Abstract
Purpose
To evaluate choroidal vasculature changes after the instillation of mydriatic parasympatholytic and sympathomimetic agents in healthy subjects.
Methods
A total of 95 healthy subjects were enrolled in this prospective, randomized comparative study. Study participants were divided into three different groups depending on the drug to be administered: tropicamide (1%) group (n = 31), tropicamide (0.5%) + phenylephrine (10%) group (n = 30) and control group receiving artificial tears (n = 34). All participants underwent a complete ophthalmological examination including best corrected visual acuity, refractive status and axial length. Subfoveal choroidal thickness (CT), total choroidal area (TCA), luminal and stromal choroidal area (LCA and SCA) and choroidal vascularity index (CVI) were measured before and after eye drops instillation.
Results
All the baseline characteristics were matched between the three groups (all P > 0.05). Before the mydriatic instillation, there were no significant differences of CT, TCA, LA, SCA, and CVI among the three groups (all P > 0.05). After drug administration, CT, TCA, LCA, SCA, and CVI did not show any significant change as well (respectively, P = 0.265; P = 0.483; 0.573; P = 0.405 and P = 0.708).
Conclusions
Instillation of mydriatic eye drops did not induce significant changes of the choroidal vasculature, suggesting that their use do not alter CT and CVI evaluation.
Similar content being viewed by others
Introduction
The choroid is a highly vascularised tissue between the retina and the sclera contributing to the majority of oxygen and other nutrients supply to the retinal pigment epithelium and the outer retina [1]. The autonomic innervation of the choroid is mediated by sympathetic and parasympathetic pathways [2]. Vascular endothelial cells contain α adrenergic receptors, whose activation by sympathetic system induces a vasoconstriction, causing a decrease in choroidal thickness (CT) [3]. There is also evidence of a parasympathetic innervation to choroidal non-vascular smooth muscle cells [2].
Mydriatic eye drops, as parasympatholytic and sympathomimetic agents, are commonly used for dilated fundoscopy examination, surgery, and cycloplegia, and they may influence the choroidal vascularity. The effect of mydriatic agents on CT has been investigated with contradictory findings. Some authors reported a significant choroidal thinning associated with both anticholinergic and sympathomimetic agents [4, 5]. Conversely, Sander et al. reported no significant changes in CT after phenylephrine instillation, and a choroidal thickening after 2% homatropine use in healthy adults [3]. The instillation of a tropicamide + phenylephrine combination (Mydrin P) showed no variations in CT [6].
Albeit the enhanced-depth imaging (EDI) modality of optical coherence tomography (OCT) allows an accurate analysis of the choroid [7], CT measurement only reflects the total choroidal vasculature with no distinctions between the stromal and luminal vascular components. Agrawal et al. proposed a new quantitative parameter called choroidal vascularity index (CVI) as a measure of vasculature status of the choroid in healthy eyes [8]. To date, there has been limited investigation on quantitative changes of choroidal vasculature induced by mydriatic agents. Therefore, the aim of the study was to evaluate possible changes of luminal and stromal choroidal areas (LCA and SCA) and of CVI after the instillation of mydriatic parasympatholytic and sympathomimetic mydriatic agents.
Methods
This was a prospective, randomized, and comparative study conducted at the Retina Center of the Eye Clinic, University of Cagliari, Italy. The investigation was approved by the Office of Research Ethics, University of Cagliari and performed according to the guidelines of the Declaration of Helsinki. After receiving a detailed explanation of the study, written informed consent was obtained from all participants before examination.
Patients and clinical examination
Ninety-five healthy subjects were recruited into this comparative study between July 2019 and November 2019. All subjects were screened for the presence of any ocular disease through a complete ophthalmologic examination encompassing best corrected visual acuity (BCVA), slit-lamp biomicroscopy, intraocular pressure, and fundus examination.
