Abstract
Purpose
Retinopexy is the most common vitreo-retinal procedure performed in the eye emergency department and significantly reduces the risk of a rhegmatogenous retinal detachment (RRD). There are various indications for retinopexy, with the most common being horseshoe-tears (HST). Multiple treatment techniques exist, ranging from slit-lamp laser-retinopexy, indirect laser-retinopexy or cryopexy. We report on our primary retinopexy 6-month RRD rate, repeat retinopexy rate and compare outcomes of different indications and treatment modalities.
Methods
Retrospective consecutive case series of 1157 patients attending Birmingham and Midlands Eye Centre, UK between January 2017 and 2020.
Results
The RRD rate at 6 months was 3.9%, with 19.1% requiring subsequent retinopexies. Multivariate Cox survival regression analysis showed that significant risk factors for RRD following primary retinopexy included male gender (p = 0.012), high myopia (≤ − 6.00D, p = 0.004), HST (compared to round holes, p = 0.026) and primary cryopexy (compared to slit-lamp laser, p = 0.014). HST was the most common indication for retinopexy (812 [70.2%]) in which 118 (14.5%) had multiple tears. Slit-lamp laser was used in 883 (76.3%) of cases. The rate for subsequent epiretinal membrane peel surgery was 3 (0.3%) and was higher in eyes that required multiple retinopexy procedures (p = 0.035).
Conclusion
With our large cohort of patients over three years, we provide additional evidence on the RRD and subsequent retinopexy rate after primary retinopexy. Further retinopexy is a common occurrence, particularly in high-risk retinal tears such as HST. Strict monitoring and prompt follow-up after retinopexy is important to prevent progression to RRD and should be of priority in the clinicians post-retinopexy management plan, particularly in those with associated risk factors.
Similar content being viewed by others
Introduction
Retinopexy to prevent rhegmatogenous retinal detachment (RRD) constitutes a large proportion of essential emergency work in any acute ophthalmic service. Without treatment, retinal tears can progress to RRD in 30–50% of cases [1, 2], which reduces to 2.1–8.8% following retinopexy [3,4,5]. Slit-lamp laser retinopexy has become widely practised as the first-choice treatment to prevent the occurrence of a RRD in an emergency setting. Indirect laser and cryopexy for retinal tears are more specialist procedures, requiring a certain level of experience, and is therefore often provided by a vitreo-retinal (VR) service. These methods are especially important for anterior breaks as they allow for adequate cover through indentation where slit-lamp retinopexy may not.
While horseshoe tears (HST) are the most common indication for patients with symptomatic posterior vitreous detachment requiring retinopexy, many patients are treated for operculated breaks, round holes and barrier laser for a localised or chronic RRD. Additionally, three-sixty barrier-laser is sometimes applied in high-risk eyes to prevent progression to a RRD [6].
In this study, we present the largest case series to date of primary retinopexies and investigate their outcomes and risk factors through multivariate regression analyses.
Methods
We present a single centre, retrospective, continuous comparative study, to analyse all patients that had a primary retinopexy from January 2017 to 2020. The research adhered to the tenets of the Declaration of Helsinki and all patient data extracted were anonymised for analysis. All data were extracted from electronic patient records (EPR, Medisoft Ophthalmology, Medisoft Limited, Leeds, UK)
Our primary outcome measure was to report RRD rate following primary retinopexy at 6 months. Secondary outcome measures included the need for repeat retinopexy in the same eye within 6 months and the risk factors that led to this, and the rate of needing subsequent epiretinal membrane (ERM) surgery following primary retinopexy. Retinopexy was performed in patients with a variety of retinal pathology (tears, holes, lattice degeneration and localised retinal detachments) to create adequate chorio-retinal adhesions and to reduce the risk of retinal detachment. Retinopexy was performed with either cryopexy or through laser photocoagulation, with the later consisting of either slit-lamp or indirect ophthalmoscopy. Furthermore, the laser delivered could be localised around the pathology or 360-degree. Laser treatment consisted of surrounding retinal breaks with two to three rows of confluent laser burns using either a contact lens or a noncontact condensing lens system.
