Abstract
Objectives
To determine the association of plasma homocysteine levels with retinal layer thickness in a large community cohort of older adults.
Methods
The Rugao Longevity and Ageing Study is an observational, prospective and community-based cohort study. A total of 989 older adults who underwent spectral-domain optical coherence tomography (SD-OCT) were included and analyzed. Foveal, macular retinal nerve fibre layer (mRNFL) and ganglion cell layer plus inner plexiform layer (GC-IPL) thicknesses were measured by SD-OCT. Plasma homocysteine levels were measured using chemiluminescence immunoassay. Linear regression analyses were performed to evaluate the relationship between plasma homocysteine and retinal layer thickness while controlling for confounding factors.
Results
Of the 989 participants, 500 (50.56%) were men. The mean age was 78.26 (4.58) years, and the mean plasma homocysteine level was 16.38 (8.05) μmol/L. In multivariable analyses, each unit increase in plasma homocysteine was associated with an 8.84 × 10−2 (95% CI: −16.54 × 10−2 to −1.15 × 10−2, p = 0.032) μm decrease in the average inner thickness of the GC-IPL after controlling for confounding factors. The association remained significant even in participants without major cardiovascular disease or diabetes (β = −10.33 × 10−2, 95% CI: −18.49 × 10−2 to −2.18 × 10−2, p = 0.013). No significant associations of plasma homocysteine levels with macular thickness or mRNFL were found in primary and sensitivity analyses (p > 0.05).
Conclusions
Increased plasma homocysteine levels are associated with a thinner GC-IPL. Plasma homocysteine may be a risk factor for thinner retinas in older adults.
Similar content being viewed by others
Introduction
Spectral-domain optical coherence tomography (SD-OCT), a rapid and noninvasive imaging technique, is commonly used to measure the retinal profile and has transformed our understanding of macular structure in health and disease [1]. Changes in retinal thickness have been increasingly recognized as potential diagnostic markers in hydroxychloroquine retinal toxicity [2, 3] and glaucoma [4,5,6]. Many cross-sectional and longitudinal studies have demonstrated the relationship between retinal thickness and Alzheimer’s disease [7,8,9]. In addition, the thinning of the retina has been associated with cerebral grey matter and white matter atrophy [10, 11], as well as worse white matter microstructure of the brain [11,12,13]. Retinal thickness was also recognized as a feasible biomarker for the early diagnosis of Alzheimer’s disease. Therefore, it is important to identify potential risk factors impacting retinal thickness and conduct interventions before clinical symptoms emerge.
Homocysteine is a sulfur-containing amino acid and acts as an intermediate product of methionine metabolism [14, 15]. It is a potential biomarker for predicting frailty [16, 17] and cardiovascular and all-cause mortality [18, 19] in older adults. Previous studies have illustrated the deleterious effects of hyperhomocysteinemia (Hhcy) on retinal ganglion cells (RGCs), including decreased RGC viability [20], dysregulation of mitochondrial dynamics [21] and increased oxidative stress [22]. Hence, it is not surprising that increased plasma homocysteine levels are associated with the risk of retinal disease [23,24,25]. Previous studies have usually focused their attention on the association of homocysteine with retinal diseases, such as age-related macular degeneration [23], glaucoma [24] and diabetic retinopathy [25]. Moreover, those studies on plasma homocysteine with retinal thickness were conducted specifically in patients with diseases that directly influenced retinal thickness. There is still a lack of studies exploring the relationship in healthy older adults. Therefore, we conducted this study to explore the relationship between plasma homocysteine levels and retinal thickness measured by SD-OCT in older adults.
Methods
Participants
The Rugao Longevity and Ageing Study (RuLAS) is an observational, prospective and population-based cohort study [26]. The baseline survey was conducted between November 2014 and December 2014 (wave 1). A total of 1788 elderly adults aged 70–84 years were recruited. Then, follow-up surveys were conducted in April 2016 (wave 2), November 2017 (wave 3) and December 2019 (wave 4). This study was approved by the Human Ethics Committee of the School of Life Sciences of Fudan University, and informed consent was obtained from each participant.
