Abstract
Aims
To assess hyperreflective foci (HF) number in angioid streaks (AS) by means of spectral-domain optical coherence tomography (SD-OCT).
Methods
Observational and cross-sectional study. Sixty-two eyes with AS and 62 controls underwent best-corrected visual acuity (BCVA), fundoscopy, and SD-OCT. HF were assessed on the horizontal scan of a six-line radial OCT, in the fovea (1500-μm diameter) and parafovea (500 μm external to the fovea), and sub-classified as retinal or choroidal, small or large. Eyes were distributed in one of four groups, as carrying foveal AS without choroidal neovascularization (CNV) (14 eyes), extra-foveal AS without CNV (14), active CNV (20), and inactive CNV (14). Primary outcome was HF assessment in AS. Secondary outcomes included their correlations with BCVA.
Results
AS-affected eyes had higher HF numbers. Our sub-analysis revealed that patients with active CNV had a larger number of retinal and choroidal HF than all the other groups, whereas retinal and choroidal foci were significantly increased in inactive CNV only with respect to controls. Interestingly, patients with foveal AS showed HF number increase in the choroid and fovea. BCVA deterioration positively correlated with the total HF number found in the fovea and the choroid.
Conclusion
HF are significantly increased in patients with AS. Despite being especially evident in active CNV, the increasing number in eyes without CNV might suggest new pathogenetic aspects of the disease.
Similar content being viewed by others
Introduction
Angioid streaks (AS) appear as irregular, dark red to gray lines under the retina resulting from breaks in the Bruch’s membrane [1], and can be idiopathic in origin or associated with systemic diseases (e.g., pseudoxanthoma elasticum, Ehlers–Danlos syndrome, Paget disease, and sickle cell anemia) [2,3,4,5]. The development of classic choroidal neovascularization (CNV) in the correspondence of the streaks represents the most common complication, occurring in 42–86% of the cases and frequently leading to severe visual loss [6,7,8]. Hyperreflective foci (HF) are a relatively new spectral domain-optical coherence tomographic (SD-OCT) finding, and have been documented in several retinal and choroidal diseases as biomarkers of disease activity or pathogenesis [9,10,11,12,13,14,15,16,17]. SD-OCT has indeed proved a valuable imaging tool in the past to assess and follow-up these patients [18, 19].
The aim of our study is to analyze the number and the location of HF in patients with AS by means of SD-OCT, and to investigate the functional correlates with the different AS presentations.
Methods
The study was designed as an observational and cross-sectional case series in patients affected by AS. All the procedures adhered to the tenets of the Declaration of Helsinki for research involving human subjects and the protocol was approved by the local institutional review board. A series of consecutive patients with recent diagnosis of AS and a group of sex- and age-matched healthy controls were enrolled in the Retinal Heredodystrophies Unit of our Department of Ophthalmology (Scientific Institute San Raffaele—Milan, Italy) from March 2015 to April 2018. Exclusion criteria included the presence of significant media opacities and of unstable fixation, precluding the acquisition of high-quality images. Written informed consent was obtained from all the study subjects.
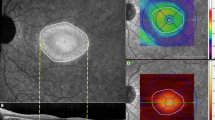
Both groups (patients with AS and control subjects) underwent a complete ophthalmologic examination, including measurement of best-corrected visual acuity (BCVA) using Early Treatment Diabetic Retinopathy Study chart, slit-lamp biomicroscopy, fundoscopy following pupil dilation with Tropicamide 1%, and SD-OCT examination. The identification of AS was based on the clinical biomicroscopic fundus examination, and subsequently confirmed by fundus fluorescein angiography. Patients were further subdivided as carrying AS crossing the fovea without CNV (foveal AS), AS not crossing the fovea without CNV (extra-foveal AS), AS with SD-OCT-documented active sub-foveal choroidal neovascularization (CNV) (showing subretinal and/or intraretinal fluid on SD-OCT scans), and SD-OCT-documented inactive sub-foveal CNV (no evidence of fluid on SD-OCT scans). SD-OCT examination was acquired using the Spectralis HRA + OCT (Heidelberg Engineering; Heidelberg, Germany). The acquisition protocol included a six-line radial SD-OCT pattern (1024 A-scans per B-line scan), centered on the fovea, at 30° distance. Eye tracking was enabled during image acquisition. Hyperreflective foci were defined as discrete, round lesions with greater reflectivity than retinal pigment epithelium, and as invisible on clinical examination [20]. They were arbitrarily subdivided according to their greatest linear dimension in two subgroups: small (if < 15 μm) or large (if > 15 μm). HF were evaluated on the horizontal scan of the six-line radial pattern, and measured in the fovea (1500-μm diameter centered on umbo) and in the para-foveal area (500 μm external to the fovea) by two independent graders (S.M. and F.R.); foci were subsequently classified according to their location as retinal or choroidal. The two examiners were unaware of the purpose of the study, and the SD-OCT scans were assessed on a high-magnification section, after being converted from “white-on-black” to “black-on-white” with contrast adjustment to enhance their visualization. The mean of the two independent measurements of the same SD-OCT scans was used for the analysis. Figure 1 thoroughly depicts the scheme employed for the assessment of HF.
