Abstract
Background
To compare the accuracy of recently developed modern intraocular lens (IOL) power formulas (Barrett Universal II, Kane and VRF-G) with existing IOL power formulas in eyes with an axial length (AL) ≤ 22 mm.
Methods
This analysis comprised 172 eyes of 172 patients operated on by one surgeon (LT) with one IQ SN60WF (Alcon Labs, Fort Worth, TX, USA) hydrophobic lens. Ten IOL formulas were evaluated: Barrett Universal II (BUII), Haigis, Hoffer Q, Holladay 1, Holladay 2, Kane, SRK/T, T2, VRF and VRF-G. The median absolute error (MedAE), mean absolute error (MAE), standard deviation (SD) and all descriptive statistics were evaluated. Percentages of eyes with a prediction error within ±0.25 D, ±0.50 D, ±0.75 D and ±1.00 D were calculated using standard optimised constants for the entire range of axial lengths.
Results
The VRF-G, Haigis and Kane produced the smallest MedAE among all formulas (0.242 D, 0.247 D and 0.263 D, respectively) and had the highest percentage of eyes with a PE within ±0.50 D (75.67%, 73.84% and 75.16%, respectively). The Barrett was less accurate (0.298 D and 68.02%, respectively). Statistically significant differences were found predominantly between the VRF-G (P < 0.05), Kane (P < 0.05) and Haigis (P < 0.05) and all other formulas. The percentage of eyes with a PE within ±0.50 D ranged from 66.28% to 75.67%.
Conclusions
In eyes with AL ≤ 22.0 mm, the VRF-G, Haigis and Kane were the most accurate predictors of postoperative refraction, and the Barrett formula was less predictable.
Similar content being viewed by others
Introduction
Accurate intraocular lens selection is mandatory for modern cataract surgery. Moreover, the increased level of patient expectation has transformed cataract surgery into a refractive procedure, with a primary aim of achieving the desired postoperative refraction. As demonstrated by previous studies, the most challenging task is to select the appropriate intraocular lens power in patients with short axial lengths [1, 2].
In the past decade, many new IOL power calculation formulas have been introduced by their authors. Some of them were described in detail, while others were not and are instead available as an element of software for PC or online web calculators [3,4,5,6]. The Barrett Universal II (Barrett) formula was elaborated as a paraxial ray-tracing approach, as it does not rely on a thin-lens assumption. This formula became widely popular in the last five years and is considered the most accurate. Recent studies have shown the Barrett formula to have the lowest absolute error among other modern methods [7]. The Kane formula was introduced by author J. Kane in 2018 [8]. It was created using several large data sets from selected high-volume surgeons uses a combination of theoretical optics, thin-lens formulas and ‘big data’ techniques to make its predictions. The Kane formula uses the AL, K, ACD (from epithelium to the lens), LT and CCT values and the gender of the patient to make its predictions. This formula is considered one of the more accurate predictors of postoperative refraction, especially in axial hyperopia, with a high-power intraocular lens of 30 or more dioptres [9]. The VRF-G formula was released as a classical vergence-based formula that incorporates both regression and ray-tracing components and does not rely on any artificial intelligence approach [10]. This method can be considered a profound modification of the original VRF formula, which was described previously [4]. It uses eight variables to predict IOL power, including mandatory AL, K, ACD (from the epithelium to the lens) and gender, while horizontal CD, LT, CCT and preoperative refractive spherical equivalent (SE) are optional. Recent investigations have shown promising results for this formula, especially in eyes with a short axial length [10, 11].
Our study aims to evaluate and compare the predictive accuracy of the classical vergence-based Haigis, Hoffer Q, Holladay 1 and 2, T2, SRK/T and VRF formulas with the recently developed modern Barrett Universal II, Kane and VRF-G methods. An additional aim of this study was to compare the performance of the VRF [4] and VRF-G [10] formulas in eyes with an axial length ≤22 mm. Furthermore, the current study sought to prospectively determine the percentage of eyes with a PE within ±0.50 D, which is crucial for practical use. To our knowledge, few published studies have assessed the Barrett Universal II, Kane, VRF and VRF-G formulas in eyes with short axial lengths [10,11,12].
