Abstract
Primary idiopathic epiretinal membrane (iERM) is a common finding, particularly so in the era of high street spectral-domain optical coherence tomography. Clinicians often face the dilemma of early versus delayed surgical intervention in the management of iERM with macular pucker, especially in those patients with good vision. The aim of this review is to assist clinicians in their understanding of the natural history of iERM to enable decision-making and optimally advising patients. We systematically searched the Medline and EMBASE databases for relevant publications from 2001 onwards using defined search terms with pre-planned inclusion and exclusion criteria. In this article, we review the epidemiology of iERM, classifications, their effect on visual function, the natural history and factors predicting progression and finally, factors which might predict the visual outcome with surgery.
Similar content being viewed by others
Introduction
Pre-macular membranes, although occurring in association with a number of retinal diseases, also occur in isolation, thought to result from pathological alterations in vitreoretinal separation [1, 2]. These primary idiopathic macular epiretinal membranes (iERM), as they are most widely known, are a common finding, with a reported prevalence of up to 34% in the over 60-year-old population [3]. In their more advanced form, they are a common indication for vitreoretinal surgery as a result of their tractional effects on retinal structure (collectively known as ‘macular pucker’), accounting for ~10% of vitrectomies in a large UK audit in 2013 [4]. They are also an important cause of visual impairment, with vitreoretinal interface disorders overall being responsible for 3–4% of monocular and binocular visual impairment in a UK biobank study [5].
Spectral-domain optical coherence tomography (SDOCT) has enabled the detection of iERM at an early stage, and with the increasing use of OCT routinely in primary care, the number of people detected and referred for a vitreoretinal opinion with the asymptomatic or minimally symptomatic disease is rising steeply.
Traditionally, the decision to operate has been based on the severity of symptoms and effect on visual function as compared with the known surgical morbidity, rather than OCT appearance. Indeed, there is a well-known mismatch between OCT appearance and visual function with some cases of extensive iERM having an excellent vision. A further consideration is that iERM is often uniocular and symptoms can be masked by good function in the fellow eye, depending on ocular dominance and an individual’s visual requirements.
Several changes in vitreoretinal practice have questioned the traditional practice of surgery only in more advanced cases with reduced vision, and earlier surgery has been advocated. It is known that visual outcomes following surgery are related to preoperative vision, and hence early surgery may offer some advantages. Furthermore, surgical morbidity has declined, and cataract surgery results have improved, making the adverse event of cataract formation less of a concern. The decision to operate may also be considered on the basis that visual function will deteriorate if left untreated, which is a key consideration to an early surgical plan. Currently, there are no end-points that can truly predict which patients would benefit from operation.
There are several questions relevant to this dilemma which we will address in this review. These include the epidemiology of iERM, their effect on visual function, the natural history and factors predicting progression and finally, factors that might predict the visual outcome with surgery. We have restricted the review to primary iERM, being the most prevalent form, and also related to the case that ERM associated with other retinal diseases will vary widely in their clinical course and features by the cause. We do not discuss the aetiology and pathogenesis in detail which have been reviewed elsewhere [6].
Methodology and search strategy
We conducted a search of the Medline and EMBASE for all publications in English language from 2001 to 2019 using the search term: (“epiretinal membrane*“ OR “macular pucker*“ OR “vitreomacular traction*“ OR “pre-retinal fibrosis” OR “pre-retinal fibrosis” OR“pre-retinal fibrosis” OR “cellophane maculopath*“ OR“ERM”)ti,ab AND (“optical coherence tomography” OR “OCT” OR “TOMOGRAPHY, OPTICAL COHERENCE”) AND (history OR aetiology OR aetiology OR risk* OR progress*).af. The exclusion criteria were: diabetic maculopathy, diabetic retinopathy, retinal vein occlusion, retinal tear, lamellar hole, macular hole, uveitis and congenital ERMs including those associated with combined hamartomas of retina and RPE. The final search yielded 398 results. The authors (PYC & TS) reviewed all the abstracts and the relevant articles. The search was also supplemented by manual search primarily using additional references from key articles.
Epidemiology and classification
iERM is one of the most common macular diseases. The prevalence varies from 2% to 34% depending on age, ethnicity, and a range of other factors but also on the sensitivity of the methodology used for detection [3, 7,8,9,10,11,12,13,14,15,16,17,18]. In studies that have used both digital colour photography and SDOCT, the prevalence has generally been higher than studies using only film-based photography and/or time-domain OCT. For example, the Beaver Dam Eye Study used digital colour photography and SDOCT in their population study of individuals aged between 63–102 years old and reported a 34% prevalence of iERM [3]. In contrast, the Blue mountain study (film-based photography) reported a prevalence of iERM of 7.2% and 11.6% for the age groups of 60–69 years old and 70–79 years old, respectively [7].
Population studies have typically classified iERM into those associated with a cellophane macular reflex (CMR) only, and those with visible pre-macular fibrosis (PMF), with distorted retinal anatomy. A systematic review published in 2017 estimated an overall prevalence of iERM of 9.2% (95% confidence interval (CI) 4.7–13.8%) with 7.1% (95% CI 3.3–10.8%) for CMR and 2.0% (95% CI 1.3–2.8%) PMF [19]. The two entities are manifestations of the same disease process.
iERM is thought to relate to pathological vitreoretinal separation that can either be partial, with vitreomacular traction or complete, with no visible vitreous adhesion on SDOCT but with vitreoschisis and cortical vitreous remnants adherent to the ILM after apparent posterior vitreous detachment [2, 20]. These residual vitreous remnants, when associated with a hyper-cellular pre-macular membrane containing several cells types including myofibroblasts, contract either centripetally or centrifugally, which can result in pseudohole or ERM foveoschisis formation, respectively. Centripedal contraction typically, however, results in a centrally thickened retina.
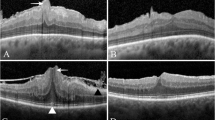
Govetto et al. [21] recently published an SDOCT-based ERM staging (stage 1–4) of these forms with progressive loss of the foveal pit, a continuous ectopic inner foveal layer (EIFL) over the fovea and then disruption of the inner retinal layers as the contraction progresses. In some cases these changes are associated with tractional consequences on the outer retina starting as a cotton ball sign in the central fovea, progressing to foveolar detachment and then the acquisition of a vitelliform type lesion with poorer VA [22]. The cotton wool ball sign-on SDOCT is the appearance of a fuzzy hyper-reflective area between the inter-digitation zone and ellipsoid zone. This is caused by centripetal traction that displaces the central cones in the fovea with loss of the normal photoreceptor alignment. More progressive traction leads to the separation of the central bouquet from the underlying RPE, with subsequent accumulation of central subretinal fluid. Chronic separation of the cone photoreceptors from the RPE disrupts the normal physiological relationship of the RPE-photoreceptor complex, which can result in progressive accumulation of metabolic debris in the subretinal space, and the vitelliform appearance described above [23]. Figure 1 shows different classifications of iERM on OCT.