In December 2019, a detailed ophthalmic examination was added to the protocol and performed at wave 4 of the cohort. A total of 2200 older adults were recruited in wave 4. Eye conditions were determined by an examining ophthalmologist (HS). Two experienced ophthalmologists (HS and WG) identified subjects with eye diseases based on the examination records and clinical history. Final adjudication was made by a senior ophthalmologist if a primary diagnosis was not definite during the initial assessment. SD-OCT was available for 1721 subjects (78.22%). Subjects who did not undergo SD-OCT (n = 479) or had ungradable OCT due to poor-quality scans (n = 538) were excluded, and 120 subjects were also excluded due to a lack of plasma homocysteine data. Furthermore, subjects with retinal-related diseases, including age-related macular degeneration (n = 14), diabetic retinopathy (n = 6), pathological myopia (n = 39) and other retinal diseases (n = 15), were excluded. Finally, 989 participants were analyzed in this study. Age-related macular degeneration was defined according to the Wisconsin Age-related Maculopathy Grading System [27]. Diabetic retinopathy was graded using the Early Treatment Diabetic Retinopathy Study (ETDRS) criteria [28]. Pathological myopia was diagnosed as an eye with refractive error exceeding −6.0 dioptres with one or more of the following findings: tessellated fundus, diffuse chorioretinal atrophy, patchy chorioretinal atrophy, macular atrophy, or presence of lacquer cracks, choroidal neovascularization, Fuchs spot or posterior staphyloma [29]. In addition, the diagnoses of retinal detachment, epiretinal membranes and other diseases followed the clinical standard.
Demographic, physiological and clinical data were collected from the RuLAS in wave 4. Demographic data included age, sex, marital status, education, smoking, and alcohol consumption. Physiological variables, including body mass index (BMI), high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride and creatinine, were also measured. Clinical variables, including high-sensitivity C-relative protein (Hs-CRP) levels and self-reported major cardiovascular disease (CVD) and diabetes mellitus, were collected. Major CVDs, including cerebral infarction, stroke, cerebral haemorrhage, coronary heart disease, myocardial infarction and heart failure, were recorded. Participants who self-reported a diagnosis of diabetes mellitus, used medication for diabetes mellitus, or had fasting glucose greater than 125 mg/dL were coded as having diabetes mellitus. In addition, mobility (measured by the timed up and go test), grip strength, depressive status (assessed by the 15-item Geriatric Depression Scale) and cognitive function (assessed by revised Hasegama’s dementia scale) were assessed. Plasma homocysteine levels were measured using a chemiluminescence immunoassay.
SD-OCT scan
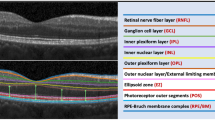
SD-OCT scanning was performed without pupil dilation using the spectral domain OCT-HS100 (Canon Inc, Tokyo, Japan) with macular 3D scans over a 10 × 10 mm area (1024 A-scan × 128 B-scan). The measurement of the right eye was used if the signal strength index was ≥5. Otherwise, the left eye was used. The ETDRS grid focused on the macula was used for OCT measurements. The ETDRS grid consisted of three concentric circles of 1-, 3-, and 6-mm diameters. The 3- and 6-mm circles were each divided into superior, inferior, nasal, and temporal quadrants. Thickness measurements were conducted by an experienced examiner (HS). To determine the intra- and interobserver repeatability of the measurements, the first 20 images were obtained twice by the same examiner (HS). Another examiner (WG) independently repeated the measurements with the same 20 measurements to determine the interobserver repeatability [30]. The intraobserver (intraclass correlation coefficient [ICC]: 0.993; 0.991–0.994) and interobserver (ICC: 0.991; 0.989–0.993) reproducibility were excellent for all measurements. The thickness was automatically segmented and manually corrected by two masked examiners (HS and WG) if necessary. The following segmented intraretinal layers were analyzed: full-thickness, macular retinal nerve fibre layer (mRNFL), ganglion cell layer plus inner plexiform layer (GC-IPL). The mean thickness was calculated as follows: average inner thickness = (superior inner + inferior inner + nasal inner + temporal inner)/4, and average outer thickness = (superior outer + inferior outer + nasal outer + temporal outer)/4.