All patients, following retinopexy, were reviewed within four weeks in a VR clinic as per our trust treatment protocol. Following this, if patients were found to have inadequate retinopexy cover, they underwent further retinopexy. In addition, patients could also have further treatment if they presented to the emergency eye clinic with deterioration of symptoms and clinically determined to require more treatment. Cryopexy was generally reserved for very anterior breaks with vitreous haemorrhage that made laser impossible to complete. Indirect laser (with local anaesthetic) was performed on patients who were intolerant of slit-lamp laser, or if the retinal break was too anterior to be adequately treated by slit-lamp retinopexy alone. Lastly, 360-indirect laser retinopexy was performed on high-risk eyes, with significant ocular co-morbidities or multiple areas of retinal pathology. Operators were all third-year residents as a minimum and were independent operators.
All patients that had prior VR surgery in the same eye were excluded. RRD rate was defined as requiring RRD surgery within 6 months of have a primary retinopexy in the same eye. As a tertiary referral centre, patients whose postcode was outside the catchment area were excluded, as these patients may have had further retinopexy or surgery at the referring unit. All RRD surgery performed in our cohort were either transconjunctival 23-gauge-PPV with vitreous-base trim, cryotherapy/laser retinopexy and gas or oil tamponade or scleral buckling. Preoperative data collection included indication of treatment, age, gender, morphology of retinal break, number of tears and presence of high myopia. High myopia in our unit is defined as the spherical equivalent of ≤−6.00 Dioptres. We also looked at the delivery system preferred for retinopexy (slit lamp, indirect laser retinopexy, indirect 360 laser retinopexy or cryopexy). Postoperative data was collected for the duration of follow-up and included the indication of further treatment in the same eye, 6-month detachment status, and subsequent ERM surgery.
Statistical analysis
Statistical significance was defined as p < 0.05. Prior to analysis, normality of continuous variables was assessed using the Shapiro–Wilk test, and found not to be normally distributed. Hence, data are primarily reported as medians and interquartile ranges (IQRs) throughout. Mann–Whitney U and Kruskal–Wallis Test were used to compare two and three independent groups respectively. Fisher exact test and Chi-Squared test were used for nominal variables. McNemar test was used for paired nominal variables. Bonferroni correction was applied for multiple statistical analysis. To account for variable operator VR experience, patient age, gender, presence of high myopia, treatment indication (HST compared to round holes), treatment modality, a multivariate Cox regression survival analysis was performed analysing both repeat retinopexy and RRD. Time in days to repeat retinopexy and RRD were used respectively, with operator grade (dichotomised to general ophthalmologist and VR specialist), gender, age, high myopia and treatment modality (cryopexy, slit-lamp and indirect retinopexy), and indication for treatment (HST vs Hole), as covariates. All statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk NY).
Results
We report on the primary retinopexy outcomes of 1157 patients. A summary of clinical characteristics of primary retinopexy are found in Table 1. We found that HSTs constitutes the majority indication for primary retinopexies, accounting for 812 (70.2%) of eyes. Sixty-four (5.5%) patients required bilateral retinopexy and 42 (3.6%) were performed at the time as fellow eye RRD repair surgery. Slit-lamp laser retinopexy was the most common treatment modality with 883 (76.3%) of patients.
A summary of the retinopexy outcomes is shown on Table 2. Repeat retinopexies were required in 19.1% of patients, on average 17 days after initial treatment, with some patients requiring up to four further sessions. Pertinent findings include an overall 6-month RRD rate of 3.9%. RRD surgery comprised of PPV 41 (3.5%), SB in 3 (0.2%) and combined PPB and SB in 4 (0.3%). RRD surgery was carried out at a median 15.0 (IQR 3.0–51.0) days after the initial retinopexy.