Pictures a–d show classification system as proposed by Govetto et al.; a Stage 1- presence of foveal pit and well-defined retinal layer; b Stage 2- absence of foveal pit with well-defined retinal layers; c Stage 3- presence of EIFL, absence of foveal pit with well-defined retinal layer; d Stage 4 presence of EIFL with no foveal pit and disrupted retinal layer; e ERM schisis; f Pseudohole with ERM. Pictures g–i show tractional abnormalities of the central foveal bouquet in ERM; g ‘Cotton ball sign’ with small, fuzzy hyper-reflective area observed between the ellipsoid zone; h Foveolar detachment with a central hyporeflective pocket of subretinal fluid under the inter-digitation zone; i Acquired vitelliform lesion with a thick dome-shaped hyper-reflective acquired vitelliform lesion between the retinal pigment epithelium and the outer retina; j VMT and ERM; k lamellar macular hole with epiretinal proliferation. EIFL ectopic inner fovea layer, VMT vitreomacular traction.
Classical iERM is seen as a linear hyper-reflective line anterior to the inner retinal surface on SDOCT often associated with retinal plication. The attachment of the iERM is further divided into global attachment (GA) and partial attachment (PA) [24, 25]. Bryon et al. [22] speculated that glial cells begin to proliferate evenly along the retinal surface and as the membrane contracts tangentially, the iERM configuration changes from the GA to PA type. More recently a layer of thick, homogeneous and iso-reflective epiretinal material over the ILM without tractional effects and referred to as epiretinal proliferation has been recognised, most commonly in association with lamellar macular holes of the degenerative type, and not covered further in this review [26].
All studies have found an increasing prevalence of iERM with age, with an odds ratio of 1.19 per year increase (95% CI 1.13–1.26) [27]. The Beaver Dam Eye Study found a prevalence of any ERM from 28% in those aged 63 to 74 years to 53.2% in those aged 85 years or more [3]. This may be partly related to the higher incidence of posterior vitreous detachment, which was recorded as 70% in those with iERM [7]. Compared with males, females have a slightly higher risk of ERM although this has not been found by all studies [27]. There is little evidence for ethnicity affecting the prevalence [19].
Cataract surgery may be a risk factor for later ERM formation relating to the vitreous changes induced by surgery, although many studies have been confounded by the fact that imaging improves following cataract extraction leading to increased detection [7, 10, 28]. Cataract surgery in iERM patients is associated with an increased rate of pseudophakic cystoid macular oedema. In a large UK clinical database study, Hardin et al. [29] showed 8.6% of the iERM patients had pseudophakic cystoid macular oedema after cataract surgery compared with 1.38% of those who do not have iERM. Similarly, a case-control study reported that pre-existing iERM was associated with a higher risk of developing pseudophakic cystoid macular oedema (OR 3.89 [95% CI 1.16–17.76]) [30]. However, there is no apparent increase in the ERM or associated contraction in the short term [31, 32]. Similarly, it is uncertain if myopia is a risk factor or not [19, 27]. Diabetic retinopathy and possibly diabetes alone increases the risk of ERM, probably related to the vitreous changes induced by hyperglycaemia [9, 13, 16]. Primary open-angle glaucoma may be associated with a higher prevalence of iERM [33].
Effects on visual function and outcomes with surgery
The effects of iERM on visual function have been widely studied. These include reduced distance visual acuity (VA), contrast sensitivity, reading speed and binocular function as well as induced metamorphopsia and anisekonia. Objective and subjective clinical measurements are often complemented with an assessment of patients’ perception of their visual function and quality of life (QOL) and many studies have used the National Eye Institute 25-item Visual Function Questionnaire (VFQ-25) to assess patients’ perception of visual function and QOL.
Reduced VA is one of the main presenting symptoms from iERM and has historically been widely used as an indication and threshold for surgery with a level of 20/40 to 20/60 being commonly quoted [34]. A number of proposed mechanisms for the decrease in VA caused by an ERM include the light-filtering and scattering effect of the membrane, distortion of photoreceptor outer segments, mechanical strain and deformation effects, intraretinal oedema, photoreceptor outer segments and RPE separation caused by traction, and obstruction of axoplasmic flow.
VA is not correlated with the degree or presence of metamorphopsia or aniseikonia, either vertical or horizontal [35]. It is not infrequent to see patients with good VA, but visually disabled with aniseikonia or metamorphopsia. Studies with SDOCT have shown that aniseikonia, typically macropsia is caused by a change in the distribution of photoreceptors, following retinal movement caused by traction of the membrane. On the other hand, metamorphopsia is related to tractional changes in the inner retinal layers. Hence the direction and magnitudes do not necessarily coincide [35]. Metamorphopsia is typically improved by peeling surgery, while aniseikonia is often not. In fact, macropsia can progress despite successful surgery [36, 37].
In a large UK audit of surgery, the median preoperative VA was logMAR 0.60 (Snellen 20/80) and this improved to logMAR 0.30 (Snellen 20/40) after surgery [38]. The improvement of VFQ-25 composite score may be correlated with the VA gain after surgery [39]. However, this depends on the level of VA before surgery [40]. Indeed, it is worth noting that whilst surgery usually leads to improvement in VA in the operated eye when VA is poor, there is a ‘ceiling effect’ that precludes substantial improvement in eyes with good VA. In one study, the changes in the VFQ-25 composite score significantly correlated with changes in the severity of metamorphopsia but not with changes in VA and contrast sensitivity [40]. Bouwens and Meurs evaluated changes of metamorphopsia, assessed with sine Amsler charts in 63 patients who underwent surgery in a prospective study [41]. Three months postoperatively, 82% of patients reported improvement of metamorphopsia and 66% showed improvement in VA. However, improvement in VA was not associated with the severity of preoperative metamorphopsia. Another prospective study comparing patients with good VA (logMAR 0.046 or better) (Snellen 20/20 or better)compared with moderate VA (logMAR 0.10–0.52) (Snellen 20/25 to 20/63), showed that early surgery was more effective for improvement in horizontal metamorphopsia, visual acuity, QOL and prevented the progressive worsening of aniseikonia seen in the moderate VA group [42]. Similarly, Krarup et al. [43] reported that the total M-chart score (vertical and horizontal) was the best end-point to predict patient-reported outcomes of ERM surgery. VA of the operated eye, binocular VA, stereoacuity, aniseikonia tolerance range or total aniseikonia were not correlated with patient-reported outcomes [43].