Exclusion criteria were patients younger than 18 or older than 70 years, history of any systemic and ophthalmic disease, previous ocular surgery, pregnancy and a spherical equivalent refractive error greater than −6 D or +3 D. In addition, patients with media opacities that could influence image quality were also excluded from the study.
Participants were randomly divided into three groups based on the application of different eye drops and only the right eye was selected for the analysis. Subjects receiving topical tropicamide 1% two times at 10 min interval were referred to as the tropicamide group, while subjects receiving topical tropicamide 0.5% + phenylephrine 10% two times at 10 min interval were defined as tropicamide + phenylephrine group. A third group named as control group received a drop of artificial tears twice at 10 min interval. All individuals required a BCVA of 20/25 or better. Axial length was measured using IOL master (Carl Zeiss Meditec, Dublin, CA) only before the mydriatics instillation.
OCT analysis
A horizontal 30° × 20° volume scan was obtained for all study eyes before and 45 min after the instillation of eye drops. Subfoveal CT was manually measured on the horizontal foveal OCT B scan using the calliper tool of the built-in automated software (Heidelberg Eye Explorer HEYEX; Heidelberg Engineering). Specifically, it was defined as the subfoveal vertical distance between the Bruch’s membrane interface and the sclerochoroidal junction. All OCT examinations were performed between 10:00 and 12:00 am to minimize the possibility of CT variations attributable to diurnal CT fluctuations.
The observer who measured subfoveal CT (F.T.) was masked as to what mydriatic was used.
The CVI was calculated using the previously reported automated algorithm [9], that included initial denoising with localization of the choroidal inner and outer boundary [10,11,12].
To allow computation of LCA and SCA, the OCT B-scan passing through the fovea was binarized and choroidal components were segmented. Automated binarization process included exponential and non-linear enhancement, and thresholding. The bright regions were labeled as SCA and the dark regions as LCA. Total choroidal area (TCA) was measured as the sum of the SCA and LCA, and the CVI was calculated as the ratio of LCA over TCA.
Statistical analysis
Data analysis was performed using the statistical package Statistical Package for the Social Sciences (SPSS) version 16. Spearman correlation analysis was used to examine the relationships among the measured variables. A one-way ANOVA was used to compare baseline continuous variables among the 3 groups. A mixed ANOVA was used to compare continuous variables before and after instillation of eye drops, using the type of eye drops as the between subjects factor. Intergroup differences were analysed using the post hoc Tukey’s test. Values of P < 0.05 were considered statistically significant.
Results
A total of 95 participants were randomly assigned to the tropicamide group (n = 31), the tropicamide + phenylephrine group (n = 30) and the control group (n = 34). All demographical and clinical characteristics of the study participants are summarized in Table 1. No significant differences of age, sex, axial length, and spherical equivalent among the three groups were observed (all P > 0.05).
All choroidal parameters in the three groups before and after the instillation of eye drops are reported in Table 2. No significant differences of CT, TCA, LCA, SCA, and CVI among the three groups were observed (all P > 0.05) before the administration of the drops (Figs. 1–3).
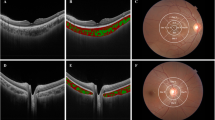
Enhanced-depth imaging OCT B-Scans and corresponding binarized images in a 30-year old man before (respectively, a and b) and after (respectively, c and d) tropicamide eye drops instillation. Subfoveal choroidal thickness and choroidal vascularity index were, respectively, 295 μm and 58.48% at baseline and 291 μm and 58.24% after drops instillation.
Enhanced-depth imaging OCT B-Scans and corresponding binarized images in a 55-year old man before (respectively, a and b) and after (respectively, c and d) tropicamide + phenylephrine eye drops instillation. Subfoveal choroidal thickness and choroidal vascularity index were, respectively, 258 μm and 68.64% at baseline and 256 μm and 67.77% after drops instillation.
