Abstract
Purpose
The management of glaucoma following cataract surgery (GFCS) in children is challenging. This study looks at the results of two-site trabeculotomy in paediatric aphakic/pseudophakic glaucoma, 1-year post operatively.
Methods
This prospective, institutional study was performed on 33 eyes with GFCS in patients aged ≤14 years. Patients underwent two-site trabeculotomy using the rigid-probe trabeculotome, through a superonasal and an inferotemporal scleral flap. Intraocular pressure (IOP), medications, complications and success rates at 1 year were reported. Success was defined as IOP < 23 mmHg or 30% IOP reduction, on the same or fewer number of medications at 1 year, without the need for another glaucoma procedure.
Results
Trabeculotomy was performed on average 3.5 years after the cataract surgery. Patients were aged 5.73 ± 1.79 years. We excluded four eyes in which >180° incision could not be achieved. A 360° incision was achieved in 14 eyes (48%). There was a significant reduction in IOP and medications at 1, 3, 6 and 12 months (p < 0.001). At 1 year, mean IOP reduction was 48.2 ± 31.5%. Success was achieved in 26 eyes (89.6%), of which 15 were controlled without medications. There was no significant difference in IOP, medications or success between aphakic and pseudophakic eyes nor between eyes that had 360° trabeculotomy and eyes that had a 180–270° incision. Three eyes (10.3%) required another glaucoma procedure. One eye required core vitrectomy for vitreous haemorrhage.
Conclusions
Two-site trabeculotomy can be used as an effective and safe first-line procedure in paediatric GFCS eyes that do not have extensive peripheral anterior synechiae.
Similar content being viewed by others
Introduction
Aphakic glaucoma is the most common secondary childhood glaucoma and one of the most serious causes of late visual loss following successful congenital cataract surgery [1,2,3,4]. Early postoperative elevation in intraocular pressure (IOP) could be caused by a steroid response, synechial angle closure, pupillary block glaucoma and/or a coexisting angle anomaly [5,6,7,8]. However, the pathogenesis of the classic, open-angle type of glaucoma that develops on average 1.3–12.2 years following uneventful cataract surgery is still obscure [9]. Mechanical collapse of the trabecular meshwork due to the loss of ciliary body tension could be one of the possible reasons. Some speculate that obstruction of the trabecular meshwork by inflammatory cells, lens remnants and vitreous-derived factors may result in delayed elevation in IOP. Arrest of postnatal angle maturation secondary to the surgical intervention could be a contributing factor, especially that aphakic glaucoma is more likely to occur in patients who had lensectomy at a younger age, usually the first year of life [10]. Its incidence varies from 6% [11] to100%2 according to the duration of follow-up and definition of glaucoma in the different studies, with a reported annual incidence of 5.25% in the first 7 years after lens extraction [9].
Management of paediatric glaucoma following cataract surgery (GFCS) is challenging. Trabeculectomy has a poor success rate and precludes the use of contact lenses, especially in the presence of thin, avascular, cystic blebs [12]. Glaucoma drainage devices have a higher chance of success than trabeculectomy, but aphakic eyes have relatively higher rates of complications, especially suprachoroidal haemorrhage, if hypotony occurs particularly if buphthalmic [13]. GDD-implanted eyes also carry a life-long risk of developing keratopathy secondary to endothelial decompensation from the tube end [14]. Cyclodestructive procedures provide a temporizing treatment with occasional long-term control after multiple treatments [15]. Yet, it is difficult to titrate with marked inflammation and a risk of phthisis, especially in microphthalmic eyes. Furthermore, it may be associated with chronic hypotony and may prejudice future surgery to failure.
Angle surgery was first described as a surgical option in GFCS by Chen et al., yielding promising results in terms of IOP lowering and surgical success [2]. Unlike bleb-based procedures, angle surgery addresses the more physiological outflow pathway through the trabecular meshwork and Schlemm’s canal. Hence the risk of bleb-based complications such as infection, bleb leak, overfiltration and bleb dysthesia is reduced. With the growing evidence that circumferential trabeculotomy yields superior results to conventional 180° angle surgery in primary congenital glaucoma [16], Freedman et al. retrospectively reported the results of microcatheter-assisted circumferential trabeculotomy in GFCS, achieving a 72% success rate. In this study, we look at the results of the two-site trabeculotomy technique, using the rigid-probe trabeculotomes in paediatric aphakic and pseudophakic glaucoma [17].