Statistical analyses
First, we describe the characteristics of the participants in our study. Continuous and categorical variables are presented as the mean with standard deviation (SD) and frequency (%), respectively. Group differences were analyzed by chi-square or t tests. Second, we evaluated the relationship between plasma homocysteine levels and retinal thickness using linear regression in four models. Model 1 was unadjusted; model 2 was adjusted for age, BMI, sex, smoking, alcohol consumption, marital status, and illiteracy; model 3 was adjusted for model 2 + fasting glucose, triglyceride, HDL, LDL, creatinine and Hs-CRP levels; and model 4 was adjusted for model 3 + CVD, hypertension, diabetes, mobility, grip strength, depressive status and cognitive function. Finally, sensitivity analyses using linear regression models were introduced to validate the relationship in participants without CVD and/or diabetes. A p value (two-tailed) less than 0.05 was considered statistically significant. All analyses were conducted by SPSS 22.0 or R (Version 3.6.1: www.r-project.org/).
Results
Characteristics of included participants
Between December 2019 and January 2020, 2200 older adults were recruited. Among them, 989 (44.958%) participants were analyzed (Supplemental Table 1). Tables 1 and 2 summarize demographic, physiological and clinical data for the participants with SD-OCT measurements. In this cross-sectional study, the mean (SD) age of the participants included in this study was 78.26 (4.58) years. The proportion of participants who were men was 50.56%, 114 (9.83%) had self-reported major CVD events, 140 (14.16%) had diabetes, 563 (56.93%) had self-reported hypertension 99(10.01%). The mean plasma homocysteine level was 16.38 (8.05) μmol/L. Table 2 shows the retinal layer thickness of the participants in the RuLAS. The detailed thicknesses of the sectors were also shown (Table 2).
Associations of plasma homocysteine with overall macular and mRNFL thicknesses
In primary (Table 3) and sensitivity (Table 4) analyses, no significant association was found between full retinal thickness and plasma homocysteine levels in either unadjusted or adjusted models (p > 0.05).
In primary analyses (Table 3), the association of plasma homocysteine levels with mRNFL thickness was significant for the average outer thickness (β = −4.32 × 10−2, 95% CI: −8.35 × 10−2 to −0.29 × 10−2, p = 0.036) but not for the average inner thickness (β = −1.43 × 10−2, 95% CI: −3.68 × 10−2 to −0.82 × 10−2, p = 0.213) in unadjusted models. The relationships between the three sectors of the mRNFL and homocysteine levels were significant (superior outer, nasal outer). However, these associations of homocysteine with the average and sector thicknesses were not significant after adjusting for age, body mass index, sex, smoking, alcohol consumption, marital status, illiteracy, self-reported CVD, hypertension, diabetes, mobility, grip strength, fasting glucose, triglyceride, HDL, LDL, and creatinine levels, depressive status and cognitive function. In sensitivity analyses (Table 4), similar results showed that there was no significant relationship between plasma homocysteine levels and mRNFL thickness (p > 0.05).
Associations of plasma homocysteine with GC-IPL thickness
In primary analyses (Table 3), the association of plasma homocysteine levels with average inner thickness was significant in unadjusted (β = −9.56 × 10−2, 95% CI: −16.28 × 10−2 to −2.83 × 10−2, p = 0.005) and adjusted models (β = −8.84 × 10−2, 95% CI: −16.54 × 10−2 to −1.15 × 10−2, p = 0.024). The association of plasma homocysteine levels with GC-IPL sectors was also significant in the superior inner, inferior inner, nasal inner, temporal inner and temporal outer sectors. In addition, the associations of plasma homocysteine levels with the superior inner layer and temporal inner layer were still significant after controlling for confounding factors.