Explanatory acquisition schemes in a control subject and a patient with active choroidal neovascularization secondary to angioid streaks. a Infrared fundus photograph with the corresponding optical coherence tomographic (OCT) six-line radial scheme of a normal subject; hyperreflective foci were divided as retinal or choroidal, and assessed in the fovea (yellow dashed line, 1500 μm) and parafovea (500 μm external to the fovea, bilaterally). Red shaded areas were excluded from the analysis. b shows the infrared photograph and structural OCT of a patient with AS and active sub-foveal CNV. Green and purple arrowheads point to the retinal and choroidal foci, respectively
The primary outcome measure was the assessment of HF in the retina and the choroid of patients affected by AS. Secondary outcomes included their correlations with BCVA and the evaluation in the different subgroups of AS patients.
Differences in the number of HF (retinal and choroidal; foveal and para-foveal) between patients and controls were analyzed by means of Student’s t test for continuous variables. Analysis of variance (ANOVA) for repeated measures and Bonferroni’s test for post hoc analysis were adopted to assess the differences among the different AS subgroups and controls. The relationship between variables was explored using Kendall’s Tau-b (non-parametric) correlation tests. The results of descriptive analyses are expressed as mean ± SD for quantitative variables, and as frequency and percentages for categorical variables. Inter-observer reproducibility for the measurement of HF was evaluated with intra-class correlation coefficients (95% confidence intervals); Shapiro–Wilk test (W score) was employed to verify the Gaussian distribution for all the continuous variables. Statistical significance was set to p < 0.05, and all the analyses were performed using SPSS Statistics Version 21.0 Software package (IBM; Armonk, NY).
Results
Overall, 31 patients (62 affected eyes), with 16 males, and 31 control subjects (62 healthy eyes) were recruited for this study. Mean age was 54.2 ± 16.2 years for the patients (range: 18–83), with a BCVA of 0.36 ± 0.41 LogMAR (~20/50 Snellen equivalents) and 54.4 ± 16.9 years for healthy controls (range: 18–82), with no significant differences in age and sex between the two cohorts (p = 0.96 and 0.99, respectively). In the patients group, 14 eyes (22.6%) revealed foveal AS, 14 (22.6%) had extra-foveal AS, 20 (32.3%) showed signs of OCT-documented active CNV, while 14 (22.6%) had SD-OCT established inactive sub-foveal CNV. Twenty patients (64.5%) had histologic or genetic diagnosis of pseudoxanthoma elasticum, whereas the remaining eyes carried idiopathic AS. Complete clinical data and demographic features of both groups are listed in Table 1.
Altogether, affected eyes did show a significantly higher number of retinal and choroidal HF, both in the fovea and parafovea, when compared with control eyes (p < 0.001). Table 2 and Fig. 2 thoroughly show the HF figures and the statistical comparison among AS subgroups and the control subjects. Of notice, retinal and choroidal HF were statistically increased in eyes with OCT-documented active CNV, with respect to all the other subgroups (p < 0.001). In addition, patients with inactive sub-foveal CNV showed a higher number of foci than control eyes (p: from < 0.001 to 0.02), but not when compared with AS patients without CNV (p: from 0.26 to 0.99 and from 0.09 to 0.99 for foveal and extra-foveal AS, respectively). No meaningful differences were noticed between controls and eyes without CNV, with the exception of foveal and total choroidal HF appearing significantly increased in the eyes carrying foveal AS with respect to controls (p = 0.001). With regard to the size of HF, they were mostly small, with large HF being especially found in foveal AS complicated by CNV. In particular, large retinal HF were detected in 85% of eyes with OCT-documented active CNV, 57% of those with inactive CNV and 7% with foveal AS; on the other hand, 80% with active CNV and 43% with inactive CNV and with foveal AS presented large choroidal HF. No patient with extra-foveal AS had any large HF, either retinal or choroidal. Inter-observer variability between the two investigators was acceptable for all the measurements (ICC = 0.919 [0.899–0.940]). Figure 3 shows HF in patients and controls.
