Abstract
Background
To evaluate the relationship between superficial, deep foveal avascular zone (FAZ) and foveal cyst areas in eyes with cystoid macular oedema (CMO) associated with gyrate atrophy of the choroid and retina (GA).
Methods
This is a retrospective collaborative multicenter study of optical coherence tomography-angiography (OCTA) images in GA. Superficial and deep FAZ and foveal cyst were measured using Image J by two independent experts. Values were corrected for myopia magnification. These values were compared with age-matched controls from normative data.
Results
Twenty-three eyes from 12 patients with GA and CMO were included in the study. The mean ± standard deviation age was 22 ± 19.7 years, mean Snellen spectacle-corrected visual acuity of 20/70 with mean myopia of 5.7 ± 4.1 dioptres. Qualitatively, no focal occlusion of superficial and deep capillary plexus was noted. Mean superficial FAZ area (0.484 ± 0.317 mm2), deep FAZ area (0.626 ± 0.452 mm2), and foveal cyst area (0.630 ± 0.503 mm2) were significantly larger than superficial and deep FAZ areas in controls of same age range (p < 0.001). Macular cyst area correlated with superficial FAZ area (R = 0.59; p = 0.0057) and more strongly with deep FAZ area (R = 0.69; p < 0.001).
Conclusions
The superficial and deep FAZ area in GA-associated CMO were noted to be significantly larger than in controls. It seems that RPE dysfunction leads to foveal cyst enlargement displacing the capillary plexus with resultant enlarged superficial and deep FAZ area.
Similar content being viewed by others
Introduction
Gyrate atrophy of the choroid and retina (GA) is a rare autosomal recessive chorioretinal degeneration caused by a mutation in the ornithine-δ-amino transferase (OAT) gene. OAT is a vitamin B6 dependent nuclear-encoded mitochondrial enzyme with the function to catalyse the interconversion of ornithine, glutamate and proline. Two clinical types of GA exist based on response to vitamin B6: 5% of patients respond to vitamin B6 and display less severe disease with slower progression compared with the rest (95%) that do not [1]. Characteristic sharply demarcated, scalloped areas of chorioretinal atrophy are noted at the equator. Visual decline in late childhood results from progressive myopia and nyctalopia [2,3,4,5,6,7,8,9,10,11,12,13,14]. Considerable variability is observed in the age at which visual acuities begin to decrease [2]. Posterior subcapsular cataract and cystoid macular oedema (CMO) start within the first 3 decades of life. GA may lead to legal blindness (visual acuity less than 6/60 or 20/200) in the fourth to seventh decades. Our study aims at further characterising the foveal anatomy and vasculature of GA-related CMO using noninvasive depth-resolved imaging of the microvasculature in the retina, optical coherence tomography-angiography (OCTA).
Methods
This is a retrospective multicenter study of optic coherence tomography angiography (OCTA) scans in GA cases with CMO. This study adhered to the tenets of the Declaration of Helsinki. This was approved by the Institutional Review Board at Rafic Hariri University Hospital in Beirut (RHUH-362020) and by the Ethical Committee of the Vita-Salute San Raffaele University in Milan (NET-2016-02363765). Signed informed consent for the examinations was obtained from each patient. Inclusion criteria included: (1) Diagnosis of GA based on the characteristic clinical picture (peripheral circumferential sharply demarcated round patches of chorioretinal atrophy) and the presence of hyperornithinemia; (2) Macular scans, usually 3 × 3 mm volume, were measured at presentation before administration of topical, oral, or intravitreal therapies and before cataract surgery. Qualitatively, all scans (3 × 3 mm) were evaluated for evidence of focal capillary occlusion. Foveal avascular zone (FAZ) area (mm2) was defined as the avascular area in the centre of the fovea, and the border of the FAZ was manually measured in each patient in both the superficial capillary plexus (SCP) (from inner limiting membrane to 8 μm below inner plexiform layer) and deep capillary plexus layers (DCP) (13–88 μm below the inner plexiform layer). Images from OCT and OCTA were scaled to mm and measured manually using Image J (to correct for differences between machines). Three measurements were obtained for each image and the average was calculated. This was done by 2 experienced investigators (AGE, C-JM) independently. The average of the two experienced graders was taken for statistical analysis after correcting for the magnification factor induced by myopia [15]. The horizontal diameter of the macular cyst was measured on horizontal OCT scan and the foveal cyst area was calculated as π x radius2. Exclusion criteria were patients with other retinal vascular disease and excessive OCTA artifacts affecting the FAZ measurements such as motion artifacts and low signal strengths.