Other visual functions that have been reported to improve following iERM surgery include stereopsis [42], contrast sensitivity [40] and reading ability [44]. It is important to note that these can improve without a concomitant improvement in VA.
With increasing evidence to suggest that VA is not the only factor associated with impaired visual function in ERM, it is clear that surgeons should take the other factors described above into consideration while counselling patients.
The natural history of ERM and factors predicting progression
A common dilemma in people with no or minimal symptoms, particularly in the presence of an overtly advanced ERM, is the risk of progression. Related to the mismatch in anatomy and function described above, we shall consider both progression in the morphological features of the ERM and retina, as well as changes in visual function. We also consider changes that have predicted progression to surgery, as these interestingly have not necessarily coincided with the objective changes mentioned above.
Anatomical characteristics and progression
Prior to the advent of OCT, the population-based Blue Mountains Eye Study showed that iERM progressed in 29% of cases, stable in 39% and regressed in 26% during 5-year follow-up using colour fundus photographs [28]. Similarly, another prospective cohort study of 207 patients who had iERM on fundus photographs after cataract surgery showed 43% progressed, 32% remained stable and 24% regressed [45].
A number of factors could be considered with regard to anatomical progression namely the extent and characteristics of the ERM itself, its effect on the retina both at an inner and outer retinal level and its effect on retinal thickness and morphology.
Byon et al. [22] reported a retrospective case series of 62 eyes with a good VA of 20/40 or better with a minimum of 24-month follow-up. They categorised ERM cases into GA versus PA, and those with and without vitreomacular traction. Overall, ERM configuration changes were observed in 24 eyes (39%), including those that progressed and resolved spontaneously. In all, 11 out of 33 eyes (33%) showed progression from baseline GA type to PA type within 24 months. Four ERMs (6%) resolved spontaneously from PA type (n = 3) and VMT (n = 1), resulting in visual improvement. Out of the 62 eyes, 4 eyes with the intact ellipsoid zone (and PA type attachment) progressed to an attenuated or disrupted one, with concurrent loss of VA > 2 lines. In a retrospective case–control study by Lee et al. of patients with iERM and VA ≥ 20/40, 15 out of 112 (13%) patients progressed with a loss ≥2 lines during a mean follow-up of 31 months. Of the patients who progressed, ERM configurational changes from a GA to PA type occurred more frequently than in the control group (without visual loss) [46]. These results suggested that iERM probably starts as GA type then gradually progresses to PA type, which is more unstable.
Vitreoretinal attachment appears to affect iERM progression. In a study by Byon et al. [22], 4 out of 10 eyes (40%) with vitreoretinal attachment at presentation showed progression and visual loss, compared with only 2 of 52 eyes with PVD (3.8%). The authors postulated that this may have been secondary to increased pro-inflammatory factors in eyes with vitreomacular or vitreopapillary adhesion.
To quantify changes in tangential traction with iERM, Lee et al. [46] measured changes in the disc-fovea-vascular (DFV) distance, central macular thickness (CMT), membrane configuration and ellipsoid zone in 45 eyes with the vision of 20/40 or better over a minimum of 24 months. Visual loss of >2 lines was observed in 15 eyes, and was significantly correlated with changes in DFV distance but not baseline DFV distance, baseline CMT, follow-up CMT and change in CMT, suggesting that tangential contractile forces influence visual loss in patients with iERM.
iERM progression can affect the integrity of inner and outer retinal layers over time and contribute to visual loss. Byon et al. [22] notice that only 4 out of 62 eyes (6%) showed decreased ellipsoid zone signal over 24 months. However, this was not observed in any of the 45 cases described by Lee at. [46]. In the case series of 143 eyes by Govetto et al. [23], 41 eyes had central foveal abnormalities, and only six of these eyes showed progression during their mean follow-up of 21 months. As far as inner retina is concerned, Govetto and associates described a continuous EIFL seen in SDOCT in ERM. Patients with such changes had poorer VA. In their case series, 10 out of 77 eyes with stage 2 iERM and without an EIFL, developed one during follow-up [21].
CMT has been widely measured in iERM studies and correlated with worsening vision [22, 47]. Although CMT has been correlated with VA at baseline, it has not been shown to be useful in predicting visual outcome over time. Li et al. [47] postulated that measuring the total area of retina thickened secondary to iERM may allow a more comprehensive evaluation of disease severity and risk of progression and showed that with every 1 mm2 of retinal thickening at baseline, the odds of having worsened vision at the last follow-up were increased.
In summary, iERM progress anatomically in 17–39% of cases over a time period of 24 months [21, 22, 46]. The anatomical factors that predict progression are no PVD at baseline, GA at baseline, early stage of iERM according to the Govetto classification and decrease of DFV distance during follow-up. In cases that do progress, the iERM typically progresses from being globally attached to only partially attached as contracture occurs. During progression, iERM usually contracts centripetally and causes the retina to thicken and the foveal disc and inter-arcade distances to reduce. The fovea pit gradually disappears, and as tissue migrates ectopic inner retina layers appear at the foveal centre. At the same time, tractional force on the outer retina results in disruption on the ellipsoid zone whilst some patients may develop a ‘cotton ball sign’ then progress to foveolar detachment and/or an acquired vitelliform lesion. The time scale for progression is likely to be slow over several years in cases that do progress.
Functional progression
A complete understanding of the functional consequences of iERM progression is unclear as most of the case series reported have been retrospectively reviewed, with only visual acuity recorded, which is known to be relatively poorly correlated with patient-reported outcomes [43]. It is also important to remember that in clinical practice, symptoms can be masked by ocular dominance.
Byron et al. [22] reported that overall visual acuity remained stable despite 1/3 of their iERM configurations having anatomically changed over 24 months. Over this period, 10% of patients had reduced vision and 7% had improved vision owing to spontaneous iERM resolution.
Visual acuity declined very slowly at a mean rate of 0.012 ± 0.003 logMAR per year from 20/30 at baseline to 20/36 at 7 years in a retrospective case series of 145 eyes of 118 patients with a visual acuity of 20/40 or better without intolerable symptom [48]. The authors also noted that the majority of phakic eyes had lower VA than pseudophakic ones at baseline, and showed greater deterioration over time, mainly related to cataract progression as CMT in both groups was not significantly different at baseline and during follow-up. The factors that were associated with rapid visual decline were the presence of metamorphopsia, lamellar hole formation and inner nuclear layer cysts on SDOCT at presentation [48].
In summary, VA typically deteriorates only slowly in those who are relatively asymptomatic and have a good VA of 20/40 or better at presentation. The factors that are associated with a more rapid visual decline include the presence of metamorphopsia at baseline and inner retinal layer cystic changes. We could not find any reports on the progression of metamorphopsia or aniseikonia in patients without surgery.