Materials and methods
All patients who were examined in a state municipal eye clinic between May 2017 and June 2021 were prospectively enroled. Informed consent was obtained from each patient, and the study was approved by the local ethics committee (IRB-CME-202013-E). The study methods adhere to the Helsinki Declaration on Human Participation in Biomedical Research.
The patients who participated in this study were selected from daily practice. All patients underwent uneventful phacoemulsification (LT) and in-the-bag implantation of one-piece soft hydrophobic acrylic posterior chamber AcrySof IQ SN60WF IOLs (172 eyes) (Alcon Labs, Fort Worth, TX, USA).
The study included patients with a diagnosis of cataracts without comorbidities. The criteria for exclusion were corneal degenerative changes, any stage glaucoma, corneal astigmatism greater than 1.5 dioptres, and prior refractive surgery. If patients had undergone bilateral IOL surgery, one eye was randomly chosen for inclusion in the study.
All patients underwent the following complex diagnostic tests before surgery: uncorrected and best-corrected visual acuity, tonometry, kerato-refractometry and optical biometry on an IOL Master 700, software version 1.7 (Carl Zeiss Meditec AG, Jena, Germany). Only measurements with good quality were included in the analysis, since measurements with exclamation marks (!) or asterisks (*) were eliminated. The Hoffer Q formula was used as the actual IOL power formula for preoperative calculation.
Preoperative intraocular lens power was calculated according to the following formulas:
-
1. Barrett Universal II (hereafter Barrett): this unpublished formula was accessible as part of the IOL Master 700 software version 1.7. (Carl Zeiss Meditec AG, Jena, Germany) and was available via an online calculator at http://calc.apacrs.org/barrett_universal2105/ (accessed on April 4th, 2021); this calculator provides the formula constant (Lens Factor) for many IOL models [5]. A Lens Factor of 1.88 was used for the SN60WF IOL as recommended by the corresponding software (IOL Master 700, software version 1.7, Carl Zeiss Meditec AG, Jena, Germany).
-
2. Haigis: this formula was programmed into Excel. The optimised constants from the User Group for Laser Interference Biometry (ULIB) website (www.ocusoft.de/ulib/c1.htm, accessed on April 4th, 2021) were used [13].
-
3. Hoffer Q: this formula was programmed into Excel according to the author’s recommendations and errata [14].
-
4. Holladay 1: this formula was programmed into Excel with the author’s corrections and recommendations [15].
-
5. Holladay 2: this unpublished formula was available as part of the IOL Master 700 software version 1.7. (Carl Zeiss Meditec AG, Jena, Germany). All required data, including preoperative refraction, were entered into the software. An ACD constant of 5.601 was used for the SN60WF IOL as recommended by the software (IOL Master 700, software version 1.7, Carl Zeiss Meditec AG, Jena, Germany).
-
6. Kane: this unpublished formula is based on theoretical optics and incorporates both AI and regression components [8]. It uses six variables to predict the IOL power, including mandatory AL, K, ACD (from the epithelium to the lens) and gender, while LT and CCT are optional. This formula is available at http://www.iolformula.com (accessed on April 4th, 2021) [6]. An A-constant for the SRK/T formula of 119.0 was used for calculation.
-
7. SRK/T: this formula was integrated into Excel following the recommendations from the original article and erratum [16].
-
8. T2: this formula was developed by R. Sheard, MD to improve the original SRK/T formula. It was programmed into Excel according to the original data [3].
-
9. VRF: this formula, which was developed and published by one of the authors (OVV) [4], is a vergence-based thin-lens formula using the optical CACD constant and four variables to calculate the IOL power: AL, K, ACD, and the horizontal CD. It was programmed into Excel by the author.
-
10. VRF-G is a new, unpublished formula that was also developed by one of us (OVV) and is based on theoretical optics with regression and ray-tracing components [10, 11]. It uses the optical A constant for the SRK/T formula and incorporates eight variables, including AL, K, ACD, LT, horizontal CD, CCT, preoperative refractive spherical equivalent (SE) and gender. It was programmed into Excel by the author.
For all formulas, we used the optimised A-constant of the SRK/T from the User Group for Laser Interference Biometry (ULIB) website (www.ocusoft.de/ulib/c1.htm, accessed on April 4th, 2021). We did not zero out PE separately for the short axial length group but instead applied the optimised A-constant for all axial length ranges as described by Wang et al. [17]. The Haigis formula was implemented using optimised constants from the ULIB website (www.ocusoft.de/ulib/c1.htm, accessed on April 4th, 2021). A CACD constant of 5.52 and an A-constant of 119.0 were used for the VRF and VRF-G formulas, respectively.