Patients macular values were compared to age-matched and dioptre-matched normal controls, age-matched and dioptre-matched eyes with retinitis pigmentosa (RP) and CMO, and to age-matched eyes with choroideremia (CHM) retrieved from one series from the literature [16]. Controls and RP data were acquired by means of swept-source Triton Topcon device (Topcon, Tokyo, Japan) and volume of 3 × 3 mm.
Statistical analyses were performed using SPSS version 22 (IBM, Chicago, IL). Paired samples statistics were performed with Pearson correlations. Data are presented as means ± standard deviations (SD). Spectacle corrected visual acuity was converted to the logarithm of the minimum angle of resolution (logMAR). Correlation was calculated between vision, central macular thickness (CMT), FAZ area and macular cyst area. Intraclass correlation coefficient (ICC) was used to assess inter-rater reliability of FAZ and cyst area measurements. For all these analyses, values of p ≤ 0.05 were considered statistically significant.
Results
A total of 23 eyes from 12 patients were collected from 6 medical centres. Mean age ± SD was 22 ± 19.7 years (median 14.5; range 6–76) with 7 female and 5 male patients. The patients were equally divided between Asian and Caucasian (6 each) race. Mean presenting spectacle-corrected visual acuity was 20/70 (median 20/63; range 20/25–20/200). Mean myopia was −5.7 ± 4.1 D (median: −5D; range 0–17D). Macular vascular anatomy was tested using Angiovue (Optovue, Fremont, CA) in 9 eyes, swept-source OCT angiography (Topcon, Tokyo, Japan) in 12 eyes and Spectralis OCT angiography (Heidelberg Engineering, Heidelberg, Germany) in 2 eyes.
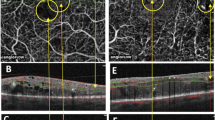
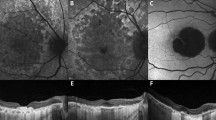
Qualitatively no focal occlusion of superficial capillary plexus (SCP) and deep capillary plexus (DCP) was noted (Fig. 1). Mean CMT was 525.6 ± 167.6 μm, superficial FAZ area 0.484 ± 0.317 mm2, deep FAZ area 0.626 ± 0.452 mm2 and macular cyst area 0.630 ± 0.503 mm2 (Table 1). Correlations were noted between best spectacle-corrected visual acuities and the following: central macular thickness (CMT) (R = 0.73; p < 0.001), superficial FAZ area (R = 0.095; p = 0.67), deep FAZ area (R = 0.27; p = 0.21), and foveal cyst area (R = 0.45; p = 0.044). Correlations between macular cyst area and the following were: CMT (R = 0.30; p = 0.21), superficial FAZ area (R = 0.59; p = 0.0057), and deep FAZ area (R = 0.69; p < 0.001) (Figs. 2, 3). Similarly, in eyes with RP with CMO, the macular cyst area correlated highly with the deep FAZ area (Table 1).
The superficial foveal avascular zone (a) is very small on 3 × 3 mm OCTA map. The superficial and capillary plexuses were well preserved with no focal losses. Fundus photograph of the macula (b) shows prominent cystoid spaces well delineated on linear OCT scan (c). Autofluorescence shows sparing of the macula. The inset (b) shows the classical scalloped areas of chorioretinal degeneration advancing from the periphery. Fluorescein transits of the equator and macula (d) shows diffuse chorioretinal atrophy sparing a sector of the midperiphery and macula.
Inter-observer reliability was excellent for FAZ area measurements. ICCs are expressed with the 95% confidence intervals in parentheses as follows: 0.929 (0.84–0.97) for superficial FAZ area, 0.945 (0.88–0.98) for deep FAZ area, and 0.984 (0.96–0.99) for macular cyst area, with all ICCs significantly different from zero (p < 0.001).
Superficial and deep FAZ areas in GA with CMO and below age 40 were found to be significantly larger than in normal eyes (age below 40) or in eyes with choroideremia CHM without maculopathy (age below 40) (Table 2). Eyes with RP CMO behaved in a similar way to GA with CMO with enlarged superficial and deep FAZ in both conditions more so in the GA disease. In contrast eyes of young patients with CH had smaller superficial and deep FAZ than in normal, RP with CMO or GA with CMO. Fluorescein angiography was available in 16 eyes and revealed no leakage of dye into the macula. Correlation between fluorescein angiography of macula and OCTA was hampered by increased xanthophyll in GA impeding view of the deep fovea and demonstrated by blockage on fundus autofluorescence (Fig. 1).