Conversion to surgery
Although the progression of symptoms or objective signs is obvious reasons to operate after a period of observation, there is evidence that this is not always the case. Baseline features and persistence of symptoms are also important, as well as potentially surgeon bias to operate on pathology which they consider to be more amenable to surgical improvement or more likely to worsen.
Luu et al. [48] retrospectively reviewed 145 eyes with good visual acuity (20/40 or better) with a median follow-up of 3.7 years. They reported that cumulative rates of progression to surgery were 2.9% at year 1, 5.6% at year 2, 12% at year 3 and 21% at year 4 based on Kaplan–Meier estimates. Patients who were symptomatic with metamorphopsia at baseline were more likely to have surgery during follow-up, irrespective of progression functionally or anatomically. The OCT predictors for surgical intervention include higher baseline CMT, presence of external limiting membrane disruption and ellipsoid zone disruption at baseline. Phakia was not associated with progression to surgery, in fact, pseudophakic had a higher surgical rate.
Kofod et al. [49] carried out a randomised clinical trial of 53 patients with symptomatic ERM and a best-corrected visual acuity at the presentation of 20/50 or better, randomised to either immediate surgery or observation. Eight of the 33 (24%) patients in their observation arm converted to surgery within 12 months, either because of the significant deterioration of visual symptoms or a VA reduction of two Snellen lines or more. They did not identify any baseline factor associated with conversion to surgery.
Chen et al. [34] retrospectively reviewed 210 eyes with visual acuity of 20/40 or better. The Kaplan–Meier survival curves show that 13% of ERMs with good vision progressed to surgery at 7 years. In addition, there appears to be a point in the curve at 4 years where eyes that had not progressed by this point, remain stable without surgery to 7 years. When categorised by baseline OCT morphology, only 5% of eyes with normal foveal contour progressed to surgery by 5.5 years, whereas 17% of eyes with incomplete and 16% of eyes with complete loss of foveal contour progressed to surgery at 6 and 7 years, respectively. Although the final rate of progression is similar between the latter two groups, eyes with complete loss of foveal contour appear to have a more rapid initial rate of progression that eventually converged with the incomplete loss of the foveal contour group.
Chen and associates also reported that a greater number of initially symptomatic eyes (15%) progressed to surgery compared with asymptomatic eyes (9%) at 7 years. However, this trend was not statistically significant (p = 0.38).
In summary, the preoperative factors that have been found to be associated with conversion to surgery in those with initial good visual acuity are higher baseline CMT, presence of external limiting membrane or ellipsoid zone disruption, loss of foveal contour and those with metamorphopsia or were otherwise visually symptomatic at presentation. Overall, 10–30% of patients that present to surgeons and are initially observed, progress to surgery within a 2–7-year period. Table 1 summarises the reported studies of the natural history of iERM progression.
Factors that predict the visual outcomes with surgery
Predictive factors for postoperative VA after ERM peeling surgery have been widely studied and recently reviewed [50, 51]. Most studies have used VA as the sole measure of visual outcome, which as discussed may not represent the true functional impact of surgery. For example, metamorphosia may improve without a corresponding increase in VA.
Person variables that have been identified as poor prognostic factors for postoperative VA are poor visual acuity at baseline [52,53,54], age >75 years old [54, 55] and longer duration of symptoms (>6 or 12 months) [54, 55]. However, patients with poorer VA preoperatively can expect a greater gain in VA postoperatively [52].
Baseline preoperative OCT features that have been suggested to predict poor postoperative VA outcomes are disruption of the ellipsoid [52], and cone outer segment tip lines [56, 57], increased parafoveal inner nuclear thickness [58], reduced photoreceptor outer segment length [53], drusen [59], presence of EIFL [60], high inner retinal irregularity index (length of the inferior border of inner plexiform layer/ length of RPE layer) [61] and conflicting evidence for preoperative central foveal thickness (CFT) [56, 62,63,64,65,66]. CFT is more highly correlated with preoperative VA than postoperative VA [51].
As mentioned already a recent OCT-based staging scheme classifies ERM into four stages with distinct morphologic characteristics. The keystone of the proposed scheme is the presence of an EIFL (stage 3) [67]. EIFL persisted in 91% of cases after surgery, and although it decreased in thickness significantly after surgery, the postoperative VA remained lower compared with cases without it at baseline [60]. In another retrospective case series, 92% of iERM patients achieved a VA of 20/40 or better after surgery, if they did not have EIFL preoperatively [68].
Intraretinal fluid spaces have been noted to be associated with disruption of ellipsoid zone preoperatively, hence associated with poorer VA postoperatively [69]. Intraretinal fluid in iERM can be present in the inner and/or outer retina. In a retrospective case series by Shiode et al. [70], 6 months after iERM surgery, intraretinal fluid had completely disappeared in 11% of their inner only group, 83% of their outer only group (p = 0.01) and 33% of a combined group. In addition, the postoperative VA significantly improved in the inner (p = 0.02) and outer group (p = 0.03) but not the combined group (p = 0.58) [70]. It should also be noted that intraretinal fluid in the inner retina is more common in eyes with associated glaucoma and less commonly resolves after surgery than in non-glaucomatous eyes [71].
Fundus autofluorescence can complement the structural information obtained from OCT. Brito et al. [72] in a prospective case series of 26 patients showed that eyes with an enlarged hypo-autofluorescent area encompassing the foveal and parafoveal area, with disruption of ellipsoid zone had lower VA 6 months after surgery. Interestingly, after iERM surgery, the integrity of ellipsoid zone recovered in those with an intact fovea autofluorescent pattern.
Several case series have also investigated factors predicting postoperative metamorphosia and aniseikonia. Significant predictors of postoperative metamorphopsia have been preoperative metamorphopsia [58, 73], increased CFT [73], ellipsoid zone disruption [73] and increased INL thickness [58, 74]. The predictors for postoperative aniseikonia have been preoperative aniseikonia [37, 75], increased baseline INL thickness [37], poorer baseline VA [76], longer duration of symptoms [76] and preoperative horizontal metamorphopsia [75]. Table 2 summarises the features identified as being associated with negative visual outcomes.
Based on all the known prognostic factors in the literature, Kaufmann et al. [54] introduced a 10-points predictive score to estimate the chance of 20/20 visual outcomes after combined cataract surgery and ERM removal. It included four parameters (age, duration of symptoms, initial VA and ellipsoid zone disruption) with a weighted number of points assigned. Patients with age <75 score 2; duration of symptoms of <12 months score 2; VA of ≥20/100 and <20/33 score 2; VA of ≥20/33 score 5 and continuous ellipsoid zone score 1. Patients with a score >5 had a >56% chance of reaching a VA of 20/20 postoperatively.