Materials and methods
This was a prospective study that included consecutive eyes of children aged 14 years or less who required surgery for glaucoma following congenital cataract surgery (GFCS), with or without intraocular lens (IOL) implantation. We excluded eyes that had synechial angle closure over ≥90°, eyes that required combined procedures, eyes that had previous procedures other than lensectomy or IOL implantation and eyes in which the trabeculotomy involved <180° of Schlemm’s canal. In patients that underwent bilateral surgery, only the first operated eye was included. Surgeries were performed by one of three surgeons (HH, GG, YE), in the paediatric ophthalmology department of Cairo University, from January 2015 to June 2018. All patients had a written informed consent signed by the parents before the operation.
All surgeries were performed using the two-site trabeculotomy technique previously described (video) [18], wherein two simultaneous, rigid-probe trabeculotomies are performed in the same sitting, one based on the superonasal quadrant and the other in the inferotemporal quadrant. A corneal traction suture was taken in the superonasal quadrant followed by dissection of a rectangular scleral flap under a conjunctival peritomy. Schlemm’s canal was identified using a crescent blade and Harms trabeculotome (Geuder, Heidelberg, Germany) was inserted into one side. A horizontal incision was created over the trabeculotome in the roof of Schlemm’s canal to expose 1–2 mm of the canal, thus making sure the trabeculotome was in the correct plane. An incision was then created over around 160–180° of Schlemm’s canal by sweeping the Harms trabeculotomes into the anterior chamber making sure the floor of the deroofed part of the canal is not incised to maintain the anterior chamber. The scleral flap and conjunctiva were then closed by 10/0 nylon sutures. The same procedure was repeated in the inferotemporal quadrant. Viscoelastic and/or air were used whenever needed to reform the anterior chamber. If a significant hyphema occurred, a paracentesis was created and the hyphema was gently washed from the anterior chamber.
Postoperatively all patients received topical tobramycin/dexamethasone combination four times daily for 1 week then twice daily for another week. Patients were generally seen on day 1, weekly for a month, monthly for 6 months, then every 3–4 months. More frequent visits were required in cases with complications or inadequate IOP control.
The following data were documented: age at surgery, age at which the patient had lensectomy, lens status (aphakic or pseudophakic), gender and laterality of glaucoma. Preoperative and postoperative examination included number of glaucoma medications, IOP measured by Perkins or Goldmann tonometer, corneal diameter, slit lamp and fundus examination. In younger children in whom examination was difficult, chloral hydrate sedation was used. Any postoperative complication or intervention was noted.
Success was defined as an IOP < 23 mmHg or 30% IOP reduction, on the same or fewer number of medications at 1 year, without the need for another glaucoma procedure. Eyes that underwent a second glaucoma procedure were excluded from the analysis subsequent to the intervention.
The study was approved by Cairo University research ethics committee and followed the tenets of the Declaration of Helsinki.
Statistical analysis
Data were entered and statistical analysis was done using SPSS (Statistical Package for the Social Science) (SPSS Inc., Chicago III, IL) for Windows version 15.0.1. Quantitative data were expressed as mean ± standard deviation (SD). Comparisons between quantitative variables were done using a paired two-sample t-test. Categorical data were summarized in percentages and compared by the Fischer’s exact test. Postoperative results were compared at 1, 3, 6, 12 months post operative visits.
Results
The study cohort included a total of 33 eyes that underwent trabeculotomy for GFCS, with at least 1-year post operative follow-up. In 4 eyes, Schlemm’s canal could not be incised over >180° of the angle, so they were excluded from the study. Accordingly, the data analysis included 29 eyes. The mean postoperative follow-up duration was 16.9 ± 1.2 months.
Preoperative characteristics
Table 1 shows patients’ baseline characteristics. None of the patients had a positive family history of childhood glaucoma. Trabeculotomy was performed on average 3.47 ± 1.1 years after the initial cataract surgery. The mean age of patients at the time of trabeculotomy was 5.73 ± 1.79 years (range; 7 months to 13 years). The mean preoperative IOP was 26.8 ± 8.2 mmHg (range; 10–36 mmHg) on 2.34 ± 1.02 topical medications (range 0–5). In 14 eyes, a complete trabeculotomy involving 4 quadrants was achieved, while 15 eyes had 2–3 quadrants incised (average 236 ± 44°; range: 180–270°), as 4-quadrant cannulation of SC was not possible intraoperatively. We retrospectively performed a subgroup analysis comparing eyes where two-site trabeculotomy was completed over four quadrants (complete trabeculotomy group) and those where it was only possible over two to three quadrants (incomplete trabeculotomy group). The preoperative IOP was significantly higher in the complete trabeculotomy group (30.14 ± 8.7 mmHg, range; 16–45 mmHg) compared to the incomplete trabeculotomy group (23.8 ± 6.6 mmHg, range;10–34 mmHg) (p = 0.04). There was no statistically significant difference between the complete and incomplete trabeculotomy groups in terms of age at time of surgery, side of surgery, gender, preoperative number of glaucoma medications or vertical cup to disc ratio (VCDR).