Differences in hyperreflective foci (HF) between different phenotypes of angioid streaks (AS) and controls. First row: box-and-whisker plots showing the mean number of HF in the fovea (retinal, choroidal, and total). Larger figures are noticed in patients with active choroidal neovascularization (CNV), when compared with all other groups and controls (blue boxes). Eyes with inactive CNV showed an increased number of HF compared with controls, whereas those carrying foveal AS had more total foveal foci than controls (green boxes). Second row: box-and-whisker plots showing the mean number of HF in the parafovea (retinal, choroidal, and total). An increased number is noticed in patients with active CNV, when compared with all other groups and controls (blue boxes). Eyes with inactive CNV showed an increased number of HF compared with controls (green boxes). Third row: box-and-whisker plots showing the mean number of total HF in the retina and the choroid. HF resulted significantly increased in patients with active CNV (blue boxes). Eyes with inactive CNV had an increased number of HF compared with controls, with total choroidal HF being increased in patients with foveal AS (green boxes)
Black-on-white optical coherence tomographic (OCT) horizontal scans of a control subject (a) and patients with different phenotypes of angioid streaks (AS; b–e). While hyperreflective foci are not visible in controls (a), their number slightly increases in patients with foveal and extra-foveal AS (b, c, respectively). Accordingly, their figures result even larger in the presence of inactive or active sub-foveal choroidal neovascularization (d, e)
With regard to the visual outcomes, the patients group showed worse BCVA when compared with controls (0.36 ± 0.41 LogMAR vs. −0.03 ± 0.03 LogMAR; p < 0.001). In particular, the subgroups of patients with active CNV and with inactive sub-foveal CNV had a significantly worse visual function (0.65 ± 0.41 LogMAR and 0.55 ± 0.32 LogMAR, respectively; p < 0.001). Interestingly, a decline in BCVA positively correlated with the total number of HF detected in the fovea (Kendall’s τ = 0.486; p < 0.001) and in the choroid (Kendall’s τ = 0.490; p < 0.001) (Supplementary Fig. 1).
Discussion
Angioid streaks have been histologically described as breaks within an abnormally thickened and calcified Bruch’s membrane, radially spreading from the optic nerve [21, 22]. They are generally observed in patients affected by PXE [2], but can be found in other systemic diseases or can be idiopathic, as well [3, 4]. While AS onset occurs between the second and the fifth decade [23], their progression is believed to arise from the reduced resistance of BM to mechanical stress [24]. Although AS does not directly affect visual function [21], the break in BM often leads to the development of Type 2 CNV and eventually to fibrotic scars, invariably damaging the photoreceptors and causing a severe deterioration of visual acuity [25]. Hyperreflective foci are, instead, discrete and dot-shaped, clinically invisible, hyperreflective lesions that have been detected by means of SD-OCT in several retinal disorders, including age-related macular degeneration, diabetic retinopathy, as well as hereditary dystrophies [9,10,11,12,13,14,15,16,17]. In particular, HF frequency has already been shown to correlate with the degree of activity in retinal vascular diseases and macular diseases complicated by choroidal neovascularization [11, 26,27,28]. HF can be found both in retina and the choroid [11], but the most attention was addressed to retinal HF. Despite the scant histological evidence pointing toward an RPE origin of these OCT-documented lesions [29], numerous etiologies have been proposed regarding their nature, including predictive signs of intraretinal fluid [8], activated microglia cells [12, 30], and RPE cells undergoing trans-differentiation [16, 31,32,33]. Our survey represents the first study to describe the presence of HF in patients with AS, and to analyze their distribution and functional correlates in different AS phenotypes. Our data suggest that HF are more frequently represented in the choroid of AS patients. In particular, choroidal HF turned out to be more numerous when the streaks pass through the fovea, and particularly when CNV is present. AS are typically characterized by a multifaceted degenerative process at the level of the Bruch’s membrane [8, 34,35,36], involving various mechanical and biochemical factors [35, 36]. A critical role seems to be played by macrophages, which can significantly contribute to the integrity and functionality of the Bruch’s membrane [35,36,37,38,39]. In this regard, we hypothesize that HF located within the retina may derive from RPE cells undergoing intraretinal migration [33], whereas choroidal HF may represent a sign of the macrophage activation. In correspondence of the AS, as measured in the foveal area, choroidal HF are more common, resulting even more numerous when CNV develops. Bearing in mind that the Bruch’s membrane typically appears irregular and fragmented in patients affected by AS, we cannot rule out that RPE cells may get through the Bruch’s membrane following a trans-differentiation process by means of epithelial-to-mesenchymal transition, thus becoming indistinguishable from the abovementioned choroidal resident macrophages [40,41,42].