Discussion
CMO in GA and other chorioretinal dystrophies is a cause for early visual loss and can be diagnosed with spectral-domain OCT. Many of such cases may not show a macular petaloid leakage pattern on fluorescein angiogram, suggesting the diagnosis of foveoschisis [7]. CMO may result from retinal pigment epithelium (RPE)-Müller cell dysfunction with a breach in the integrity of the blood-retinal barrier and accumulation of fluid in the macular area [17, 18]. In the present study, best spectacle-corrected visual acuity correlated with CMT and macular cyst area; also, the macular cyst area strongly correlated with the area of the deep FAZ. Accordingly, we speculate that cyst distension mechanically displaces the foveal vasculature, hence the apparently enlarged FAZ in GA with CMO and also in RP with CMO. We did not detect evidence of a primary occlusive disorder of the SCP or the DCP in GA. The changes in the equatorial area (i.e. chorioretinal atrophy) do not occur in the fovea at a young age. Wilson et al. [19] documented an abrupt transition from a normal-appearing retina to a zone of near-total atrophy of the retina, RPE, and choroid in the fundus midperiphery of an autopsy eye in a 98-year-old woman with GA. The posterior pole had focal areas of photoreceptor atrophy with underlying RPE hyperplasia, but this finding could also relate to an aging process. Similar late-stage retinal histopathology was described in an adult domestic cat with OAT deficiency [20].
Braham et al. [7] noted perifoveal microvascular alterations with choriocapillaris changes in GA. OCTA revealed apparent areas of capillary drop out in the macula at the level of the SCP and DCP not seen on fluorescein angiography [14]. These changes were attributed to ischaemic macular changes secondary to neurodegenerative disorder. Raval et al. [6] noted macular schisis present in both the inner and outer retinal layers in the parafoveal region with enlargement of the FAZ area in both the SCP and DCP layers. Raval et al. [6] noted an apparent loss of the capillary bed in the DCP at the central foveal areas. These findings were in accordance with the hypothesis of RPE degeneration with photoreceptor loss as the earliest changes in the pathogenesis of GA.
OAT is a mitochondrial enzyme serving to form glutamate from ornithine in the liver, brain, and kidney, and serving to form citrulline or arginine in the intestine. Its main function is to control the production of signalling molecules and mediators, such as glutamate, citrulline, and gamma-aminobutyric acid, in the retina and elsewhere. OAT utilizes pyridoxal 5-phosphate which is the active form of vitamin B6 as a co-factor and is expressed in most tissues, including the retina [17]. Deficiency of the enzyme OAT results in a tenfold rise in plasma ornithine, which is toxic to the RPE and choroid. In vivo studies using a mouse model of GA has shown the earliest pathological changes to be in the RPE cells. It appears that the RPE is sensitive to ornithine accumulation, and the OAT gene is expressed at higher levels in the RPE than in the photoreceptors [17]. The focal degeneration and malfunction of the RPE cells likely results in overlying photoreceptor abnormalities and secondary choriocapillaris atrophy. The regional pattern of photoreceptor degeneration in the OAT-deficient mice resembles the patterns found in other toxic and inherited conditions in which the RPE cells are the primary site of pathology, such as the Royal College of Surgeons rat [17]. Three to 7 days following intravitreal injection of 1-ornithine hydrochloride into monkeys, Takeuchi et al. [21] noted RPE oedema by fundus photography, and hyperfluorescence of the equator without retinal changes at the posterior pole by fluorescein angiography. Histologically, equatorial RPE showed severe damage while minimal RPE and inner retina changes were seen in the posterior pole.