The VCDR was reduced from a mean of 0.55 ± 0.2 preoperatively to 0.49 ± 0.22 at 1 year (p = 0.3). Snellen visual acuity could be obtained preoperatively and postoperatively in 11 eyes, however, no statistically significant difference was demonstrated between pre- and postoperative values (p = 0.6). Eyes requiring another glaucoma surgery were excluded from the analysis after the intervention.
IOP and glaucoma medications
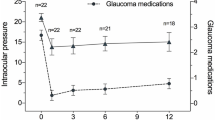
There was a statistically significant reduction in the mean IOP at 1 month (13.9 ± 4.1 mmHg, range; 8–22 mmHg, p < 0.001), 3 months (14.6 ± 4.3 mmHg, range; 10–28 mmHg, p < 0.001), 6 months (14.1 ± 4.7 mmHg, range; 6–23 mmHg p < 0.001) and 12 months post operatively (14.1 ± 3.1 mmHg, range; 10–21 mmHg, p < 0.001) compared to preoperative values. The mean IOP reduction was 42.8 ± 25.3% at 1 month, 41.7 ± 31.8% at 3 months, 48 ± 26.6% at 6 months and 48.2 ± 31.5% at 12 months.
The number of glaucoma medications was significantly reduced from 2.34 ± 1.02 (range 0–5) preoperatively to 0.6 ± 1.1 (range; 0–4) at 1 month, 0.6 ± 1.2 (range; 0–4) at 3 month, 0.7 ± 1.1 (range; 0–4) at 6 month and 0.9 ± 1.1 (range; 0–4) at 12 months post operatively (p < 0.001). At 1-year follow-up, 11 eyes (37.9%) needed topical glaucoma medications for IOP control. The mean time at which medications was started postoperatively was 2.4 ± 2.5 months (range: 1–8 months).
There was no statistically significant difference between the complete and incomplete trabeculotomy groups in the mean postoperative IOP at 1 (p = 0.5), 3 (p = 0.8), 6 (p = 0.5) and 12 months (p = 0.5). There was no significant difference in the number of glaucoma medications needed postoperatively at 1 (p = 0.9), 3 (p = 0.7), 6 (p = 0.6) and 12 months (p = 0.9) (Table 2).
Stratifying patients according to preoperative lens status (pseudophakic or aphakic) did not demonstrate any statistically significant difference between both groups in terms of mean preoperative IOP (27.9 ± 7.2 mmHg in pseudophakics vs 27.2 ± 9.1 mmHg in aphakics, p = 0.5) or mean number of preoperative glaucoma medications (2.3 ± 0.82 in pseudophakics vs 2.3 ± 1 in aphakics, p = 0.8). Postoperatively, there was no significant difference in terms of the mean IOP at 1 (p = 0.3), 3 (p = 0.3), 6 (p = 0.4) and 12 months (p = 0.9). There was no significant difference in the mean number of glaucoma medications needed postoperatively at 1 (p = 0.07), 3 (p = 0.07), 6 (p = 0.2) and 12 months (p = 0.3). At 1 year, the mean IOP in the pseudophakic group was 14.2 ± 3.5 mmHg (range; 10–21 mmHg) compared to 14 ± 2.8 (range; 10–20 mmHg) in the aphakic group (p = 0.9).
Success rates
At 1-year follow-up, success was achieved in 26 eyes (89.6%), of which 15 were controlled without medications. Three eyes were considered as failure because of the need for another glaucoma procedure to control their IOP and were excluded from the analysis following the intervention. In the complete trabeculotomy group, one eye required Ahmed glaucoma valve implantation 9 months after trabeculotomy. In the incomplete trabeculotomy group, two eyes underwent diode laser cyclophotocoagulation at 9 and 12 months after trabeculotomy. All pseudophakic patients had surgical success, while the success rate in aphakic group was 81.25% (13 out of 16 eyes) with 3 eyes requiring further glaucoma procedures to control their IOP (p = 0.2).