Identification of HF related to CNV activity in AMD patients has been previously described [11, 43]; likewise, the higher number of HF in our AS cohort with sub-foveal CNV might be explained by a progressive recruitment of inflammatory cells (e.g., monocytes), or activated microglia cells from the interaction with Muller cells. The expansion of choroidal foci in patients without CNV might, in this sense, reflect a sub-clinical inflammatory process; moreover, the presence of large choroidal HF in a sub-group of AS patients might be another sign of this process. Therefore, we speculate that HF pathogenesis may be related to the progression of the disease, and foci might represent important biomarkers to predict CNV development in patients affected by AS.
We acknowledge that our study carries several limitations, including the limited size of our cohorts and the lack of a longitudinal follow-up in time to assess the changes in the number of HF. In addition, the foci were manually measured by two masked examiners as no automated software to count them is currently available, and our statistical analyses included both eyes of the same patients, variably introducing a source of bias [44]. However, AS is a rare retinal condition, making a profuse recruitment of patients challenging to achieve. We also acknowledge that the interpretation of HF origin may be simply matter of speculation in the absence of specific histopathologic studies.
In conclusion, HF seem to be a valuable sign of neovascular exudation in angioid streaks; moreover, foci appear increased in the fovea and the choroid of eyes with foveal AS, even in the absence of CNV. Their number correlates with the visual function of these patients and might help identifying eyes that are at greater risk of developing choroidal neovascularization, anticipating the appearance of clinically evident exudation. Further studies are warranted to investigate the changes of HF distribution in time, possibly enrolling a larger number of subjects in the study, and thus validate our hypotheses.
Summary
What was known before
Different angioid streaks phenotypes disclose an increased number of hyperreflective foci on SD-OCT, especially in the presence of CNV.
These foci might help unveiling new pathogenetic aspects and be predictive of angioid streaks evolution.
What this study adds
New insights in angioid streaks pathogenesis.
References
Doyne RW. Choroidal and retinal changes. The result of blows on the eyes. Trans Ophthalmol Sok UK. 1889;9:128.
Gliem M, De Zaeytijd J, Finger RP, et al. An update on the clinical phenotype in patients with pseudoxanthoma elasticum. Front Genet. 2013;4:14.
Aessopos A, Voskaridou E, Kavouklis E, et al. Angioid streaks in sickle-thalassemia. Am J Ophthalmol. 1994;117:589–92.
Dabbs TR, Skjodt K. Prevalence of angioid streaks and other ocular complications of Paget’s disease of bone. Br J Ophthalmol. 1990;74:579–82.
Gurwood AS, Mastrangelo DL. Understanding angioid streaks. J Am Optom Assoc. 1997;68:309–24.
Lim JI, Bressler NM, Marsh MJ, Bressler SB. Laser treatment of choroidal neovascularization in patients with angioid streaks. Am J Ophthalmol. 1993;116:414–23.
Battaglia Parodi M, Iacono P, La Spina C, et al. Intravitreal bevacizumab for nonsubfoveal choroidal neovascularization associated with angioid streaks. Am J Ophthalmol 2014;157:374–7.e2.
Clarkson JG, Altman RD. Angioid streaks. Surv Ophthalmol 1982;26:235–46.
Bolz M, Schmidt-Erfurth U, Deak G, Diabetic Retinopathy Research Group Vienna. et al. Optical coherence tomographic hyper-reflective foci: a morphological sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–20.
Christenbury JG, Folgar FF, O’Connell R, et al. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120:1038–45.
Coscas G, De Benedetto U, Coscas F, et al. Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica. 2013;229:32–7.
Kuroda M, Hirami Y, Hata M, et al. Intraretinal hyperreflective foci on spectral-domain optical coherence tomography images of patients with retinitis pigmentosa. Clin Ophthalmol. 2014;8:435–40.
Piri N, Nesmith BLW, Schaal S. Choroidal hyperreflective foci in Stargardt disease shown by spectral-domain optical coherence tomography imaging. Correlation with disease severity. JAMA Ophthalmol. 2015;133:398–405.
De Benedetto U, Sacconi R, Pierro L, et al. Optical coherence tomographic hyperreflective foci in early stages of diabetic retinopathy. Retina. 2015;35:449–53.