No accessible and known therapy halts the photoreceptor cell degeneration in inherited retinopathies. Nonetheless, the CMO that occurs in such patients can be often managed. The macular region has a predilection to develop these changes because of its unique structural features that include the absence of multiple supportive retinal layers. RPE dysfunction results in disrupted outer blood-retinal barrier that is responsible for diffusion of intraretinal fluid in various retinal dystrophies. However, many such cases show no petaloid leakage on fluorescein angiography suggesting foveoschisis [7]. Other proposed minor causes for intraretinal fluid accumulation have included tangential vitreous forces and disruption of the retinal cell-to-cell adhesion. Arginine-restricted diet [5, 11] and vitamin B6 supplementation [5, 11] can be effective but when this strategy alone fails [12, 13], additional approaches have been used to treat the intraretinal cysts in GA, including carbonic anhydrase inhibitors [8, 9], topical non-steroid anti-inflammatory drugs [8, 9], and intravitreal (or periocular [4]) corticosteroid [3] or vascular endothelial growth factor antagonists [14], either separately or in combination. Results using these other agents have shown variable and transient efficacy depending on the stage of the disease, with a better response seen in early stages of the disease and little response in advanced stages. This variable response to these alternative therapies could also be explained by the small sample size, short follow-up, different diet regimens, variable compliance, and genetic differences [22].
Retinitis pigmentosa (RP), choroideremia (CHM), and GA are the most important retinal dystrophy subtypes [22,23,24,25,26,27] with prevalent peripheral involvement. Battaglia Parodi et al. [28] noted a normal superficial capillary plexus and more involvement of the DCP, esp. in RP. This was confirmed by projection-resolved OCTA: Hagag et al. [29] verified the preservation of the superficial capillary plexus while most of the changes were in the deep capillary plexus. Several researchers compared the FAZ in the SCP and DCP in RP vs. controls with contradicting results. Battaglia Parodi et al. [28] found that the deep FAZ area but not the superficial FAZ area to be larger in RP eyes, while Takagi et al. [30] found that the superficial FAZ area but not the deep FAZ area is larger in RP eyes. In contrast, Koyanagi et al. [31] found no difference between the superficial or deep FAZ area between RP and control eyes. Moreover, Sugahara et al. [32] found larger FAZ in RP than in controls for both superficial and deep plexuses. The superficial FAZ area was increased in 17 eyes of 9 males with CHM with an age range 12–57 years, but the increase was not significant [33]. In CHM, the SCP was preserved in the foveal region in 6 male subjects between 28 and 52 years of age, yet with alterations in the DCP [34]. Murro et al. [16] examined 7 young patients (age range 12–28 years) with 20/20 (6/6) vision and CHM and found the following: decreased CMT, smaller superficial FAZ area, smaller deep FAZ area, impaired SCP and impaired DCP compared with controls. Table 2 highlights the enlargement at a young age of the superficial and deep FAZ in GA with CMO compared to normal controls and subjects with CHM. Spaide [35] observed repeatedly that the region of CMO in most retinal or retinovascular diseases shows a decreased or absent DCP flow. Hence alterations in DCP may not be specific to retinal dystrophies. Müller cells appear to also play a role in the pathogenesis of CMO in many retinal disorders including GA [27, 35].
In retinal vascular diseases (diabetic macular oedema or retinal vein occlusion), fluorescein angiography shows increased leakage into macular cysts. There is enlargement of superficial FAZ by fluorescein angiography and OCTA in retinal vascular diseases [35]. Moreover, OCTA demonstrates areas of decreased flow in the deep vascular plexus colocalizing with the cystoid spaces [35]. The pathogenesis of CMO in hereditary chorioretinal degenerations is still poorly understood and includes Müller cell dysfunction [35], damage to retinal pigment epithelium pumping mechanism, breakdown of the blood-retinal barrier, disruption of retinal cell-to-cell adhesion, antiretinal antibodies, and vitreous traction. Unlike retinal vascular cystoid macular oedema, fluorescein angiography shows very minimal or no leakage into these cystoid spaces in degenerative retinopathies, suggesting little role of vascular leakage in these degenerative disorders.
We acknowledge that the present study is affected by several limitations, including, first of all, the small number of patients. Nevertheless, GA is a very rare disease, and thus, just a wider multicenter trial would be able to collect a larger sample. In addition, our study is retrospective, and based on different OCTA machines. In a study conducted by Sacconi et al. [36], 7 different OCTA devices presented measurements with different mean values for foveal avascular zone. In order to reduce the variability between machines, we used the same image segmentation, corrected for refractive error, scaled the images, and used manual measurements by 2 graders. Thus, the present analysis can be considered as a pilot study, designed to stimulate the interest about the OCTA application in GA. A prospective study assessing the OCTA changes over the follow-up would shed more light on the pathophysiology of the disease.