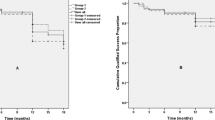
The mean survival time was 11.1 months (standard error 0.51), 95% confidence interval (CI) 10.09–12.11 months. Kaplan–Meier survival analysis was used to compare the survival time, without the use of medications, in eyes that had a complete compared to those that had incomplete trabeculotomy (Fig. 1). Although there was a tendency towards shorter survival time for eyes that had incomplete trabeculotomy, the difference was not statistically significant (p = 0.69).
Postoperative interventions and complications
All eyes had intraoperative hyphema, which resolved on the first week post operatively, except two eyes in each group where the hyphema persisted for 2 weeks and resolved on conservative treatment. One eye developed vitreous haemorrhage that required pars plana vitrectomy. At 12 months post operatively, its IOP was 20 mmHg on two topical medications. One eye developed postoperative corneal oedema, which resolved spontaneously in 3–5 days. One eye underwent scleral rupture repair 2 weeks following trabeculotomy, after the patient withstood blunt trauma by falling on the edge of a table. The rupture occurred at the site of the superonasal scleral flap and was repaired without further sequelae and the patient continued to maintain good vision in this eye.
One pseudophakic patient underwent IOL exchange because of progressive myopic shift resulting in 10 D of anisometropia. At the last follow-up, 13 months after IOL exchange, IOP was 12 mmHg without medications. Two eyes in the aphakic group underwent secondary IOL implantation and both eyes met success criteria at the final follow-up. No serious postoperative complications such as retinal detachment, hypotony, endophthalmitis or loss of vision were identified in our series.
Discussion
Trabeculotomy and goniotomy are the standard first-line procedures in primary congenital glaucoma as well as some secondary paediatric glaucomas and have more recently been described as a first-line procedure in glaucoma following congenital cataract surgery [2, 17, 19,20,21,22]. In a retrospective study on 14 eyes of 11 aphakic glaucoma patients, Bothun et al. showed that conventional goniotomy and trabeculotomy can yield reasonable success rates [23]. With success defined as a final IOP of ≤24 mmHg, 42.8% of eyes achieved surgical success after one procedure and 57.1% achieved success after multiple angle surgeries, suggesting the likely superiority of treating a bigger extent of the angle. Recent evidence from studies on primary congenital glaucoma has shown significantly lower IOPs and higher success rates with circumferential compared to traditional 120–160° angle surgeries [16, 24,25,26,27,28]. The retrospective results of circumferential trabeculotomy using the illuminated microcatheter in GFCS were reported by Freedman et al. With success defined as an IOP ≤ 22 mmHg and >20% IOP reduction, circumferential trabeculotomy achieved a 72% success rate in 25 eyes with aphakic/pseudophakic glaucoma [17, 21].
Our study prospectively looked at the results of the two-site, rigid-probe trabeculotomy technique in 33 eyes of 33 patients with GFCS. Success was achieved in 26 of the 29 eyes (89.6%) in which >180° trabeculotomy was performed. IOP and glaucoma medications were significantly reduced at all postoperative time points in the first postoperative year, with a 48.2 ± 31.5% IOP reduction at 1 year. There was no significant difference in the outcome between eyes that had a complete, four-quadrant trabeculotomy and those that had two to three quadrants incised (incomplete trabeculotomy group). However, the number of eyes in the complete and incomplete trabeculotomy groups may have been too small to reach any statistically significant results. There was also no significant difference in the final IOP of aphakic and pseudophakic eyes.
We have previously compared the two-site trabeculotomy technique to microcatheter-assisted trabeculotomy in primary congenital glaucoma [18] and found that both techniques yield the similar results. The reason we prefer the two-site technique, in addition to being less costly, is that it is performed through two scleral flaps fashioned in the superonasal and inferotemporal quadrants, leaving the superior and superotemporal conjunctiva untouched in case it is needed for a subsequent trabeculectomy or glaucoma shunt. Microcatheter-assisted trabeculotomy, on the other hand, despite being performed through a single scleral flap, additional scleral flaps/cutdowns need to be created in as many as 50% of cases in order to retrieve a microcatheter that has reached an obstruction or become misdirected. These flaps are often created in sites that are inaccessible or preferably avoided, like the superior or superotemporal quadrants.