Enhanced-depth imaging OCT B-Scans and corresponding binarized images in a 45-year old woman man before (respectively, part a and part b) and after (respectively, part c and part d) artificial tears instillation. Subfoveal choroidal thickness and choroidal vascularity index were, respectively, 330 μm and 58.21% at baseline and 329 μm and 58.22% after drops instillation.
After the instillation of eye drops, CT, TCA, LCA, SCA, and CVI did not show any significant change (respectively, P = 0.265; P = 0.483; P = 0.573; P = 0.405; and P = 0.708). In particular, CVI values before and after the eye drops instillation, respectively, were 64.25 ± 4.59% and 64.81% ± 4.85 in the tropicamide group, 62.80 ± 3.75 and 63.27 ± 3.82 in the tropicamide + phenylephrine group, and 65.58 ± 4.67 and 64.96 ± 4.66 in the control group.
Regarding correlation analysis, in the overall group, age was negatively correlated with CT (R = −0.256, P = 0.015) but not with CVI (P = 0.796). Similarly, spherical equivalent was correlated with CT (R = 0.229, P = 0.026) but not with CVI (P = 0.957).
Discussion
In the present study, we evaluated the effect of both sympathomimetic and parasympatholytic agents on the choroidal vasculature by measuring subfoveal CT and CVI. Mydriatic agents are widely used in ophthalmologic practise to allow a better evaluation of refraction, to dilate pupil before fundus and retinal imaging examinations and before surgery. Tropicamide is a parasympatholytic agent with antimuscarinic effects. Phenylephrine is an α-agonist agent with sympathomimetic properties and in Italy it is available in combination with 0.5% tropicamide. As a α-agonist, phenylephrine promotes the contraction of the choroidal vasculature bed due to vasoconstricting effects and the contraction of non-vascular smooth muscle cells innervated by sympathetic system [13]. Tropicamide as an antimuscarinic molecule affects the choroidal vasculature by inducing a posterior movement of the ciliary muscle and decreasing mechanical traction after cycloplegia [5]. Furthermore, non-vascular smooth muscle cells are thought to be innervated by both sympathetic and parasympathetic inputs [14, 15].
From a theoretical point of view, choroidal structure may show some changes following the mydriatic agents use. We found no significant differences in terms of CT, TCA, LCA, SCA, and CVI between subjects receiving tropicamide alone or tropicamide + phenylephrine and control group. As expected, the group receiving the combination of tropicamide 0.5% + phenylephrine 10% with both sympathomimetic and parasympatholytic properties, showed the highest variation of the CT (4.8 μm) but still not significant.
Prior literature shows inconsistent findings on CT variations after the use of mydriatic eye drops. Regarding antimuscarinic agents effects, several authors reported a significant increase of subfoveal CT after homatropine [3] and cyclopentolate 1% use [16]. By contrast, a choroidal thinning was reported after instillation of tropicamide 1% [4, 5], and cyclopentolate 1% [5]. Öner et al. reported no variation of CT after tropicamide administration [16]. Among adrenergic molecules, phenylephrine 2.5% was associated with choroidal thinning [4, 5, 13]. Nevertheless, Sander et al. found no variation in CT after phenylephrine 2.5% use [3]. Only Kim and co-workers analysed the potential influence of a combination of tropicamide and phenylephrine (Mydrin-P) on the CT, reporting no changes after the eye drops instillation [6]. The lack of any significant variation of the choroidal structure in our study, was further confirmed by the evaluation of the CVI.
Since CVI is emerging as a new imaging tool for the analysis of the choroidal vascular system [17, 18], it is important to assess whether it is influenced by dilations eye drops or not. The automated or semi-automated software used to measure CVI provide the capability to calculate quantitative parameters of the choroid and stratify the stromal and vascular components. To date, CVI has been proved to be very useful in both healthy [8, 19,20,21], and pathological eyes [22,23,24,25,26]. Current literature suggests that CVI has a lesser variability and is influenced by fewer physiologic factors as opposed to CT [8, 27]. Indeed, although CT is considered a robust tool in clinical research, it only reflects the total choroidal vasculature with no distinctions between the two stromal and luminal vascular components [28,29,30]. Moreover, CT is manually measured and therefore errors of few microns should be taken into account.