In sensitivity analyses (Table 4), the relationship between plasma homocysteine levels and average inner thickness was significant in unadjusted (β = −11.12 × 10−2, 95% CI: −18.46 × 10−2 to −3.78 × 10−2, p = 0.003) and adjusted models (β = −10.33 × 10−2, 95% CI: −18.49 × 10−2 to −2.18 × 10−2, p = 0.013) after excluding participants with major CVD and/or diabetes. The associations of plasma homocysteine levels with particular GC-IPL sectors (superior inner, inferior inner, nasal inner, temporal inner, nasal outer, temporal outer layers) were significant in unadjusted models, with the exception of the inferior outer layer. Novelty, the association of plasma homocysteine levels with GC-IPL thickness remained significant after adjusting for confounding factors in participants without CVD and/or diabetes in the superior inner layer, temporal inner layer and temporal outer layer.
Discussion
To our knowledge, this is the first study to identify that increased plasma homocysteine levels were associated with thinner GC-IPL after adjustment for confounding factors in a large, healthy community cohort of older adults (Fig. 1). This association remained significant in the sensitivity analyses that excluded participants with major CVD and/or diabetes. In contrast, no significant associations of plasma homocysteine level with mRNFL thickness were observed. Our findings suggested that increased homocysteine might be a risk factor for decreased retinal thickness.
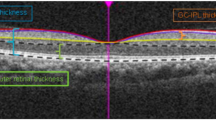
A A 80-year-old male with plasma homocysteine of 13.5 μmol/L. B A 87-year-old male with plasma homocysteine of 17.6 μmol/L. C A 86-year-old male with plasma homocysteine of 21.8 μmol/L. These examples are representative of results observed in this study that increased plasma homocysteine was associated with thinner GC-IPL.
There is still a lack of research exploring the precise measurement of retinal thickness and plasma homocysteine levels in older adults. Previous studies focused their attention on the association of homocysteine with age-related macular degeneration [23, 31], diabetic retinopathy [32,33,34] and glaucoma [24, 35]. Our results added complementary knowledge; we found a significant association between plasma homocysteine levels and GC-IPL thickness in healthy older adults, who were specifically not patients with a disease that directly influences retinal thickness. Notably, we used precise measurements of retinal thickness by SD-OCT to evaluate the associations. Our results provide direct evidence that increased plasma homocysteine concentrations were associated with a thinner GC-IPL.
Interestingly, clinical studies have illustrated the potential benefit of Hcy-lowering interventions (such as vitamin B and folic acid supplementation). A pilot study found that intervention with Metanx® vitamin tablets appeared to reduce retinal oedema and increase light sensitivity in subjects with nonproliferative diabetic retinopathy [36]. In addition, high vitamin B6 intake was associated with a lower incidence of diabetic retinopathy in Japanese patients with type 2 diabetes [37]. Similarly, participants with serum vitamin B-12 deficiency or folate deficiency had a higher risk of incident early and late AMD [31, 38], while participants who reported supplementary vitamin B-12 intake had a reduced risk of incident AMD (OR = 0.53; 95% CI: 0.33, 0.85) [31]. Therefore, homocysteine-lowering interventions may be beneficial for the retina. Conversely, similar to the unclear association of Hcy with CVD [39], the refined impact of Hcy-lowering therapy regarding the benefits of macular thickness needs to be validated in intervention studies in the future.
The most likely mechanism for the increased plasma homocysteine levels being associated with thinner macular GC-IPL is that Hcy induced disorder in the structure and function of RGCs. It has been demonstrated that Hhcy could induce death of RGCs in vitro. The potential mechanisms of death include dysregulation of mitochondrial dynamics [21], elevated intracellular calcium and increased oxidative stress in the form of increased superoxide and nitric oxide levels [20, 34, 40]. In in vivo studies, homozygous and heterozygous deletion of the cystathionine-β-synthase (cbs) gene in mice (resulting in increased plasma homocysteine and representing a model for severe homocysteinemia [41]) also had elevated retinal homocysteine [22]. The retinas of cbs+/− mice maintained on a high-methionine diet (cbs+/− HM) demonstrated an approximate 20% decrease in cells of the ganglion cell layer and increased expression of Aven, Egr1, and Bat3 in the retina [42]. Mthfr+/− mice with Hhcy also demonstrated reduced RGC function and a thinner ganglion cell layer [43, 44]. The lesion may be due to the death of RGCs caused by stimulation of n-methyl-D-aspartate receptors [45], reduced levels of NRF2 and the capacity of Müller cells to maintain RGCs in mice [20].