Vitreous haemorrhage secondary to intraoperative hyphema is a serious complication following trabeculotomy in aphakic eyes, where the lack of compartmentalization allows the blood to access the vitreous cavity. Amblyopia may develop if vitreous haemorrhage persists for a few weeks in eyes of younger children and is an indication to intervene early with pars plana vitrectomy to clear the visual axis. In our study, eyes that developed moderate to severe intraoperative hyphema had the blood gently aspirated with an irrigating solution and air was injected into the anterior chamber. Despite this, 1 aphakic eye (3%) required pars plana vitrectomy for non-clearing vitreous haemorrhage. Dao et al. reported 2 aphakic eyes (8%) out of 25 eyes with GFCS developing vitreous haemorrhage following microcatheter-assisted trabeculotomy and in both cases a pars plana vitrectomy was also required [21]. Bothun et al. reported three cases that developed vitreous haemorrhage after trabeculotomy in aphakic eyes, of which two required vitrectomy [29]. It may be worthwhile considering to fill the anterior chamber with viscoelastic at the end of the surgery, particularly in aphakic eyes, to avoid the posterior percolation of blood in cases of hyphema. The drawback would be the risk of developing an IOP spike, which should be treated timely to avoid further glaucoma progression.
Our study had some limitations. The study was non-comparative and had a relatively short follow-up period. It would be useful to compare the two-site technique with an illuminated microcatheter-assisted trabeculotomy as well as with standard, 180°, rigid-probe trabeculotomy. The relatively small number of patients may have precluded reaching statistically significant results when comparing the outcomes in aphakic and pseudophakic eyes, as well as comparing eyes that had a complete versus incomplete trabeculotomy.
Trabeculotomy using the two-site technique is a viable option for the management of GFCS, yielding similar results in both aphakic and pseudophakic eyes. Being less invasive than trabeculectomy and glaucoma drainage devices, it can be considered as a first-line procedure in eyes that do not have extensive synechial angle closure. Non-clearing vitreous haemorrhage is the most serious possible complication which, although uncommon, may require pars plana vitrectomy.
Summary
What was known before
-
Traditionally, glaucoma drainage devices and diode CPC were the main surgical treatment methods, but more recently there has been a rising interest to address the more physiological outflow pathway, through Schlemm’s canal.
What this study adds
-
The paper describes a prospective study evaluating the results of two-site trabeculotomy in paediatric aphakic and pseudophakic glaucoma.
References
Asrani SG, Wilensky JT. Glaucoma after congenital cataract surgery. Ophthalmology. 1995;102:863–7.
Chen TC, Walton DS, Bhatia LS. Aphakic glaucoma after congenital cataract surgery. Arch Ophthalmol. 2004;122:1819–25.
Kirwan C, O’Keefe M. Paediatric aphakic glaucoma. Acta Ophthalmol Scand. 2006;84:734–9.
Parks MM, Johnson DA, Reed GW. Long-term visual results and complications in children with aphakia. A function of cataract type. Ophthalmology. 1993;100:826–40.
Chen TC, Bhatia LS, Halpern EF, Walton DS. Risk factors for the development of aphakic glaucoma after congenital cataract surgery. J Pediatr Ophthalmol Strabismus. 2006;43:274–80.
Gawdat IG, Youssef MM, Bahgat NM, Elfayoumi DM, Eddin AM. Incidence and risk factors of early-onset glaucoma following pediatric cataract surgery in egyptian children: one-year study. J Curr Glaucoma Pract. 2017;11:80–5.
Gouda J, Elhusseiny A, Tomairek R, El-Fayoumi D, Awadein A, Gawdat G, et al. Changes in intraocular pressure and anterior chamber angle after congenital cataract extraction. J Am Assoc Pediatr Ophthalmol Strabismus. 2019;23:e30–1.
Esmael A, Ismail YM, Elhusseiny AM, Fayed AE, Elhilali HM. Agreement profiles for rebound and applanation tonometry in normal and glaucomatous children. Eur J Ophthalmol. 2019;29:379–85.
Chak M, Rahi JS. Incidence of and factors associated with glaucoma after surgery for congenital cataract: findings from the british congenital cataract study. Ophthalmology. 2008;115:1013–8.e2.
Lundvall A, Kugelberg U. Outcome after treatment of congenital bilateral cataract. Acta Ophthalmol Scand. 2002;80:593–7.