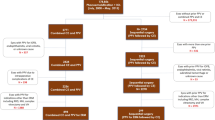
Risk factors that led to RRD are found in Table 3. In our study, significant risk factors associated with RRD included a younger age group, high myopia, and male gender (p = 0.046, p = 0.029 and p = 0.022, respectively). We found a similar proportion of patients required repeat retinopexy between the group of patients that did not detach and those that developed RRD (212 [19.0%] and 10 [22.2%] respectively, p = 0.565) and so requiring repeat retinopexy was not identified as a risk factor for developing RRD. HST compared to round holes were found to have significantly higher RRD rate of 35 (4.3%) compared to 2 (1.1%) respectively (p = 0.031). No operculated breaks that had primary retinopexy required RRD surgery at 6 months. Barrier retinopexy for RRD was successful in 53 (93.0%) of cases. Patients that developed a RRD had a shorter time interval between primary and secondary retinopexy (p = 0.012). Cryopexy compared to laser retinopexy was associated with a higher rate of RRD (8 (8.2%) compared to 37 (3.5%) respectively, p = 0.047). However, cryopexy was performed in significantly more HST than holes compared to laser (p < 0.001) and was more utilised with multiple HST (p = 0.003). A breakdown of laser compared to cryopexy use by indication can be found in Fig. 1A.
Repeat retinopexy was performed in 187 (23.0%) of HST compared to 15 (8.0%) of round holes (p < 0.001). Figure 1B shows the distribution of delivery method used in primary retinopexy compared to the subsequent second treatment. Significantly, more cryotherapy is used for further retinopexy than laser (p < 0.001, McNemar test). Patients undergoing multiple retinopexy procedures compared to a single retinopexy were associated with an increased rate of needing subsequent ERM surgery (p = 0.035). There was no difference in the number of patients having ERM surgery between laser and cryopexy (p = 0.231).
Following 360-laser indirect retinopexy, only one of the 22 patients detached (4.5%). Two patients however did need further retinopexy (one indirect and one slit lamp).
Cox multivariate regression survival analysis
Due to high number of risk factors, a multivariate Cox survival regression analysis was performed on the risk factors for RRD and further retinopexy following primary retinopexy. Our Cox regression survival analysis can be found in Table 4. HST (relative to round holes, p < 0.001) and high myopia were associated with significantly higher repeat retinopexy rate (p = 0.042).
HST (p = 0.026), male gender (0.012), high myopia (p = 0.004), general ophthalmologists (compared to VR specialist, p = 0.014) and cryopexy (relative to slit-lamp laser, p = 0.014) were all associated with higher RRD rate following primary retinopexy.
Discussion
To the best of our knowledge, this represents the largest case series of retinopexy outcomes available. In addition, we use a multivariate Cox survival regression analysis to identify risk factors for repeat retinopexy and RRD rate. Our RRD rate of 45 (3.9%) is consistent with that found in the literature, which is reported as between 2.1 and 8.8%. [3,4,5, 7,8,9,10,11] Garoon et al., in a study of 401 eyes reported comparable results with a RRD rate of 5.7% [10]. We demonstrate that HST, compared to retinal holes, lead to a significantly higher risk of RRD. However, we also demonstrated that higher risk patients are more likely to undergo cryopexy for primary retinopexy, including those with multiple HST and localised RRD (Fig. 1A). Within our unit, cryopexy is usually reserved for anterior, more difficult tears, breaks obscured by vitreous haemorrhage, and those with multiple breaks that would carry a poorer prognosis. Due to the retrospective nature of this study, it is not possible to make conclusions on the safety profile of cryopexy compared to laser retinopexy from these data alone and further prospective studies would be required. We did not find that further retinopexy after primary retinopexy increased the risk of RRD. Retinopexy for barrier RRD was used to good effect in our cohort, avoiding RRD surgery in 53 (93.0%) of cases. This offers a better primary success rate than most literature reports [12,13,14]. Furthermore, with regards to 360-degree laser, we found that only one out of the 22 patients had a detached retina. Chauhan et al. conversely found a 76% RRD rate following 360 prophylactic laser in eyes that did not have a posterior vitreous detachment [15], although this may represent a different group of patients to ours.