In conclusion, CMO can be identified early in the course of GA, and OCTA can contribute to the clinical characterisation of the disease. A large macular cyst is associated with enlarged foveal avascular zone in GA and RP probably from centrifugal expansion of the cyst.
Summary
What was known before
-
In eyes with cystoid macular oedema associated with gyrate atrophy, foveal avascular zone is thought to be enlarged from a primary occlusive disorder.
What this study adds
-
In eyes with cystoid macular oedema, macular cyst area correlated with an enlarged superficial and deep foveal avascular zone in gyrate atrophy and retinitis pigmentosa raising the possibility that the macular cyst is displacing mechanically away the central foveal vasculature without capillary occlusion.
References
Javadzadeh A, Gharabaghi D. Gyrate atrophy of the choroid and retina with hyperornithinemia responsive to vitamin B6: a case report. J Med Case Rep. 2007;1:27. https://doi.org/10.1186/1752-1947-1-27.
Takki KK, Milton RC. The natural history of gyrate atrophy of the choroid and retina. Ophthalmology. 1981;88:292–301.
Abdelmassih Y, El-Khoury S, Cherfan CG. Dexamethasone implant for the treatment of gyrate atrophy associated macular edema. J Fr Ophtalmol. 2019;42:e1–e4. https://doi.org/10.1016/j.jfo.2018.03.029.
Alparslan Ş, Fatih MT, Muhammed Ş, Adnan Y. Cystoid macular edema secondary to gyrate atrophy in a child treated with sub-tenon injection of triamcinolone acetonide. Rom J Ophthalmol. 2018;62:246–9.
Casalino G, Pierro L, Manitto MP, Michaelides M, Bandello F. Resolution of cystoid macular edema following arginine-restricted diet and vitamin B6 supplementation in a case of gyrate atrophy. J AAPOS. 2018;22:321–3.
Raval V, Kapoor A, Nayak S, Rao S, Das T. Optical coherence tomography angiography and macular vessel density analysis of cystoid macular edema in gyrate atrophy. Ophthalmic Surg Lasers Imaging Retin. 2019;50:423–7.
Zhioua Braham I, Ammous I, Maalej R, Boukari M, Mili Boussen I, Errais K. et al. Multimodal imaging of foveoschisis and macular pseudohole associated with gyrate atrophy: a family report. BMC Ophthalmol. 2018;18:89. https://doi.org/10.1186/s12886-018-0755-9.
Çavdarlı C, Şahlı E, Çavdarlı B, Alp MN. Regression of macular edema with topical brinzolamide and nepafenac alone and identification of a novel gyrate atrophy mutation. Arq Bras Oftalmol. 2020;83:149–52.
Piozzi E, Alessi S, Santambrogio S, Cillino G, Mazza M, Iggui A, et al. Carbonic anhydrase inhibitor with topical NSAID therapy to manage cystoid macular edema in a case of gyrate atrophy. Eur J Ophthalmol. 2017;27:e179–e183.
Oliveira TL, Andrade RE, Muccioli C, Sallum J, Belfort R Jr. Cystoid macular edema in gyrate atrophy of the choroid and retina: a fluorescein angiography and optical coherence tomography evaluation. Am J Ophthalmol. 2005;140:147–9.
Heller D, Weiner C, Nasie I, Anikster Y, Landau Y, Koren T, et al. Reversal of cystoid macular edema in gyrate atrophy patients. Ophthalmic Genet. 2017;38:549–54.
Feldman RB, Mayo SS, Robertson DM, Jones JD, Rostvold JA. Epiretinal membranes and cystoid macular edema in gyrate atrophy of the choroid and retina. Retina. 1989;9:139–42.
Doguizi S, Sekeroglu MA, Anayol MA, Yilmazbas P. Arginine-restricted therapy resistant bilateral macular edema associated with gyrate atrophy. Case Rep. Ophthalmol Med. 2015;2015:137270.
Elnahry AG, Hassan FK, Abdel-Kader AA. Bevacizumab for the treatment of intraretinal cystic spaces in a patient with gyrate atrophy of the choroid and retina. Ophthalmic Genet. 2018;39:759–62.
Mansour AM. Measuring fundus landmarks. Invest Ophthalmol Vis Sci. 1990;31:41–2.
Murro V, Mucciolo DP, Giorgio D, Sodi A, Passerini I, Virgili G, et al. Optical Coherence Tomography Angiography (OCT-A) in young choroideremia (CHM) patients. Ophthalmic Genet. 2019;40:201–6.