Chrousos GA, Parks MM, O’Neill JF. Incidence of chronic glaucoma, retinal detachment and secondary membrane surgery in pediatric aphakic patients. Ophthalmology. 1984;91:1238–41.
Mandal AK, Bagga H, Nutheti R, Gothwal VK, Nanda AK. Trabeculectomy with or without mitomycin-c for paediatric glaucoma in aphakia and pseudophakia following congenital cataract surgery. Eye. 2003;17:53–62.
Kirwan C, O’Keefe M, Lanigan B, Mahmood U. Ahmed valve drainage implant surgery in the management of paediatric aphakic glaucoma. Br J Ophthalmol. 2005;89:855–8.
Koo EB, Hou J, Keenan JD, Stamper RL, Jeng BH, Han Y. Effects of glaucoma tube surgery on corneal endothelial cells: a review. Eye Contact Lens. 2016;42:221–4.
Kirwan JF, Shah P, Khaw PT. Diode laser cyclophotocoagulation: role in the management of refractory pediatric glaucomas. Ophthalmology. 2002;109:316–23.
El Sayed Y, Gawdat G. Two-year results of microcatheter-assisted trabeculotomy in paediatric glaucoma: a randomized controlled study. Acta Ophthalmol. 2017;95:e713–9.
Lim ME, Dao JB, Freedman SF. 360-degree trabeculotomy for medically refractory glaucoma following cataract surgery and juvenile open-angle glaucoma. Am J Ophthalmol. 2017;175:1–7.
El Sayed YM, Gawdat GI. Microcatheter-assisted trabeculotomy versus 2-site trabeculotomy with the rigid probe trabeculotome in primary congenital glaucoma. J Glaucoma. 2018;27:371–6.
Beck AD, Lynn MJ, Crandall J, Mobin-Uddin O. Surgical outcomes with 360-degree suture trabeculotomy in poor-prognosis primary congenital glaucoma and glaucoma associated with congenital anomalies or cataract surgery. J AAPOS. 2011;15:54–8.
Bothun ED, Hansen EK. Fiber-optic microcatheter trabeculotomy combined with anterior segment surgery in children: report of three cases. J AAPOS. 2011;15:193–5.
Dao JB, Sarkisian SR Jr., Freedman SF. Illuminated microcatheter-facilitated 360-degree trabeculotomy for refractory aphakic and juvenile open-angle glaucoma. J Glaucoma. 2014;23:449–54.
Mendicino ME, Lynch MG, Drack A, Beck AD, Harbin T, Pollard Z, et al. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. J AAPOS. 2000;4:205–10.
Bothun ED, Guo Y, Christiansen SP, Summers CG, Anderson JS, Wright MM, et al. Outcome of angle surgery in children with aphakic glaucoma. J AAPOS. 2010;14:235–9.
Elhusseiny AM, El Sayed YM, El Sheikh RH, Gawdat GI, Elhilali HM. Circumferential schlemm’s canal surgery in adult and pediatric glaucoma. Curr Eye Res. 2019;44:1281–90.
Lim ME, Neely DE, Wang J, Haider KM, Smith HA, Plager DA. Comparison of 360-degree versus traditional trabeculotomy in pediatric glaucoma. J AAPOS. 2015;19:145–9.
Neustein RF, Beck AD. Circumferential trabeculotomy versus conventional angle surgery: comparing long-term surgical success and clinical outcomes in children with primary congenital glaucoma. Am J Ophthalmol. 2017;183:17–24.
Shi Y, Wang H, Yin J, Li M, Zhang X, Xin C, et al. Microcatheter-assisted trabeculotomy versus rigid probe trabeculotomy in childhood glaucoma. Br J Ophthalmol. 2016;100:1257–62.
Elhusseiny AM, Jamerson EC, Menshawey R, Tam EK, El Sayed YM. Collector channels: role and evaluation in schlemm’s canal surgery. Curr Eye Res. 2020:1–7.
Bothun ED, Groth SL, Freedman SF. Vitreous hemorrhage after trabeculotomy in aphakic eyes. J AAPOS. 2013;17:307–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
El Sayed, Y.M., Elhusseiny, A.M., Gawdat, G.I. et al. One-year results of two-site trabeculotomy in paediatric glaucoma following cataract surgery. Eye 35, 1637–1643 (2021). https://doi.org/10.1038/s41433-020-01138-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01138-w