Within our own unit, we have previously reported repeat retinopexy rates: i) Petrou et al. in 2014 found that their study of 100 consecutive retinopexies in September 2010–2011, resulted in a repeat retinopexy rate of 40% with a 0% RRD rate [8], and ii) Ghosh et al. in 2005, in a study of 100 consecutive trainee surgeons found a repeat retinopexy rate of 24% over a period between August 2000 to December 2002 [7]. This is compared to our current study rate of 19.1%. The reasons for these fluctuations within the same unit are manifold. Firstly, those papers focused on retinopexies performed only within the emergency department (ED), whereas we captured all primary retinopexy (including prophylactic 360 laser indirect retinopexy). Typically, in the ED, primary retinopexy is performed by residents who are not VR specialists, compared to our cohort, where we captured primary retinopexies also performed by VR fellows and consultants. Additionally, 20 years on from the initial study by Ghosh et al., we are performing much higher numbers of retinopexy in our unit. This may be due to a possible change in indication and clinical management of tears within our unit. This could also be further exacerbated by an increase in referrals for asymptomatic holes from primary care. In addition to a variable repeat retinopexy rate reported by our unit, this figure is considerably variable in the literature, Levin et al. reported additional laser treatment in 15% of eyes and a RRD rate of 1.2% [16]. However, they also reported that 56.6% of patients were asymptomatic at the time of retinopexy. Garoon et al., reported further retinopexy use in 18.7% of eyes [10].
In our cohort we found that 3 (0.3%) of our patients required subsequent ERM surgery consistent with that of other reports (0.2–10%) [16,17,18]. This was significantly higher in patients requiring repeat retinopexy than those treated once (0.9% and 0.3% respectively, p = 0.035).
Study limitations and strengths
The limitations of our study include its retrospective nature and lack of case randomisation. Additionally, we used RRD surgery as a determinant of failed primary retinopexy at 6 months. Patients may also have had their surgery at another eye unit, although patients were excluded by postcode to minimise this probability. Due to the retrospective nature of this study, we had no standardised protocol to follow and retinopexy was performed at the discretion of the reviewing clinician. However, this study has several strengths. A retrospective analysis allowed us to collate a large case series for adequate numbers even within smaller subgroups. By excluding patients outside our catchment area and ensuring data capture when all clinicians were utilising electronic patient records, we can present consecutive cases. Decision for further retreatment of retinopexy is typically performed by experienced VR clinicians. Additionally, a multivariate analysis helps reduce confounders of multiple investigators with various VR experience, multiple mode of delivery of treatment, and variations in clinical presentations.
Conclusions
With a larger cohort, our study gives more conclusive evidence of the RRD rate following retinopexies, as well as the rate of subsequent retinopexies performed. Further retinopexy is a common occurrence following primary retinopexy and we demonstrate that HST have a higher risk profile than holes and require more retinopexy treatments and a higher RRD rate. Cryopexy is reserved for higher risk indications and consequently has a higher RRD rate than laser retinopexy. Strict monitoring and adequate prompt follow-up after retinopexy are important to prevent progression to RRD and should be of priority in the clinicians post-retinopexy management plan, particular in those with associated risk factors.
What was known before
-
Retinopexy can be delivered through various techniques such as slit-lamp laser retinopexy, indirect laser retinopexy and cryopexy for various types of retinal breaks and indications.
-
Few large case series exist to assess outcomes of primary retinopexy by indication and subtype of retinopexy and their success in preventing RRD.
What this paper adds
-
This three-year study, with the largest cohort of patients, provided additional evidence of RRD and subsequent retinopexy rate following primary retinopexy with multivariate regression analysis.