Does observation, with surgery only carried out after progression, affect outcome?
The visual outcomes for delayed epiretinal membrane surgery in patients who are symptomatic but with relatively good vision have not been widely reported. Kofod et al. [49] randomised 53 eyes with good presenting visual acuity (20/50 or better) and mild symptoms to early or delayed surgery. After 12 months, the mean VA was not significantly different between the groups, although 24% of patients randomised to the watchful waiting group had crossed over to surgery. In a retrospective case series of 94 patients with a VA of 20/30 or better by Pareja et al. [77] with a minimum of 2-years follow-up, almost 30% of the patients underwent vitrectomy because of increasing symptoms. They also showed that there was no difference in mean VA improvement if patients had surgery within 6 months, compared with >6 months after diagnosis (p = 0.10).
Peeling of the internal limiting membrane during surgery
iERM typically adheres strongly with ILM, and surgical specimens of apparently isolated iERM removed during vitreous surgery reveal varying degrees of interspersed ILM fragments [78]. Park et al. [79] conducted an initial non-comparative case series of eyes that underwent ERM peeling with or without additional ILM peeling. They concluded that ILM peeling did not cause deleterious effects and benefited from reduced recurrence rate. Since then, multiple studies including randomised controlled trials and meta-analyses have shown that ILM peeling does not confer any additional benefit to the final visual acuity in iERM cases [80,81,82,83]. Aside from visual acuity, Tranos et al. [80] in a randomised controlled trial showed no improvement in metamorphopsia with additional ILM peeling. The only clear and consistent benefit shown by meta-analyses has been a reduction in ERM recurrence. This is related to the remaining ILM acting as a scaffold for recurrent ERM growth and the fact that 80% of ILM peeled after apparent complete ERM peeling contains ERM remnants histologically [84]. However, recurrent iERM may not be clinically significant, in a case series of 104 patients, 16% had recurrence after surgery but only 5.6% required reoperation [85].
Peeling of ILM removes variable quantities of Muller cell fragments, so it is reasonable to expect some Muller cell dysfunction after ILM peeling [81]. Indeed peeling of ILM has been shown to induce a characteristic late postoperative appearance called a dissociated optic nerve fibre layer appearance, sometimes with more acute swelling of arcuate retinal nerve fibre layer [86,87,88]. Functionally, one study has shown a decreased retinal sensitivity and formation of microscotomas with additional ILM peeling in iERM surgery, compared with iERM peeling only [89].
In summary, the current evidence suggested that peeling of ILM during iERM surgery does not affect the final visual acuity and metamorphosia. However, the recurrent rate is lower with ILM peeling. In many cases, ILM peeling occurs concurrently with ERM peeling either pre-planned with ‘en bloc’ peeling or spontaneously, and the question of whether to then additionally peel the ILM is not a consideration. In cases, however, where the ERM separates easily, leaving an apparently clean ILM surface, surgeons should consider the risk of retinal trauma by additionally peeling the ILM, against the risk of recurrent ERM, particularly in iERM cases where the recurrence rate is low.
Risks of surgery
Any decision to operate on iERM has to be made with knowledge of the surgical risk. The intraoperative complication rate in a large case series of 1131 cases treated in the United Kingdom, with predominantly narrow-gauge surgery, was recorded as 9.8% [38]. The three most common complications were retinal tears (4.9%), iatrogenic retina trauma (1%) and lens touch (1%). Postoperatively, excluding cataract surgery, 3% had repeat ERM surgery at a median of 5.5 months after primary surgery and 1% had retinal detachment surgery at a median of 3.2 months. These data are consistent with another narrow-gauge case series by Rizzo et al. [90], where they reported 1.2% with retinal detachment. As far as post-vitrectomy cataract is concerned, the 1, 2 and 3-year cataract surgery rates were 52%, 73% and 76%, respectively. Patients with worse postoperative BCVA following surgery have ranged from 10% to 15% [38, 91]. It is unclear how much of this was due to cataract, intraoperative and/or postoperative complications. The most severe postoperative complication endopthalmitis, is rare and recorded as 0.14–0.84% in several large cases series [92,93,94].
Conclusion
People with iERM presenting to retinal specialists can display a number of anatomical features, with very variable symptom severity. It is important to note that the anatomical changes at baseline may not correlate with symptoms, and similarly, these do not necessarily progress concomitantly. If symptoms are mild and observation is preferred, overall, ~15–40% progress slowly over a period of years with increasing or persisting retinal changes and symptoms, resulting in the decision to operate. Conversely, up to 5–7% of cases can improve with a spontaneous partial or complete avulsion of the ERM from the retinal surface. Visual acuity alone can incompletely represent a patient’s visual disabilities due to the condition and other measures of visual function should be considered. In particular, metamorphopsia and anisekonia can be prominent symptoms, but their progression over time is understudied. Metamorphopisa is more often improved by surgery than anisekonia. Prediction of visual deterioration with observation is uncertain. However, based on the literature, those with good vision (≥20/40) and asymptomatic are less likely to progress rapidly and require surgery. On the other hand, those who are symptomatic with inner and outer retinal changes are more likely to progress and require surgery. Observation, with surgery carried out if progression occurs, is a reasonable strategy with a low risk of a worsened prognosis.
References
Sebag J. Anomalous posterior vitreous detachment: a unifying concept in vitreo-retinal disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:690–8.
Gupta P, Yee KM, Garcia P, Rosen RB, Parikh J, Hageman GS, et al. Vitreoschisis in macular diseases. Br J Ophthalmol. 2011;95:376–80.
Meuer SM, Myers CE, Klein BE, Swift MK, Huang Y, Gangaputra S, et al. The epidemiology of vitreoretinal interface abnormalities as detected by spectral-domain optical coherence tomography: the beaver dam eye study. Ophthalmology. 2015;122:787–95.
Jackson TL, Donachie PHJ, Sparrow JM, Johnston RL. United Kingdom national ophthalmology database study of vitreoretinal surgery: report 1; case mix, complications, and cataract. Eye (Lond, Engl). 2013;27:644–51.
McKibbin M, Farragher TM, Shickle D. Monocular and binocular visual impairment in the UK Biobank study: prevalence, associations and diagnoses. BMJ open Ophthalmol. 2018;3:e000076–e000076.
Bu SC, Kuijer R, Li XR, Hooymans JM, Los LI. Idiopathic epiretinal membrane. Retina. 2014;34:2317–35.
Mitchell P, Smith W, Chey T, Wang JJ, Chang A. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–40.