After the operation, all patients underwent postoperative examinations at 1 month (LT). Postoperative examinations included uncorrected and best-corrected visual acuity, tonometry, automatic kerato-refractometry, and direct or indirect ophthalmoscopy. Patients who had a postoperative corrected distance visual acuity of at least 6/9 were included in the study.
Refraction was first assessed by an automatic kerato-refractometer (RT-7000, Ver. 1.7, Tomey, Japan). Using a Jackson cross-cylinder set at ±0.25 D, we then verified the automatic refractometry data, first according to the cylinder axis, then by its power and SE. The red-green duochrome test was used at the end of the subjective refraction examination to refine the spherical refraction. The obtained value was used as the basis for postoperative manifest refraction, which was measured at 4 m and then adjusted to 6 m by adding 0.08 D, as recommended by Simpson and Charman [18].
The difference between the spherical equivalent of the obtained (PostRef) and the preoperative predicted refraction (PredRef) was calculated and analysed for each of the formulas and defined as prediction error (PE), median absolute error (MedAE), mean absolute error (MAE), SD, MIN and MAX error values.
The mean PE, MedAE and MAE were calculated and analysed. The percentages of eyes that had a prediction error within ±0.25 D, ± 0.50 D, ± 0.75 D and ±1.00 D were calculated for each formula.
Statistical analysis
All results were statistically analysed using GraphPad software (version 3.1; Instat) and MedCalc (version 12.3.0; MedCalc Software Inc.). The Kolmogorov–Smirnov test was applied to assess the normality of the distributions of the data. The results indicated that the PE data of each formula were normally distributed (P > 0.05) but not their absolute value (each P < 0.05). Homogeneity of variance was checked with the F test for each sample pair. The differences between all of the formulas were assessed using one-way analysis of variance (ANOVA) for PEs and Friedman’s ANOVA for absolute errors. In the event of a significant result, paired analysis was performed with the Dunn post-test. The nonparametric Cochran Q test with the McNemar post hoc test was used to compare the percentage of eyes with a PE within ±0.5 D. Correlation with the Spearman coefficient was calculated to assess the relationship between PE and preoperative ACD. A P value less than 0.05 was considered statistically significant.
A minimum sample size of 109 eyes was calculated using the PS programme (version 3.0.12; DuPont WD, Plummer WD Jr. 2012). This was sample size necessary to detect a difference in MedAE of 0.04 D with a power of 95% at a significance level of 5%, given a within-subject SD for simulated keratometry equal to 0.05 D.
Results
Data from 181 eyes of 181 patients were evaluated; 9 eyes of 9 patients were excluded for different reasons: high intraocular pressure (3), macular oedema (1) and low visual acuity (5). Table 1 contains the measured parameters of the eyes and demographic characteristics of the patients.
All data analyses, including refractive outcomes obtained by each formula and optimised constants, are shown in Table 2.
For the mean prediction error, no statistically significant differences were found (one-way ANOVA, P = 0.1142). In contrast, a comparison of the absolute values of predicted errors found a statistically significant difference between formulas (Friedman’s ANOVA, P = 0.0000). The Dunn post-test for paired comparisons showed statistically significant differences between absolute errors of the formulas. Table 3 shows the multiple pair comparisons of formulas with statistically significant differences (P < 0.05). Significant differences were predominantly found between the Haigis (P < 0.05), Kane (P < 0.05), and VRF-G formulas (P < 0.05) and all other formulas.
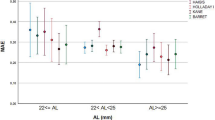
Figure 1a demonstrates the box-and-whisker plots and the distribution around the MedAE for the investigated formulas.
Distribution of the median absolute errors (a), stacked histogram comparing the percentage of cases with a given prediction error (b) and line graph (c) of prediction error (in diopters) versus anterior chamber depth (in millimeters) for SN60WF lens for BUII, Haigis, Hoffer Q, Holladay 1, Holladay 2, Kane, SRK/T, T2, VRF and VRF-G formulas.
