these 2 diseases have not been priorly evaluated.
-
There is a continuous search for OCT biomarkers for these diseases and improvement of OCT ability for diagnosis.
What this study adds
-
This study evaluates Parkinson disease, essential tremor and healthy subjects, a swept source OCT device is used for the first time in these patients.
-
Retinal vascular plexus is evaluated in these patients for the first time using SS OCT-Angiography A Linear Discriminant Function is calculated for SS-OCT for the diagnosis of Parkinson and Essential tremor, yielding good results for Parkinson disease.
Data availability
Data supporting the conclusions of this study will be available upon reasonable request to the corresponding author.
References
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20:725–7.
Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord. 2009;24:626–7.
Rao AK, Louis ED. Ataxic Gait in Essential Tremor: A Disease-Associated Feature? Tremor Other Hyperkinet Mov (NY). 2019 9. https://doi.org/10.7916/d8-28jq-8t52
Benito-Leon J, Labiano-Fontcuberta A. Linking Essential Tremor to the Cerebellum: Clinical Evidence. Cerebellum 2016;15:253–62.
Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–61.
Marin-Lahoz J, Gironell A. Linking Essential Tremor to the Cerebellum: Neurochemical Evidence. Cerebellum 2016;15:243–52.
Khoo TK, Yarnall AJ, Duncan GW, Coleman S, O’Brien JT, Brooks DJ, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–81.
Kwon DH, Kim JM, Oh SH, Jeong HJ, Park SY, Oh ES, et al. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol. 2012;71:267–77.
Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009;127:737–41.
Fekete R, Jankovic J. Revisiting the relationship between essential tremor and Parkinson’s disease. Mov Disord. 2011;26:391–8.
Minen MT, Louis ED. Emergence of Parkinson’s disease in essential tremor: a study of the clinical correlates in 53 patients. Mov Disord. 2012;23:1602–5.
Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson’s disease. Vis Res. 2004;44:2793–97.
Satue M, Obis J, Alarcia R, Rodrigo MJ, Vilades E. Gracia et al. Retinal and choroidal changes in patients with Parkinson’s disease detected by Swept source Optical coherence tomography. Curr Eye Res. 2018;43:109–15.
Tsokolas G, Tsaousis KT, Diakonis VF, Matsou A, Tyradellis S. Optical Coherence Tomography Angiography in Neurodegenerative Diseases: A Review. Eye Brain. 2020;12:73–87.
Cordon B, Vilades E, Orduna E, Satue M, Perez-Velilla J, Sebastian B, et al. Angiography with optical coherence tomography as a biomarker in multiple sclerosis. PLoS ONE. 2020;15:e0243236.
Tugcu B, Melikov A, Yildiz GB, Gökcal E, Ercan R, Uysal O, et al. Evaluation of retinal alterations in Parkinson disease and tremor diseases. Acta Neurol Belg. 2020;120:107–13.
Cubo E, Tedejo RP, Rodriguez-Mendez V, López-Peña MJ, Trejo-Gabriel Y, Galán JM. Retina thickness in Parkinson’s disease and essential tremor. Mov Disord. 2010;25:2461–2.
Turkel Y, Ornek N, Dag E, Ornek K, Alpua M, Ogurel T, et al. Retinal nerve fiber layer thickness in patients with essential tremor. Neurol Asia. 2015;20:363–6.
Reichmann H. Clinical criteria for the diagnosis of Parkinson’s disease. Neurodegenerative Dis. 2010;7:284–90. https://doi.org/10.1159/000314478
Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Dolz I, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. EYE (Lond). 2013;27:507–14.
Polo V, Satue M, Rodrigo MJ, Otin S, Alarcia R, Bambo MP, et al. Visual dysfunction and its correlation with retinal changes in patients with Parkinson’s disease: an observational cross-sectional study. BMJ Open. 2016;6:e009658.
Bodis-Wollner I. Retinopathy in Parkinson disease. J Neural Transm. 2009;116:1493–501.
Tak AZA, Şengül Y, Karadağ AS. Evaluation of thickness of retinal nerve fiber layer, ganglion cell layer, and choroidal thickness in essential tremor: can eyes be a clue for neurodegeneration? Acta Neurol Belg. 2018;118:235–41.
Herrero R, Garcia-Martin E, Almarcegui C, Ara JR, Rodriguez-Mena R, Martin J, et al. Progressive degeneration of the retinal nerve fiber layer in patients with multiple sclerosis. Investig Ophthalmol Vis Sci. 2012;53:8344–9.
Garcia-Martin E, Pablo LE, Herrero R, Satue M, Polo V, Larrosa JM, et al. Diagnostic ability of a linear discriminant function for Spectral domain optical coherence tomography in multiple sclerosis patients. Ophthalmology 2012;119:1705–11.
Garcia-Martin E, Polo V, Bambo MP, Pinilla J, Larrosa JM, Satue M, et al. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. EYE (Lond). 2014;28:680–90.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 2007;130:3297–307.
Kwapong WR, Ye H, Peng C, Zhuang X, Wang J, Shen M, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Investig Ophthalmol Vis Sci. 2018;59:4115–22.
Garcia-Martin E, Satue M, Otin S, Fuertes I, Alarcia R, Larrosa JM, et al. Retina measurements for diagnosis of Parkinson disease. Retina 2014;34:971–80.
Funding
This research received no specific funding from any agency in the commercial or not-for-profit sectors. This paper was supported by PI17/01726, PI17/01946 and PI20/00437 (Carlos III Health Institute), and by MAT2017-83858-C2-2 and PID2020-113281RB-C22 MINECO/AEI/ERDF, EU. MS was supported by the Juan Rodes program (Carlos III Health Institute, CM17/00010).
Author information
Authors and Affiliations
Contributions
MS was responsible for the conception and design, interpretation of data, drafting of paper and final approval of the version to be published. LC, AP, EV, BC and JME carried out the acquisition, analysis and interpretation of data, critical revision of draft and the final approval of the version to be published. EMC and EGM were responsible for the conception and design, interpretation of data, critical revision of draft and final approval of the version to be published: All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors report no competing interests. The authors alone are responsible for the content and writing of the paper. The authors have no proprietary or commercial interest in any materials discussed in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Satue, M., Castro, L., Vilades, E. et al. Ability of Swept-source OCT and OCT-angiography to detect neuroretinal and vasculature changes in patients with Parkinson disease and essential tremor. Eye 37, 1314–1319 (2023). https://doi.org/10.1038/s41433-022-02112-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02112-4