Wang T, Milam AH, Steel G, Valle D. A mouse model of gyrate atrophy of the choroid and retina. Early retinal pigment epithelium damage progressive retinal degeneration. J Clin Invest. 1996;97:2753–62.
Scruggs BA, Chen CV, Pfeifer W, Wiley JS, Wang K, Drack AV. Efficacy of topical brinzolamide in children with retinal dystrophies. Ophthalmic Genet. 2019;40:350–8.
Wilson DJ, Weleber RG, Green WR. Ocular clinicopathologic study of gyrate atrophy. Am J Ophthalmol. 1991;111:24–33.
Valle D, Boison A, Jezyk J, Aguirre G. Gyrate atrophy of the choroid and retina in a cat. Invest Ophthalmol Vis Sci. 1981;20:251–5.
Takeuchi M, Itagaki T, Takahashi K, Uyama M. Retinal degeneration after intravitreal injection of ornithine. 1. Early change after administration. Nippon Ganka Gakkai Zasshi. 1990;94:1012–23.
Ong SS, Patel TP, Singh MS. Optical coherence tomography angiography imaging in inherited retinal diseases. J Clin Med. 2019;8:2078.
Ganesh A, Stroh E, Manayath GJ, Al-Zuhaibi S, Levin AV. Macular cysts in retinal dystrophy. Curr Opin Ophthalmol. 2011;22:332–9.
Salvatore S, Fishman GA, Genead MA. Treatment of cystic macular lesions in hereditary retinal dystrophies. Surv Ophthalmol. 2013;58:560–84.
Cicinelli MV, Marchese A, Bordato A, Manitto MP, Bandello F, Battaglia Parodi M. Reviewing the role of ultra-widefield imaging in inherited retinal dystrophies. Ophthalmol Ther. 2020;9:249–63.
Yoshida S, Ishibashi T, Sonoda K-H, Ikeda Y. Optical coherence tomography angiography of the macular microvasculature changes in retinitis pigmentosa. Acta Ophthalmol 2018;96:e59–e67.
Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245:627–36.
Battaglia Parodi M, Cicinelli MV, Rabiolo A, Pierro L, Gagliardi M, Bolognesi G, et al. Vessel density analysis in patients with retinitis pigmentosa by means of optical coherence tomography angiography. Br J Ophthalmol. 2017;101:428–32.
Hagag AM, Wang J, Lu K, Harman G, Weleber RG, Huang D, et al. Projection-resolved optical coherence tomographic angiography of retinal plexuses in retinitis pigmentosa. Am J Ophthalmol. 2019;204:70–9.
Takagi S, Hirami Y, Takahashi M, Fujihara M, Mandai M, Miyakoshi C, et al. Optical coherence tomography angiography in patients with retinitis pigmentosa who have normal visual acuity. Acta Ophthalmol. 2018;96:e636–e642.
Koyanagi Y, Murakami Y, Funatsu J, Akiyama M, Nakatake S, Fujiwara K, et al. Optical coherence tomography angiography of the macular microvasculature changes in retinitis pigmentosa. Acta Ophthalmol. 2018;96:e59–e67.
Sugahara M, Miyata M, Ishihara K, Gotoh N, Morooka S, Ogino K, et al. Optical coherence tomography angiography to estimate retinal blood flow in eyes with retinitis pigmentosa. Sci Rep. 2017;7:46396.
Abbouda A, Dubis AM, Webster AR, Moosajee M. Identifying characteristic features of the retinal and choroidal vasculature in choroideremia using optical coherence tomography angiography. Eye. 2018;32:563–71.
Battaglia Parodi M, Arrigo A, MacLaren RE, Aragona E, Toto L, Mastropasqua R, et al. Vascular alterations revealed with optical coherence tomography angiography in patients with choroideremia. Retina. 2019;39:1200–5.
Spaide RF. Retinal vascular cystoid macular edema: review and new theory. Retina. 2016;36:1823–42.
Sacconi R, Borrelli E, Querques G. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018;192:252–3.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mansour, A.M., Elnahry, A.G., Tripathy, K. et al. Analysis of optical coherence angiography in cystoid macular oedema associated with gyrate atrophy. Eye 35, 1766–1774 (2021). https://doi.org/10.1038/s41433-020-01166-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01166-6