All formulas demonstrated good outcomes, with the largest outliers occurring with the T2 and SRK/T formulas. Interestingly, some of the classical vergence formulas (Haigis) outperformed the new, last generation formulas (Barrett, Kane) and showed good accuracy and reproducibility. Thus, Haigis (MedAE 0.247 D) worked better than Kane (MedAE 0.263 D) and Barrett (MedAE 0.298 D) and outperformed all other formulas with the exception of the VRF-G (MedAE 0.242 D). Additionally, we found that the new formula that uses AI showed promising results (Kane MedAE 0.263 D).
Analysing MAE, the best results were achieved with the VRF-G (MAE 0.359 D), Kane (MAE 0.363 D), Haigis (MAE 0.372 D) and VRF (MAE 0.377 D), with the top performers remaining the same.
Most of the formulas showed similar results in terms of a prediction error within ±0.50 D and were between 66% and 71%, which is a good indicator of their accuracy. We also investigated the percentage of errors within ±0.25 D. Only five formulas achieved results of 45.0% or more: VRF-G (53.49%), Haigis (51.16%), Kane (49.42%), VRF (46.51%) and Barrett (45.93%) (Fig. 1b).
Overall, the results were good across all formulas, and 91.0% achieved errors up to 1.0 D. Statistically significant differences among formulas with PE within ±0.5 D according to the Cochrane Q test with McNemar post hoc test shown in Table 4.
Differences were found between the Haigis, Kane and VRF-G formulas and all other formulas.
The lowest SD values were achieved by the Kane (0.484 D), VRF-G (0.486 D), Holladay 2 (0.489 D), VRF (0.493 D) and Haigis (0.501 D) formulas, whereas the highest SD was achieved by the T2 (0.553 D) and SRK/T (0.584 D) formulas.
We also analysed the correlation between the individual PE obtained by each formula and the preoperative ACD (Fig. 1c).
A statistically significant correlation (p < 0.001) was found for the Barrett (r = 0.196, P < 0.000), Hoffer Q (r = 0.226, P < 0.000), Holladay 1 (r = 0.264, P < 0.000), T2 (r = 0.307, P < 0.000) and SRK/T (r = 0.345, P < 0.000) formulas. It is obvious that more accurate formulas use algorithms that take into account the preoperative ACD to avoid any correlation with the PE.
Discussion
In our investigation, the VRF-G, Haigis and Kane formulas had the lowest MedAE and MAE and may therefore be the most precise. In contrast, T2 and SRK/T had significantly larger absolute PEs and therefore should not be selected when calculating the IOL power in eyes with AL ≤ 22.00 mm.
Several studies investigated formula accuracy in eyes with an AL ≤ 22.00 mm [1, 2, 11, 12].
In the comparison by Roh et al. [19] of 4 formulas in a sample of 25 hyperopic eyes, the MAEs were 0.37 D, 0.53 D, 0.56 D and 0.62 D for the Haigis, SRK/T, SRK II and Hoffer Q, respectively. The proportion of absolute errors less than ±1.0 D was more than 80% for all formulas. The calculation with the Haigis formula showed the best results. The results from our study confirm that the Haigis (MAE 0.372 D) was one of the most accurate formulas for eyes with short axial lengths.
Eom et al. [20] compared the accuracy of the Hoffer Q and Haigis formulas according to the anterior chamber depth in cases of short axial length. There were no significant differences in the MedAE predicted by the Hoffer Q and Haigis formulas (0.40 and 0.40 D, respectively). However, the difference between the formulas increased significantly as the anterior chamber depth decreased (R² = 0.644, P < 0.001). Therefore, ACD should be considered when evaluating the accuracy of the IOL power calculation formulas in short eyes. In contrast, the analysis of the absolute prediction errors in our study revealed statistically significant differences, and the Haigis (MedAE 0.247 D) was more accurate than the Hoffer Q (MedAE 0.314 D).
Recently, Voytsekhivskyy [4] scrutinised seven formulas on a large sample (n = 823) of eyes. He did not find any statistically significant differences among the absolute errors of the 7 formulas in 53 short eyes; where he used the optimised constants, which are calculated for the whole range of ALs and not specifically for short eyes. The most accurate formula was the VRF (MedAE 0.345 D). The Hoffer Q (MedAE 0.350 D) and Holladay 2 (MedAE 0.361 D) also showed good results. The Haigis (MedAE 0.386 D), T2 (MedAE 0.418 D) and SRK/T (MedAE 0.426 D) formulas were significantly worse. Interestingly, in our study, Haigis (MedAE 0.247 D) was one of the most accurate formulas and showed statistically significant differences. This discrepancy is likely due to the much larger sample in our data set.