To our knowledge, this is the first study to investigate the potential effects of topical mydriatics on the choroidal vasculature by means of CVI evaluation. The automatic software used in the present study, reduces at least the possibility of measurements inaccuracies.
Limitations of the current study include the relatively small number of study participants. In addition, we did not determine CT at different points, but the TCA evaluation that reflects the entire choroidal area of the OCT scan lessens this limitation. Lastly, we did not perform a volumetric analysis of the macular area, though it has been demonstrated that CVI measured from a single scan encompassing the fovea is representative of the whole posterior pole choroidal vascularity [12].
In conclusion, this study provided evidence that both tropicamide and tropicamide + phenylephrine topical administration do not induce significant variations of the choroidal vasculature. CVI can be measured with reliability with pupil dilation. Further studies are warranted to investigate the influence of different mydriatics, or mydriatics with different concentrations in patients with chorioretinal diseases.
Summary
What was known before
-
Mydriatics do not affect choroidal thickness.
What this study adds
-
Tropicamide and phenylephrine do not induce variations of choroidal vasculature.
-
Choroidal vascularity index can be measured with reliability after pupil dilation.
References
Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–68.
Lütjen-Drecoll E. Choroidal innervation in primate eyes. Exp Eye Res. 2006;82:357–61.
Sander BP, Collins MJ, Read SA. The effect of topical adrenergic and anticholinergic agents on the choroidal thickness of young healthy adults. Exp Eye Res. 2014;128:181–9.
Kara N, Demircan A, Karatas G, Ozgurhan EB, Tatar G, Karakucuk Y, et al. Effects of two commonly used mydriatics on choroidal thickness: direct and crossover effects. J Ocul Pharmacol Ther. 2014;30:366–70.
Yuvac I, Pangal E, Yuvac S, Bayram N, Ataş M, Başkan B, et al. An evaluation of effects of different mydriatics on choroidal thickness by examining anterior chamber parameters: the scheimpflug imaging and enhanced depth imaging-OCT study. J Ophthalmol. 2015;2015:981274.
Kim M, Kwon HJ, Lee SC. Influence of mydriatics on choroidal thickness measurement using enhanced depth imaging-OCT. Optom Vis Sci. 2012;89:1150–5.
Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008;146:496–500.
Agrawal R, Gupta P, Tan KA, Cheung CMG, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;6:21090.
Vupparaboina KK, Nizampatnam S, Chhablani J, Richhariya A, Jana S. Automated estimation of choroidal thickness distribution and volume based on OCT images of posterior visual section. Comput Med Imaging Graph. 2015;46:315–27.
Rasheed MA, Sahoo NK, Goud A, Vupparaboina KK, Chhablani J. Qualitative comparison of choroidal vascularity measurement algorithms. Indian J Ophthalmol. 2018;66:1785–9.
Tan R, Agrawal R, Taduru S, Gupta A, Vupparaboina K, Chhablani J. Choroidal vascularity index in retinitis pigmentosa: an OCT study. Ophthalmic Surg, Lasers Imaging Retin. 2018;49:191–7.
Agrawal R, Wei X, Goud A, Vupparaboina KK, Jana S, Chhablani J. Influence of scanning area on choroidal vascularity index measurement using optical coherence tomography. Acta Ophthalmol. 2017;95:e770–5.
Casado A, López-de-Eguileta A, Gaitán J, Fonseca S, Gordo-Vega MA. Peripapillary and macular choroidal thickness before and after phenylephrine instillation. Eye. 2019;33:1741–7.
Schrödl F, De Laet A, Tassignon MJ, Van Bogaert PP, Brehmer A, Neuhuber WL, et al. Intrinsic choroidal neurons in the human eye: Projections, targets, and basic electrophysiological data. Investig Ophthalmol Vis Sci. 2003;44:3705–12.