You Q, Xu L, Jonas JB. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing eye study. Eye (Lond). 2008;22:874–9.
Ng CH, Cheung N, Wang JJ, Islam AF, Kawasaki R, Meuer SM, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118:694–9.
Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–25. discussion 425–30
Fraser-Bell S, Ying-Lai M, Klein R, Varma R. Prevalence and associations of epiretinal membranes in latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2004;45:1732–6.
Kawasaki R, Wang JJ, Mitchell P, Aung T, Saw SM, Wong TY. Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol. 2008;92:1320–4.
Kawasaki R, Wang JJ, Sato H, Mitchell P, Kato T, Kawata S, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata study. Eye (Lond). 2009;23:1045–51.
Duan XR, Liang YB, Friedman DS, Sun LP, Wei WB, Wang JJ, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009;50:2018–23.
Koh V, Cheung CY, Wong WL, Cheung CM, Wang JJ, Mitchell P, et al. Prevalence and risk factors of epiretinal membrane in Asian Indians. Invest Ophthalmol Vis Sci. 2012;53:1018–22.
Zhu XF, Peng JJ, Zou HD, Fu J, Wang WW, Xu X, et al. Prevalence and risk factors of idiopathic epiretinal membranes in Beixinjing blocks, Shanghai, China. PLoS ONE. 2012;7:e51445.
Aung KZ, Makeyeva G, Adams MK, Chong EW, Busija L, Giles GG, et al. The prevalence and risk factors of epiretinal membranes: the Melbourne Collaborative Cohort Study. Retina. 2013;33:1026–34.
Delyfer MN, Legout P, Le Goff M, Blaizeau M, Rougier MB, Schweitzer C, et al. Prevalence of epiretinal membranes in the ageing population using retinal colour images and SD-OCT: the Alienor Study. Acta Ophthalmol. 2020;98:e830–8.
Xiao W, Chen X, Yan W, Zhu Z, He M. Prevalence and risk factors of epiretinal membranes: a systematic review and meta-analysis of population-based studies. BMJ Open. 2017;7:e014644.
Sebag J, Gupta P, Rosen RR, Garcia P, Sadun AA. Macular holes and macular pucker: the role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Trans Am Ophthalmol Soc. 2007;105:121–9.
Govetto A, Lalane RA 3rd, Sarraf D, Figueroa MS, Hubschman JP. Insights into epiretinal membranes: presence of ectopic inner foveal layers and a new optical coherence tomography staging scheme. Am J Ophthalmol. 2017;175:99–113.
Byon IS, Pak GY, Kwon HJ, Kim KH, Park SW, Lee JE. Natural history of idiopathic epiretinal membrane in eyes with good vision assessed by spectral-domain optical coherence tomography. Ophthalmologica. 2015;234:91–100.
Govetto A, Bhavsar KV, Virgili G, Gerber MJ, Freund KB, Curcio CA, et al. Tractional abnormalities of the central foveal bouquet in epiretinal membranes: clinical spectrum and pathophysiological perspectives. Am J Ophthalmol. 2017;184:167–80.
Wilkins JR, Puliafito CA, Hee MR, Duker JS, Reichel E, Coker JG, et al. Characterization of epiretinal membranes using optical coherence tomography. Ophthalmology. 1996;103:2142–51.
Mori K, Gehlbach PL, Sano A, Deguchi T, Yoneya S. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24:57–62.
Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br. J. Ophthalmol. 2020;104:1741–7.
Quinn NB, Steel DH, Chakravarthy U, Peto T, Hamill B, Muldrew A, et al. Assessment of the vitreomacular interface using high-resolution OCT in a population-based cohort study of older adults. Ophthalmol Retin. 2020;4:801–13.
Fraser-Bell S, Guzowski M, Rochtchina E, Wang JJ, Mitchell P. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110:34–40.
Hardin JS, Gauldin DW, Soliman MK, Chu CJ, Yang YC, Sallam AB. Cataract surgery outcomes in eyes with primary epiretinal membrane. JAMA Ophthalmol. 2018;136:148–54.
Schaub F, Adler W, Enders P, Koenig MC, Koch KR, Cursiefen C, et al. Preexisting epiretinal membrane is associated with pseudophakic cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 2018;256:909–17.
Vallejo-Garcia JL, Romano M, Pagano L, Montericcio A, Borgia A, Morenghi E, et al. OCT changes of idiopathic epiretinal membrane after cataract surgery. Int J Retin Vitreous. 2020;6:37.
Hayashi K, Hayashi H. Influence of phacoemulsification surgery on progression of idiopathic epiretinal membrane. Eye (Lond). 2009;23:774–9.
Mavrommatis MA, De Cuir N, Reynaud J, De Moraes CG, Xin D, Rajshekhar R, et al. An examination of the frequency of paravascular defects and epiretinal membranes in eyes with early glaucoma using en-face slab OCT images. J Glaucoma. 2019;28:265–9.
Chen X, Klein KA, Shah CP, Heier JS. Progression to surgery for patients with idiopathic epiretinal membranes and good vision. Ophthalmic Surg Lasers Imaging Retin. 2018;49:S18–s22.
Tanikawa A, Shimada Y, Horiguchi M. Comparison of visual acuity, metamorphopsia, and aniseikonia in patients with an idiopathic epiretinal membrane. Jpn J Ophthalmol. 2018;62:280–5.
Enoch JM, Schwartz A, Chang D. Hirose H. Aniseikonia, metamorphopsia and perceived entoptic pattern: some effects of a macular epiretinal membrane, and the subsequent spontaneous separation of the membrane. Ophthalmic Physiol Opt. 1995;15:339–43.
Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Time course of changes in aniseikonia and foveal microstructure after vitrectomy for epiretinal membrane. Ophthalmology. 2014;121:2255–60.
Jackson TL, Donachie PH, Williamson TH, Sparrow JM, Johnston RL. The royal college of ophthalmologists’ national ophthalmology database study of vitreoretinal surgery: report 4, epiretinal membrane. Retina. 2015;35:1615–21.
Ghazi-Nouri SM, Tranos PG, Rubin GS, Adams ZC, Charteris DG. Visual function and quality of life following vitrectomy and epiretinal membrane peel surgery. Br J Ophthalmol. 2006;90:559–62.
Okamoto F, Okamoto Y, Hiraoka T, Oshika T. Effect of vitrectomy for epiretinal membrane on visual function and vision-related quality of life. Am J Ophthalmol. 2009;147:869–74.e861.
Bouwens MD, Van Meurs JC. Sine Amsler Charts: a new method for the follow-up of metamorphopsia in patients undergoing macular pucker surgery. Graefes Arch Clin Exp Ophthalmol. 2003;241:89–93.