Hipólito-Fernandes et al. [10] compared the results of 13 formulas for a large database, where 82 short eyes were implanted with one type of lens (IQ SN60WF). Overall, in short eyes, the VRF-G (MAE 0.345 D), EVO 2.0 (MAE 0.347 D) and Kane (MAE 0.348 D) were the more accurate formulas, as they provided mean PEs closer to zero, whereas the Barrett (MAE 0.367 D), Haigis (MAE 0.397 D) and Hoffer Q (MAE 0.478) were less accurate. In our study, the VRF-G (MAE 0.359 D) and Kane (MAE 0.363 D) demonstrated similar outcomes and outperformed all other formulas, while the Barrett (MAE 0.392 D) was less predictable, with the Haigis (MAE 0.372 D) formula being more accurate. This discrepancy is likely due to the much larger sample in our data set.
Connell and Kane investigated six formulas in 846 eyes. They did not find any statistically significant differences among the absolute prediction errors of the formulas in the short AL group (n = 46). The Kane (MAE 0.441 D) and Olsen (MAE 0.442 D) formulas were the most accurate predictors of postoperative refractions. The Barrett (MAE 0.479 D) was less accurate than the Haigis (MAE 0.472 D) and Hoffer Q (MAE 0.476 D) formulas [21]. Our data are in good agreement, where the Kane (MAE 0.363 D) was one of the most accurate formulas, while the Barrett (MAE 0.392 D) was less predictable, in contrast with the Haigis (MAE 0.372 D), which was the second-most accurate formula.
Recently, Darcy et al. [22] found a statistically significant difference in the absolute error achieved by 9 formulas with a larger sample of short eyes (n = 766). The Kane formula providing the lowest MAE (0.441 D), and the Barrett Universal II achieved the highest one (0.493 D). The Haigis (MAE 0.486 D) was less predictable than the Hoffer Q (MAE 0.478 D) and Holladay 1 (MAE 0.461 D) formulas. The authors did not specifically optimise the constants for short eyes. In our study, the Kane (MAE 0.363 D) had the second lowest absolute error value and outperformed all other formulas except the VRF-G (MAE 0.359 D). Interestingly, in the previous study, the Barrett was the least predictable among all formulas.
A study by Wendelstein et al. [12] of 150 hyperopic eyes showed that the lowest MedAE was found with the Pearl-DGS (0.26 D), Castrop (0.27 D), Okulix (0.30 D) and Kane (0.30 D) formulas, while the highest values were obtained with the Hoffer Q (0.38 D) and SRK/T (0.42 D) formulas. The Barrett formula (0.33 D) was less accurate than the Haigis formula (0.32 D). Their data are in good agreement with our study, as the Kane (MedAE 0.263 D) and Haigis (MedAE 0.247 D) formulas achieved better outcomes than the Barrett (MedAE 0.298 D), Hoffer Q (MedAE 0.314 D) and SRK/T (MedAE 0.340 D) formulas.
In a recent investigation, Kane and Melles analysed the results of various formulas in axial hyperopia with a high-power intraocular lens of 30 or more dioptres [9]. The Kane (MedAE 0.371 D) and Haigis (MedAE 0.436 D) formulas were more accurate than the Barrett (MedAE 0.650 D), Hoffer Q (MedAE 0.656 D) and SRK/T (MedAE 0.561 D) formulas. Their findings are in good accordance with our study, where the Haigis (MedAE 0.247 D) and Kane (MedAE 0.263 D) formulas had lower absolute errors than the Barrett (MedAE 0.298 D) and SRK/T (MedAE 0.340 D) formulas.
Analysis of the results from our investigation and the aforementioned studies led to similar conclusions for some formulas, such as the Haigis, Kane and VRF-G, which performed better for short eyes than all other formulas. Additionally, we found that all formulas achieved a high level of accuracy, with a prediction error within ±0.50 D. The best value was obtained with the VRF-G (75.67%) and Kane (75.16%) formulas, and the worst was obtained with the SRK/T (66.28%) and T2 (67.44%); however, all formulas obtained a value of 65.00%.