May CA, Neuhuber W, Lütjen-Drecoll E. Immunohistochemical classification and functional morphology of human choroidal ganglion cells. Investig Ophthalmol Vis Sci. 2004;45:361–7.
Öner V, Bulut A, Öter K. The effect of topical anti-muscarinic agents on subfoveal choroidal thickness in healthy adults. Eye. 2016;30:925–8.
Agrawal R, Ding J, Sen P, Rousselot A, Chan A, Nivison-Smith L, et al. Exploring choroidal angioarchitecture in health and disease using choroidal vascularity index. Prog Retin Eye Res. 2020;10:100829.
Iovino C, Pellegrini M, Bernabei F, Borrelli E, Sacconi R, Govetto A, et al. Choroidal vascularity index: an in-depth analysis of this novel optical coherence tomography parameter. J Clin Med. 2020;9:595.
Yip VCH, Laude A, Tan KA, Ding J, Wong E, Agrawal R. A longitudinal study of choroidal changes following cataract surgery in patients with diabetes. Diabetes Vasc Dis Res. 2019;16:369–77.
Singh SR, Invernizzi A, Rasheed MA, Cagini C, Goud A, Vupparaboina KK, et al. Wide-field choroidal vascularity in healthy eyes. Am J Ophthalmol. 2018;193:100–5.
Singh SR, Rasheed MA, Goud A, Sahoo NK, Vupparaboina KK, Chhablani J. Diurnal variation in subfoveal and peripapillary choroidal vascularity index in healthy eyes. Indian J Ophthalmol. 2019;67:1667–72.
Agrawal R, Chhablani J, Tan KA, Shah S, Sarvaiya C, Banker A. Choroidal vascularity index in central serous chorioretinopathy. Retina. 2016;36:1646–51.
Ratra D, Tan R, Jaishankar D, Khandelwal N, Gupta A, Chhablani J, et al. Choroidal structural changes and vascularity index in stargardt disease on swept source optical coherence tomography. Retina. 2018;38:2395–400.
Iovino C, Au A, Hilely A, Violanti S, Peiretti E, Gorin MB, et al. Evaluation of the choroid in eyes with retinitis pigmentosa and cystoid macular edema. Investig Opthalmology Vis Sci. 2019;60:5000.
Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Moscardelli F, Iovino C, et al. Choroidal vascularity index quantification in geographic atrophy using binarization of enhanced-depth imaging optical coherence tomographic scans. Retina. 2020;40:960–5.
Pellegrini M, Giannaccare G, Bernabei F, Moscardelli F, Schiavi C, Campos EC. Choroidal vascular changes in arteritic and nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2019;205:43–49.
Oh J, Baik DJ, Ahn J. Inter-relationship between retinal and choroidal vasculatures using optical coherence tomography angiography in normal eyes. Eur J Ophthalmol. 2018;30:48–57.
Tan KA, Gupta P, Agarwal A, Chhablani J, Cheng CY, Keane PA, et al. State of science: choroidal thickness and systemic health. Surv Ophthalmol. 2016;61:566–81.
Sezer T, Altınışık M, Koytak İA, Özdemir MH. The choroid and optical coherence tomography. Turk Oftalmoloiji Derg. 2016;46:30–37.
Laviers H, Zambarakji H. Enhanced depth imaging-OCT of the choroid: a review of the current literature. Graefe’s Arch Clin Exp Ophthalmol. 2014;252:1871–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iovino, C., Chhablani, J., Rasheed, M.A. et al. Effects of different mydriatics on the choroidal vascularity in healthy subjects. Eye 35, 913–918 (2021). https://doi.org/10.1038/s41433-020-0995-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-0995-9
This article is cited by
-
Influence of protocol scan on choroidal vascularity measurements: a spectralis optical coherence tomography study
Eye (2023)
-
Corneal morphology correlates with choriocapillaris perfusion in myopic children
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)