The association of plasma homocysteine with mRNFL thickness was not significant after adjusting for potential confounding factors. The RNFL is composed of RGC axons, while the GC-IPL is composed of RGC bodies and dendrites [35, 46]. We speculated that Hhcy-induced RGC death occurs over a gradual time course and that RNFL thickness may gradually decrease over long periods of time. A similar condition was found in previous studies on the association of retinal thickness with dementia [7] and obstructive sleep apnoea syndrome [47]. Thinner GC-IPL thickness rather than RNFL thickness was correlated with prevalent dementia and obstructive sleep apnoea syndrome in those studies. Due to the cross-sectional nature of our study in community-based older adults and the lack of published studies to compare and explore the reasons for the different roles of the GC-IPL and mRNFL in retinal thickness, it remains unclear whether the RNFL is associated with plasma homocysteine. Our study was the first to explore the relationship between plasma homocysteine levels and retinal thickness. More importantly, it illustrated the consistent and robust relationship between them.
Limitations
Several limitations were presented in this study. First, we conducted a cross-sectional study to evaluate the association of plasma homocysteine levels with retinal thickness. Our findings only suggested an association but not a causative relationship. Therefore, longitudinal studies should be conducted to validate our findings in the future. Second, the mean age of the participants was relatively high at 78.37 (4.53) years, and the mean plasma Hcy was as high as 16.38 (8.05) μmol/L in our study, which limits the generalizability of our results. Considering this, we performed sensitivity analyses after excluding participants with CVD and/or diabetes. Further studies should be conducted in younger populations to verify our conclusions. Third, a very large number of participants were excluded due to our strict inclusion criteria. Therefore, many uncontrolled factors might have affected our findings. Fourth, vitamin B and folic acid affect plasma Hcy in vivo. However, we could not eliminate the influence of these factors because of the lack of data in our study. Fifth, we measured and explored the relationship between macular thickness and plasma Hcy, but choroidal thickness was not measured in our study, which could be an interesting area of study in the future. Last, the multiple testing was not analyzed and it may increase false-positive results.
The strengths of our study included a community-based setting in older adults, a large sample size and extensive data on covariates.
Conclusions
In this cross-sectional study of 1060 older adults with SD-OCT measurements in the RuALS, increased plasma homocysteine levels were associated with a thinner GC-IPL after adjustment for demographic, physiological and clinical confounding factors. We provided direct evidence that increased plasma homocysteine levels are associated with a thinner GC-IPL. Plasma homocysteine may be a risk factor for a thinner GC-IPL.
Summary
What was known before
-
Plasma homocysteine levels are associated with macular thickness in patients with age-related macular degeneration, diabetic retinopathy and glaucoma.
What this study adds
-
Thinner ganglion cell layer plus inner plexiform layer (GC-IPL) rather than macular retinal nerve fibre layer (mRNFL) thickness was associated with increased plasma homocysteine levels in healthy older adults, who were specifically not patients with disease that directly influence the macular thickness.