The results of this study show promising outcomes. The VRF-G, Kane and Haigis formulas were the most accurate predictors of postoperative refraction in hyperopic eyes and outperformed all other methods. The Barrett Universal II formula demonstrated average performance among all methods and outperformed the existing Hoffer Q, Holladay 1 and 2, T2 and SRK/T formulas, with the Haigis formula as an exception. However, the absolute error of the Barrett formula was significantly higher than that of the Kane, VRF and VRF-G formulas. One of the best formulas, in our opinion, was the Haigis, which was the second-most accurate formula and can be recommended for calculation in hyperopic eyes. This is supported by many other authors and can thus help clinicians choose the formula for determining IOL power in eyes with short axial lengths [11, 12, 19,20,21,22,23,24,25].
A restriction of this study was the small number of eyes with an axial length of less than 21.00 mm (15). Moreover, the accuracy of the new VRF-G and Kane formulas must be further investigated and tested for other axial length ranges.
In conclusion, the findings of the present investigation support the idea that the new methods as a rule outperform the original formulas for eyes with AL ≤ 22.00 mm. Overall, the VRF-G, Kane and Haigis were the most accurate predictors in short eyes.
Summary
What was known before
-
The IOL power calculation in short eyes remains a problem.
-
Contradictory outcomes have been reported by several studies as far as which formula yields the most accurate refractive results in short eyes.
What this study adds
-
The new, previously untested formulas VRF and VRF-G have been assessed in eyes with AL ≤ 22.0 mm.
-
The newest generation formulas (Kane and VRF-G) provided the best outcomes and outperformed all classical formulas except for the Haigis.
-
The Barrett Universal II formula showed intermediate result and outperformed existing formulas with the Haigis, Kane, VRF and VRF-G as an exception.
References
Carifi G, Aiello F, Zygoura V, Kopsachilis N, Maurino V. Accuracy of the refractive prediction determined by multiple currently available intraocular lens power calculation formulas in small eyes. Am J Ophthalmol. 2015;159:577–83. https://doi.org/10.1016/j.ajo.2014.11.036
Gökce SE, Zeiter JH, Weikert MP, Koch DD, Hill W, Wang L. Intraocular lens power calculations in short eyes using 7 formulas. J Cataract Refract Surg. 2017;43:892–7. https://doi.org/10.1016/j.jcrs.2017.07.004
Sheard RM, Smith GT, Cooke DL. Improving the prediction accuracy of the SRK/T formula: the T2 formula. J Cataract Refract Surg. 2010;36:1829–34. https://doi.org/10.1016/j.jcrs.2010.05.031
Voytsekhivskyy O. Development and clinical accuracy of a new intraocular lens power formula (VRF) compared to other formulas. Am J Ophthalmol. 2018;185:56–67. https://doi.org/10.1016/j.ajo.2017.10.020
Barrett G. Barrett Universal II Formula. Singapore, Asia-Pacific Association of Cataract and Refractive Surgeons. http://calc.apacrs.org/barrett_universal2105/. Accessed 4 April 2021.
Kane J. Kane Formula. https://www.iolformula.com. Accessed 4 April 2021.
Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125:169–78. https://doi.org/10.1016/j.ophtha.2017.08.027
Melles RB, Kane JX, Olsen T, Chang WJ. Update on intraocular lens power calculation formulas. Ophthalmology. 2019;126:1334–5. https://doi.org/10.1016/j.ophtha.2019.04.011
Kane JX, Melles RB. Intraocular lens formula comparison in axial hyperopia with a high-power intraocular lens of 30 or more diopters. J Cataract Refract Surg. 2020;46:1236–9. https://doi.org/10.1097/j.jcrs.0000000000000235
Hipólito-Fernandes D, Luís ME, Gil P, Maduro V, Feijão J, Yeo TK, et al. VRF-G, a new intraocular lens power calculation formula: a 13-formulas comparison study. Clin Ophthalmol. 2020;14:4395–402. https://doi.org/10.2147/OPTH.S290125
Voytsekhivskyy O, Hoffer KJ, Savini G, Tutchenko L, Hipólito-Fernandes D. Clinical accuracy of 18 IOL power formulas in 241 short eyes. Curr Eye Res. 2021; May 20. https://doi.org/10.1080/02713683.2021.1933056. Online ahead of print.