Nakashizuka H, Kitagawa Y, Wakatsuki Y, Tanaka K, Furuya K, Hattori T, et al. Prospective study of vitrectomy for epiretinal membranes in patients with good best-corrected visual acuity. BMC Ophthalmol. 2019;19:183.
Krarup T, Nisted I, Christensen U, Kiilgaard JF, la Cour M. Monocular and binocular end-points after epiretinal membrane surgery and their correlation to patient-reported outcomes. Acta Ophthalmol 2020;98:716–25.
Mieno H, Kojima K, Yoneda K, Kinoshita F, Mizuno R, Nakaji S, et al. Evaluation of pre- and post-surgery reading ability in patients with epiretinal membrane: a prospective observational study. BMC Ophthalmol. 2020;20:95.
Fong CS, Mitchell P, Rochtchina E, Hong T, de Loryn T, Wang JJ. Incidence and progression of epiretinal membranes in eyes after cataract surgery. Am J Ophthalmol. 2013;156:312–18.e311.
Lee SM, Pak KY, Kwon HJ, Park SW, Lee JE, Byon IS. Association between tangential contraction and early vision loss in idiopathic epiretinal membrane. Retina. 2018;38:541–9.
Li DQ, Rudkin AK, Altomare F, Giavedoni L, Wong DT. Predicting progression of untreated macular pucker using retinal surface en face optical coherence tomography. Ophthalmologica 2020;243:323–33.
Luu KY, Koenigsaecker T, Yazdanyar A, Mukkamala L, Durbin-Johnson BP, Morse LS, et al. Long-term natural history of idiopathic epiretinal membranes with good visual acuity. Eye (Lond). 2019;33:714–23.
Kofod M, Christensen UC, la Cour M. Deferral of surgery for epiretinal membranes: is it safe? Results of a randomised controlled trial. Br J Ophthalmol. 2016;100:688–92.
Miguel AI, Legris A. Prognostic factors of epiretinal membranes: a systematic review. J Fr Ophtalmol. 2017;40:61–79.
Scheerlinck LM, van der Valk R, van Leeuwen R. Predictive factors for postoperative visual acuity in idiopathic epiretinal membrane: a systematic review. Acta Ophthalmol. 2015;93:203–12.
Inoue M, Morita S, Watanabe Y, Kaneko T, Yamane S, Kobayashi S, et al. Preoperative inner segment/outer segment junction in spectral-domain optical coherence tomography as a prognostic factor in epiretinal membrane surgery. Retina. 2011;31:1366–72.
Shiono A, Kogo J, Klose G, Takeda H, Ueno H, Tokuda N, et al. Photoreceptor outer segment length: a prognostic factor for idiopathic epiretinal membrane surgery. Ophthalmology. 2013;120:788–94.
Kauffmann Y, Ramel JC, Lefebvre A, Isaico R, De Lazzer A, Bonnabel A, et al. Preoperative prognostic factors and predictive score in patients operated on for combined cataract and idiopathic epiretinal membrane. Am J Ophthalmol. 2015;160:185–92.e185.
Song SJ, Kuriyan AE, Smiddy WE. Results and prognostic factors for visual improvement after pars plana vitrectomy for idiopathic epiretinal membrane. Retina. 2015;35:866–72.
Shimozono M, Oishi A, Hata M, Matsuki T, Ito S, Ishida K, et al. The significance of cone outer segment tips as a prognostic factor in epiretinal membrane surgery. Am J Ophthalmol. 2012;153:698–704.e691.
Itoh Y, Inoue M, Rii T, Hirota K, Hirakata A. Correlation between foveal cone outer segment tips line and visual recovery after epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2013;54:7302–8.
Kim JH, Kang SW, Kong MG, Ha HS. Assessment of retinal layers and visual rehabilitation after epiretinal membrane removal. Graefes Arch Clin Exp Ophthalmol. 2013;251:1055–64.
Wilde C, Awad M, Dua H, Gandhewar R, Chen HC, Amoaku WM. Epiretinal membrane surgery outcomes in eyes with subretinal drusenoid deposits: a case control study. Ophthalmol Retina. 2018;2:1218–26.
Govetto A, Virgili G, Rodriguez FJ, Figueroa MS, Sarraf D, Hubschman JP. Functional and anatomical significance of the ectopic inner foveal layers in eyes with idiopathic epiretinal membranes: surgical results at 12 months. Retina. 2019;39:347–57.
Cho KH, Park SJ, Cho JH, Woo SJ, Park KH. Inner-retinal irregularity index predicts postoperative visual prognosis in idiopathic epiretinal membrane. Am J Ophthalmol. 2016;168:139–49.
Suh MH, Seo JM, Park KH, Yu HG. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147:473–80.e473.
Kim JH, Kim YM, Chung EJ, Lee SY, Koh HJ. Structural and functional predictors of visual outcome of epiretinal membrane surgery. Am J Ophthalmol. 2012;153:103–10.e101.
Kunikata H, Abe T, Kinukawa J, Nishida K. Preoperative factors predictive of postoperative decimal visual acuity ≥ 1.0 following surgical treatment for idiopathic epiretinal membrane. Clin Ophthalmol. 2011;5:147–54.
Kinoshita T, Imaizumi H, Okushiba U, Miyamoto H, Ogino T, Mitamura Y. Time course of changes in metamorphopsia, visual acuity, and OCT parameters after successful epiretinal membrane surgery. Invest Ophthalmol Vis Sci. 2012;53:3592–7.
Mitamura Y, Hirano K, Baba T, Yamamoto S. Correlation of visual recovery with presence of photoreceptor inner/outer segment junction in optical coherence images after epiretinal membrane surgery. Br J Ophthalmol. 2009;93:171–5.
Iuliano L, Fogliato G, Gorgoni F, Corbelli E, Bandello F, Codenotti M. Idiopathic epiretinal membrane surgery: safety, efficacy and patient related outcomes. Clin Ophthalmol. 2019;13:1253–65.
González-Saldivar G, Berger A, Wong D, Juncal V, Chow DR. Ectopic inner foveal layer classification scheme predicts visual outcomes after epiretinal membrane surgery. Retina. 2020;40:710–7.
Cobos E, Arias L, Ruiz-Moreno J, Rubio M, Garcia-Bru P, Caminal J, et al. Preoperative study of the inner segment/outer segment junction of photoreceptors by spectral-domain optical coherence tomography as a prognostic factor in patients with epiretinal membranes. Clin Ophthalmol (Auckl, NZ). 2013;7:1467–70.
Shiode Y, Morizane Y, Toshima S, Kimura S, Kumase F, Hosokawa M, et al. Surgical outcome of idiopathic epiretinal membranes with intraretinal cystic spaces. PLoS ONE. 2016;11:e0168555.