References
Wolf S, Wolf-Schnurrbusch U. Spectral-domain optical coherence tomography use in macular diseases: a review. Ophthalmologica. 2010;224:333–40. https://doi.org/10.1159/000313814
Pasadhika S, Fishman GA, Choi D, Shahidi M. Selective thinning of the perifoveal inner retina as an early sign of hydroxychloroquine retinal toxicity. Eye. 2010;24:756–62. https://doi.org/10.1038/eye.2010.21
Kan E, Yakar K, Demirag MD, Gok M. Macular ganglion cell-inner plexiform layer thickness for detection of early retinal toxicity of hydroxychloroquine. Int Ophthalmol. 2018;38:1635–40. https://doi.org/10.1007/s10792-017-0635-y
Springelkamp H, Lee K, Wolfs RC, Buitendijk GH, Ramdas WD, Hofman A, et al. Population-based evaluation of retinal nerve fiber layer, retinal ganglion cell layer, and inner plexiform layer as a diagnostic tool for glaucoma. Investig Ophthalmol Vis Sci. 2014;55:8428–38. https://doi.org/10.1167/iovs.14-15506
Nakatani Y, Higashide T, Ohkubo S, Sugiyama K. Influences of the inner retinal sublayers and analytical areas in macular scans by spectral-domain OCT on the diagnostic ability of early glaucoma. Investig Ophthalmol Vis Sci. 2014;55:7479–85. https://doi.org/10.1167/iovs.14-15530
Pazos M, Dyrda AA, Biarnés M, Gómez A, Martín C, Mora C, et al. Diagnostic accuracy of spectralis SD OCT automated macular layers segmentation to discriminate normal from early glaucomatous eyes. Ophthalmology. 2017;124:1218–28. https://doi.org/10.1016/j.ophtha.2017.03.044
Mutlu U, Colijn JM, Ikram MA, Bonnemaijer PWM, Licher S, Wolters FJ, et al. Association of retinal neurodegeneration on optical coherence tomography with dementia: a population-based study. JAMA Neurol. 2018;75:1256–63. https://doi.org/10.1001/jamaneurol.2018.1563
López-de-Eguileta A, Lage C, López-García S, Pozueta A, García-Martínez M, Kazimierczak M, et al. Ganglion cell layer thinning in prodromal Alzheimer’s disease defined by amyloid PET. Alzheimer’s Dement. 2019;5:570–8. https://doi.org/10.1016/j.trci.2019.08.008
Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology. 2019;126:497–510. https://doi.org/10.1016/j.ophtha.2018.08.009
Casaletto KB, Ward ME, Baker NS, Bettcher BM, Gelfand JM, Li Y, et al. Retinal thinning is uniquely associated with medial temporal lobe atrophy in neurologically normal older adults. Neurobiol Aging. 2017;51:141–7. https://doi.org/10.1016/j.neurobiolaging.2016.12.011
Mutlu U, Bonnemaijer PWM, Ikram MA, Colijn JM, Cremers LGM, Buitendijk GHS, et al. Retinal neurodegeneration and brain MRI markers: the Rotterdam Study. Neurobiol Aging. 2017;60:183–91. https://doi.org/10.1016/j.neurobiolaging.2017.09.003
Mutlu U, Ikram MK, Roshchupkin GV, Bonnemaijer PWM, Colijn JM, Vingerling JR, et al. Thinner retinal layers are associated with changes in the visual pathway: a population-based study. Hum Brain Mapp. 2018;39:4290–301. https://doi.org/10.1002/hbm.24246
Méndez-Gómez JL, Pelletier A, Rougier MB, Korobelnik JF, Schweitzer C, Delyfer MN, et al. Association of retinal nerve fiber layer thickness with brain alterations in the visual and limbic networks in elderly adults without dementia. JAMA Netw Open. 2018;1:e184406 https://doi.org/10.1001/jamanetworkopen.2018.4406
Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol. 2000;32:385–9. https://doi.org/10.1016/s1357-2725(99)00138-7
Hannibal L, Blom HJ. Homocysteine and disease: causal associations or epiphenomenons? Mol Asp Med. 2017;53:36–42. https://doi.org/10.1016/j.mam.2016.11.003
Wong YY, Almeida OP, McCaul KA, Yeap BB, Hankey GJ, Flicker L. Homocysteine, frailty, and all-cause mortality in older men: the health in men study. J Gerontol Ser A Biol Sci Med Sci. 2013;68:590–8. https://doi.org/10.1093/gerona/gls211