-
Our large cohort identifies the safety profile of performing primary retinopexy for various indications, from round holes to RRD. Retinopexy was an effective treatment with an overall 3.9% 6-month RRD rate and a 19.1% retinopexy retreatment rate, but increased retinopexy episodes were associated with a higher need for subsequent epiretinal membrane surgery.
References
Davis MD. Natural history of retinal breaks without detachment. Arch Ophthalmol. 1974;92:183–194.
Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye. 2002;16:411–421.
Colyear BH, Pischel DK. Clinical tears in the retina without detachment. Am J Ophthalmol. 1956;41:773–792.
Blindbæk S, Grauslund J. Prophylactic treatment of retinal breaks - a systematic review. Acta Ophthalmol. 2015;93:3–8.
Lankry P, Loewenstein A, Moisseiev E. Outcomes following laser retinopexy for retinal tears: a comparative study between trainees and specialists. Ophthalmologica. 2020;243:355–359.
Koh HJ, Cheng L, Kosobucki B, Freeman WR. Prophylactic intraoperative 360° laser retinopexy for prevention of retinal detachment. Retina. 2007;27:744–749.
Ghosh YK, Banerjee S, Tyagi AK. Effectiveness of emergency argon laser retinopexy performed by trainee doctors. Eye. 2005;19:52–54.
Petrou P, Lett KS. Effectiveness of emergency argon laser retinopexy performed by trainee physicians: 10 Years later. Ophthalmic Surg Lasers Imaging Retin. 2014;45:194–196.
Khan AA, Mitry D, Goudie C, Singh J, Bennett H. Retinal detachment following laser retinopexy. Acta Ophthalmol. 2016;94:e76.
Garoon. R, Flynn. HW. Laser retinopexy for retinal tears: clinical course and outcomes. Investig Ophthalmol Vis Sci. 2018;59:4236.
Smiddy WE, Flynn HW, Nicholson DH, Clarkson JG, Gass JDM, Olsen KR, et al. Results and complications in treated retinal breaks. Am J Ophthalmol. 1991;112:623–631.
Orlin A, Hewing NJ, Nissen M, Lee S, Kiss S, D’Amico DJ, et al. Pars plana vitrectomy compared with pars plana vitrectomy combined with scleral buckle in the primary management of noncomplex rhegmatogenous retinal detachment. Retina 2014;34:1069–1075.
Thelen U, Amler S, Osada N, Gerding H. Success Rates of Retinal Buckling Surgery: Relationship to Refractive Error and Lens Status: Results from a Large German Case Series. Ophthalmology 2010;117:785–790.
Kinori M, Moisseiev E, Shoshany N, Fabian ID, Skaat A, Barak A, et al. Comparison of pars plana vitrectomy with and without scleral buckle for the repair of primary rhegmatogenous retinal detachment. Am. J. Ophthalmol. 2011;152:291–297.e2.
Chauhan DS, Downie JA, Eckstein M, Aylward GW. Failure of prophylactic retinopexy in fellow eyes without a posterior vitreous detachment. Arch Ophthalmol. 2006;124:968–971.
Levin M, Naseri A, Stewart JM. Resident-performed prophylactic retinopexy and the risk of retinal detachment. Ophthalmic Surg Lasers Imaging. 2009;40:120–126.
Saran BR, Brucker AJ. Macular epiretinal membrane formation and treated retinal breaks. Am J Ophthalmol. 1995;120:480–485.
Mester U, Volker B, Kroll P, Berg P. Complications of prophylactic argon laser treatment of retinal breaks and degenerations in 2,000 eyes. Ophthalmic Surg. 1988;19:482–484.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moussa, G., Samia-Aly, E., Ch’ng, S.W. et al. Primary retinopexy in preventing retinal detachment in a tertiary eye hospital: a study of 1157 eyes. Eye 36, 1080–1085 (2022). https://doi.org/10.1038/s41433-021-01581-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01581-3