Govetto A, Su D, Farajzadeh M, Megerdichian A, Platner E, Ducournau Y, et al. Microcystoid macular changes in association with idiopathic epiretinal membranes in eyes with and without glaucoma: clinical insights. Am J Ophthalmol. 2017;181:156–65.
Brito PN, Gomes NL, Vieira MP, Faria PA, Fernandes AV, Rocha-Sousa A, et al. Possible role for fundus autofluorescence as a predictive factor for visual acuity recovery after epiretinal membrane surgery. Retina. 2014;34:273–80.
Bae SH, Kim D, Park TK, Han JR, Kim H, Nam W. Preferential hyperacuity perimeter and prognostic factors for metamorphopsia after idiopathic epiretinal membrane surgery. Am J Ophthalmol. 2013;155:109–17.e103.
Okamoto F, Sugiura Y, Okamoto Y, Hiraoka T, Oshika T. Inner nuclear layer thickness as a prognostic factor for metamorphopsia after epiretinal membrane surgery. Retina. 2015;35:2107–14.
Takabatake M, Higashide T, Udagawa S, Sugiyama K. Postoperative changes and prognostic factors of visual acuity, metamorphopsia, and aniseikonia after vitrectomy for epiretinal membrane. Retina. 2018;38:2118–27.
Han J, Han SH, Kim JH, Koh HJ. Restoration of retinally induced aniseikonia in patients with epiretinal membrane after early vitrectomy. Retina. 2016;36:311–20.
Pareja J, Coronado A, Contreras I. Epiretinal membrane surgery in daily clinical practice: results of a proposed management scheme. J Ophthalmol. 2019;2019:8246858.
Trese M, Chandler DB, Machemer R. Macular pucker. II. Ultrastructure. Graefes Arch Clin Exp Ophthalmol. 1983;221:16–26.
Park DW, Dugel PU, Garda J, Sipperley JO, Thach A, Sneed SR, et al. Macular pucker removal with and without internal limiting membrane peeling: pilot study. Ophthalmology. 2003;110:62–64.
Tranos P, Koukoula S, Charteris DG, Perganda G, Vakalis A, Asteriadis S, et al. The role of internal limiting membrane peeling in epiretinal membrane surgery: a randomised controlled trial. Br J Ophthalmol. 2017;101:719–24.
Díaz-Valverde A, Wu L. To peel or not to peel the internal limiting membrane in idiopathic epiretinal membranes. Retina. 2018;38:S5–s11.
Huang Q, Li J. With or without internal limiting membrane peeling during idiopathic epiretinal membrane surgery: A meta-analysis. PLoS One. 2021;16:e0245459.
Christodoulou E, Batsos G, Galanis P, Kalogeropoulos C, Katsanos A, Alamanos Y, et al. Vitrectomy for the removal of idiopathic epiretinal membrane with or without internal limiting membrane peeling: a meta-analysis. Ther Adv Ophthalmol. 2020;12:2515841420927133.
Beyazyildiz Ö, Tirhiş MH, Hekimoğlu ER, Beyazyildiz E, Kaymaz F, Yilmazbaş P, et al. Histopathological Analysis of Internal Limiting Membrane Surgically Peeled From Eyes with Epiretinal Membrane. Curr Eye Res. 2016;41:258–65.
Shimada H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, Yuzawa M. Double staining with brilliant blue G and double peeling for epiretinal membranes. Ophthalmology. 2009;116:1370–6.
Mitamura Y, Ohtsuka K. Relationship of dissociated optic nerve fiber layer appearance to internal limiting membrane peeling. Ophthalmology. 2005;112:1766–70.
Tadayoni R, Paques M, Massin P, Mouki-Benani S, Mikol J, Gaudric A. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology. 2001;108:2279–83.
Clark A, Balducci N, Pichi F, Veronese C, Morara M, Torrazza C, et al. Swelling of the arcuate nerve fiber layer after internal limiting membrane peeling. Retina. 2012;32:1608–13.
Deltour JB, Grimbert P, Masse H, Lebreton O, Weber M. Detrimental effects of active internal limiting membrane peeling during epiretinal membrane surgery: microperimetric analysis. Retina. 2017;37:544–52.
Rizzo S, Belting C, Genovesi-Ebert F, di Bartolo E. Incidence of retinal detachment after small-incision, sutureless pars plana vitrectomy compared with conventional 20-gauge vitrectomy in macular hole and epiretinal membrane surgery. Retina. 2010;30:1065–71.
Dawson SR, Shunmugam M, Williamson TH. Visual acuity outcomes following surgery for idiopathic epiretinal membrane: an analysis of data from 2001 to 2011. Eye (Lond, Engl). 2014;28:219–24.
Chen JK, Khurana RN, Nguyen QD, Do DV. The incidence of endophthalmitis following transconjunctival sutureless 25- vs 20-gauge vitrectomy. Eye (Lond). 2009;23:780–4.
Czajka MP, Byhr E, Olivestedt G, Olofsson EM. Endophthalmitis after small-gauge vitrectomy: a retrospective case series from Sweden. Acta Ophthalmol. 2016;94:829–35.
Scott IU, Flynn HW Jr., Dev S, Shaikh S, Mittra RA, Arevalo JF, et al. Endophthalmitis after 25-gauge and 20-gauge pars plana vitrectomy: incidence and outcomes. Retina. 2008;28:138–42.
Acknowledgements
We would like to thank Angela Hall, Library Manager from Royal Liverpool University Hospital for conducting a literature search.
Author information
Authors and Affiliations
Contributions
P.Y.C., M.T.S. and D.H.S. contributed equally to designing the protocol, reviewing the selected articles, writing and revising manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chua, P.Y., Sandinha, M.T. & Steel, D.H. Idiopathic epiretinal membrane: progression and timing of surgery. Eye 36, 495–503 (2022). https://doi.org/10.1038/s41433-021-01681-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01681-0
This article is cited by
-
OCTDL: Optical Coherence Tomography Dataset for Image-Based Deep Learning Methods
Scientific Data (2024)
-
Visual outcomes and complications of combined versus sequential pars plana vitrectomy and phacoemulsification for epiretinal membrane
Eye (2024)
-
Anatomical changes in idiopathic epiretinal membrane at 2-year follow-up assessed using spectral domain optical coherence tomography and optical coherence tomographic angiography
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Patient-reported outcome measures in vitreoretinal surgery: a systematic review
Eye (2023)
-
Patient satisfaction after EDOF intraocular lens implantation in vitrectomized eyes
Graefe's Archive for Clinical and Experimental Ophthalmology (2023)