Ma T, Sun XH, Yao S, Chen ZK, Zhang JF, Xu WD, et al. Genetic variants of homocysteine metabolism, homocysteine, and frailty—Rugao Longevity and Ageing Study. J Nutr Health Aging. 2020;24:198–204. https://doi.org/10.1007/s12603-019-1304-9
de Ruijter W, Westendorp RG, Assendelft WJ, den Elzen WP, de Craen AJ, le Cessie S, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083 https://doi.org/10.1136/bmj.a3083
Fan R, Zhang A, Zhong F. Association between homocysteine levels and all-cause mortality: a dose-response meta-analysis of prospective studies. Sci Rep. 2017;7:4769 https://doi.org/10.1038/s41598-017-05205-3
Navneet S, Zhao J, Wang J, Mysona B, Barwick S, Ammal Kaidery N, et al. Hyperhomocysteinemia-induced death of retinal ganglion cells: the role of Müller glial cells and NRF2. Redox Biol. 2019;24:101199 https://doi.org/10.1016/j.redox.2019.101199
Ganapathy PS, Perry RL, Tawfik A, Smith RM, Perry E, Roon P, et al. Homocysteine-mediated modulation of mitochondrial dynamics in retinal ganglion cells. Investig Ophthalmol Vis Sci. 2011;52:5551–8. https://doi.org/10.1167/iovs.11-7256
Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, et al. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circulation Res. 2001;88:1203–9. https://doi.org/10.1161/hh1101.092180
Pinna A, Zaccheddu F, Boscia F, Carru C, Solinas G. Homocysteine and risk of age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. 2018;96:e269–76. https://doi.org/10.1111/aos.13343
Xu XY, Xiao H, Luo JY, Liu X. Evaluation of spectral domain optical coherence tomography parameters in discriminating preperimetric glaucoma from high myopia. Int J Ophthalmol. 2019;12:58–65. https://doi.org/10.18240/ijo.2019.01.09
Srivastav K, Saxena S, Mahdi AA, Shukla RK, Meyer CH, Akduman L, et al. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol Vision. 2016;22:1352–60.
Liu Z, Wang Y, Zhang Y, Chu X, Wang Z, Qian D, et al. Cohort profile: the Rugao Longevity and Ageing Study (RuLAS). Int J Epidemiol. 2016;45:1064–73. https://doi.org/10.1093/ije/dyv101
Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34. https://doi.org/10.1016/s0161-6420(91)32186-9
Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs-an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98:786–806.
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, Verhoeven VJ, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877–83.e7. https://doi.org/10.1016/j.ajo.2015.01.022
Chen Q, He J, Yin Y, Zhou H, Jiang H, Zhu J, et al. Impact of the morphologic characteristics of optic disc on choroidal thickness in young myopic patients. Investig Ophthalmol Vis Sci. 2019;60:2958–67. https://doi.org/10.1167/iovs.18-26393
Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. Am J Clin Nutr. 2013;98:129–35. https://doi.org/10.3945/ajcn.112.057091
Brazionis L, Rowley K Sr., Itsiopoulos C, Harper CA, O’Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31:50–6. https://doi.org/10.2337/dc07-0632
Tawfik A, Mohamed R, Elsherbiny NM, DeAngelis MM, Bartoli M, Al-Shabrawey M Homocysteine: a potential biomarker for diabetic retinopathy. J Clin Med. 2019;8. https://doi.org/10.3390/jcm8010121
Dong N, Shi H, Tang X. Plasma homocysteine levels are associated with macular thickness in type 2 diabetes without diabetic macular edema. Int Ophthalmol. 2018;38:737–46. https://doi.org/10.1007/s10792-017-0528-0
Begum VU, Addepalli UK, Yadav RK, Shankar K, Senthil S, Garudadri CS, et al. Ganglion cell-inner plexiform layer thickness of high definition optical coherence tomography in perimetric and preperimetric glaucoma. Investig Ophthalmol Vis Sci. 2014;55:4768–75. https://doi.org/10.1167/iovs.14-14598
Smolek MK, Notaroberto NF, Jaramillo AG, Pradillo LR. Intervention with vitamins in patients with nonproliferative diabetic retinopathy: a pilot study. Clin Ophthalmol. 2013;7::1451–58. https://doi.org/10.2147/opth.S46718