Wendelstein J, Hoffmann P, Hirnschall N, Fischinger IR, Mariacher S, Wingert T, et al. Project hyperopic power prediction: accuracy of 13 concepts for intraocular lens calculation in short eyes. Br J Ophthalmol. 2021;0:1–7. https://doi.org/10.1136/bjophthalmol-2020-318272
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for IOL calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–73. https://doi.org/10.1007/s004170000188
Hoffer KJ. The Hoffer Q formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19:700–12. https://doi.org/10.1016/s0886-3350(13)80338-0. errata 1994; 20:677. 10.1016/s0886-3350(13)80338-0,
Holladay JT, Prager TC, Chandler TY, Musgrove KH, Lewis JW, Ruiz RS. A three-part system for refining intraocular lens power calculations. J Cataract Refract Surg. 1988;14:17–24. https://doi.org/10.1016/s0886-3350(88)80059-2
Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16:333–40. https://doi.org/10.1016/s0886-3350(13)80705-5. erratum: 528
Wang L, Koch DD, Hill W, Abulafia A. Pursuing perfection in intraocular lens calculations: III. Criteria for analyzing outcomes. J Cataract Refract Surg. 2017;43:999–1002. https://doi.org/10.1016/j.jcrs.2017.08.003
Simpson MJ, Charman WN. The effect of testing distance on intraocular lens power calculation. J Refract Surg. 2014;30:726. https://doi.org/10.3928/1081597X-20141021-01
Roh YR, Lee SM, Han YK, Kim MK, Wee WR, Lee JH. Intraocular lens power calculation using IOLMaster and various formulas in short eyes. Korean J Ophthalmol. 2011;25:151–5. https://doi.org/10.3341/kjo.2011.25.3.151
Eom Y, Kang SY, Song JS, Kim YY, Kim HM. Comparison of Hoffer Q and Haigis formulae for intraocular lens power calculation according to the anterior chamber depth in short eyes. Am J Ophthalmol. 2014;157:818–824. https://doi.org/10.1016/j.ajo.2013.12.017. e2
Connell BJ, Kane JX. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4:e000251. https://doi.org/10.1136/bmjophth-2018-000251
Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane JX. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK National Health Service. J Cataract Refract Surg. 2020;46:2–7. https://doi.org/10.1016/j.jcrs.2019.08.014
Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42:1157–64. https://doi.org/10.1016/j.jcrs.2016.06.029
Cooke DL, Cooke TL. Prediction accuracy of preinstalled formulas on 2 optical biometers. J Cataract Refract Surg. 2016;42:358–62. https://doi.org/10.1016/j.jcrs.2015.11.040
Zuberbuhler B, Morell AJ.Errata in printed Hoffer Q formula [letter].Cataract Refract Surg.2007;33(1):2–3. https://doi.org/10.1016/j.jcrs.2006.08.054. reply by KJ Hoffer.
Acknowledgements
Larysa Tutchenko, MD, PhD for performing all phacoemulsification operations.
Author information
Authors and Affiliations
Contributions
OVV was responsible for designing the study protocol, writing the protocol and report, conducting the calculation and statistical analysis, collecting and analysing data, interpreting results, updating reference lists and creating VRF and VRF-G formulas. LT was responsible for performing all operation, designing the review protocol and screening potentially eligible studies. She contributed to writing the draft of the article and analysing data. DHF was responsible for collecting and analysing data, writing the protocol and report and interpreting results, updating reference lists.
Corresponding author
Ethics declarations
Competing interests
Voytsekhivskyy is the inventor and sole owner of the VRF and VRF-G formulas and has a patent on the method of estimation of postoperative lens position (ELP) and the calculation of optical power and is the author and copyright holder of a computer programme ViOL Commander. Tutchenko declares no potential conflict of interest. Hipólito-Fernandes declares no potential conflict of interest. We have disclosed those interests fully to the journal, and have in place an approved plan for managing any potential conflicts arising from this arrangement.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Voytsekhivskyy, O.V., Tutchenko, L. & Hipólito-Fernandes, D. Comparison of the Barrett Universal II, Kane and VRF-G formulas with existing intraocular lens calculation formulas in eyes with short axial lengths. Eye 37, 120–126 (2023). https://doi.org/10.1038/s41433-021-01890-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01890-7