Horikawa C, Aida R, Kamada C, Fujihara K, Tanaka S, Tanaka S, et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: analysis of data from the Japan Diabetes Complications Study (JDCS). Eur J Nutr. 2020;59:1585–94. https://doi.org/10.1007/s00394-019-02014-4
Rochtchina E, Wang JJ, Flood VM, Mitchell P. Elevated serum homocysteine, low serum vitamin B12, folate, and age-related macular degeneration: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:344–6. https://doi.org/10.1016/j.ajo.2006.08.032
Martí-Carvajal AJ, Solà I, Lathyris D, Dayer M. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2017;8:Cd006612 https://doi.org/10.1002/14651858.CD006612.pub5
Ganapathy PS, White RE, Ha Y, Bozard BR, McNeil PL, Caldwell RW, et al. The role of N-methyl-D-aspartate receptor activation in homocysteine-induced death of retinal ganglion cells. Investig Ophthalmol Vis Sci. 2011;52:5515–24. https://doi.org/10.1167/iovs.10-6870
Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. https://doi.org/10.1073/pnas.92.5.1585
Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, et al. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Investigative Ophthalmol Vis Sci. 2009;50:4460–70. https://doi.org/10.1167/iovs.09-3402
Markand S, Saul A, Roon P, Prasad P, Martin P, Rozen R, et al. Retinal ganglion cell loss and mild vasculopathy in methylene tetrahydrofolate reductase (Mthfr)-deficient mice: a model of mild hyperhomocysteinemia. Investig Ophthalmol Vis Sci. 2015;56:2684–95. https://doi.org/10.1167/iovs.14-16190
Markand S, Saul A, Tawfik A, Cui X, Rozen R, Smith SB. Mthfr as a modifier of the retinal phenotype of Crb1(rd8/rd8) mice. Exp Eye Res. 2016;145:164–72. https://doi.org/10.1016/j.exer.2015.11.013
Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1997;94:5923–8. https://doi.org/10.1073/pnas.94.11.5923
Shin HY, Park HL, Jung KI, Choi JA, Park CK. Glaucoma diagnostic ability of ganglion cell-inner plexiform layer thickness differs according to the location of visual field loss. Ophthalmology. 2014;121:93–9. https://doi.org/10.1016/j.ophtha.2013.06.041
Kara N, Sayin N, Bayramoglu SE, Savas AU. Peripapillary retina nerve fiber layer thickness and macular ganglion cell layer thickness in patients with obstructive sleep apnea syndrome. Eye. 2018;32:701–6. https://doi.org/10.1038/eye.2017.279
Acknowledgements
We acknowledge all participants involved in the present study from the RuLAS. We acknowledge the support from the government of Rugao, the Public Health Bureau of Jiang’an township and Rugao city, the Bureau of Civil Affairs, the Rugao People’s Hospital and all the local village physicians.
Funding
This work was financially supported by grants from the National Key R&D Program of China (2018YFC2000400), the National Natural Science Foundation of China (81571372), the National Key R&D Program of China (2016YFC0904800, 2019YFC0840607), the National Science and Technology Major Project of China (2017ZX09304010), and the Natural Science Foundation of China (81970810). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HZ, HS, XX, DL and XW: study design, interpretation of results, preparation and editing manuscript. HZ: data analysis. JW and XJ: study design. HZ, HS, XS, XX, DL, GW and XW: data collection. All authors were involved in the preparation of the manuscript and final approval of the submitted and published versions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, H., Shen, H., Gong, W. et al. Plasma homocysteine and macular thickness in older adults—the Rugao Longevity and Aging Study. Eye 36, 1050–1060 (2022). https://doi.org/10.1038/s41433-021-01549-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01549-3