Battaglia Parodi M, Iacono P, Romano F, et al. Optical coherence tomography in best vitelliform macular dystrophy. Eur J Ophthalmol 2017;27:201–4.
Parodi MB, Romano F, Sacconi R, et al. Intraretinal hyperreflective foci in best vitelliform macular dystrophy. Retina. 2018;38:2379–86. https://doi.org/10.1097/IAE.0000000000001893.
Battaglia PM, Sacconi R, Romano F, Bandello F. Hyperreflective foci in Stargardt disease: 1-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2018. https://doi.org/10.1007/s00417-018-4167-6. [e-pub ahead of print].
Charbel Issa P, Finger RP, Holz FG, Scholl HP. Multimodal imaging including spectral domain OCT and confocal near infrared reflectance for characterization of outer retinal pathology in pseudoxanthoma elasticum. Invest Ophthalmol Vis Sci. 2009;50:5913–84.
Ellabban AA, Tsujikawa A, Matsumoto A, et al. Macular choroidal thickness and volume in eyes with angioid streaks measured by swept source optical coherence tomography. Am J Ophthalmol. 2012;153:1133–43.
Lee H, Lee J, Chung H, Kim HC. Baseline spectral domain optical coherence tomographic hyperreflective foci as a predictor of visual outcome and recurrence for central serous chorioretinopathy. Retina 2016;36:1372–80.
Dreyer R, Green WR. The pathology of angioid streaks: a study of twenty-one cases. Trans Pa Acad Ophthalmol Otolaryngol. 1978;31:158–67.
Jensen OA. Bruch’s membrane in pseudoxanthoma elasticum. Histochemical, ultrastructural, and x-ray microanalytical study of the membrane and angioid streak areas. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;203:311–20.
Scholz R. Angioid streaks. Arch Ophthalmol. 1941;26:677–95.
Hagedoorn A. Angioid streaks and traumatic ruptures of Bruch’s membrane. Br J Ophthalmol. 1975;59:267.
Gliem M, Finger RP, Fimmers R, et al. Treatment of choroidal neovascularization due to angioid streaks: a comprehensive review. Retina. 2013;33:1300–14.
Abri Aghdam K, Pielen A, Framme C, Junker B. Correlation between hyperreflective foci and clinical outcomes in neovascular age-related macular degeneration after switching to aflibercept. Invest Ophthalmol Vis Sci. 2015;56:6448–55. https://doi.org/10.1167/iovs.15-17338.
Parodi MB, Arrigo A, Romano F, et al. Hyperreflective foci number correlates with choroidal neovascularization activity in angioid streaks. Invest Ophthalmol Vis Sci. 2018;59:3314–9. https://doi.org/10.1167/iovs.18-24291.
Schreur V, Altay L, van Asten F, et al. Hyperreflective foci on optical coherence tomography associate with treatment outcome for anti-VEGF in patients with diabetic macular edema. PLoS One 2018;13:e0206482. https://doi.org/10.1371/journal.pone.0206482.
Chen KC, Jung JJ, Curcio CA, et al. Intraretinal hyperreflective foci in acquired vitelliform lesions of the macula: clinical and histologic study. Am J Ophthalmol. 2016;164:89–98.
Turgut B, Yildirim H. The causes of hyperreflective dots in optical coherence tomography excluding diabetic macular edema and retinal venous occlusion. Open Ophthalmol J. 2015;9:36–40.
Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405.
Kim JW, Kang KH, Burrola P, et al. Retinal degeneration triggered by inactivation of PTEN in the retinal pigment epithelium. Genes Dev. 2008;22:3147–57.
Zanzottera EC, Messinger JD, Ach T, Smith RT, Freund KB, Curcio CA. The project MACULA retinal pigment epithelium grading system for histology and optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015;56:3253–68.
Killingsworth MC, Sarks JP, Sarks SH. Macrophages related to Bruch’s membrane in age-related macular degeneration. Eye (Lond). 1990;4:613–21.
Hu X, Plomp AS, van Soest S, Wijnholds J, de Jong PT, Bergen AA. Pseudoxanthoma elasticum: a clinical, histopathological, and molecular update. Surv Ophthalmol. 2003;48:424–38.
Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch’s membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010;94:918–25.
Chan CY, Papakostas TD, Vavvas DG. Choroidal changes associated with Bruch membrane pathology in pseudoxanthoma elasticum. Am J Ophthalmol. 2014;158:644–9.
Gliem M, Fimmers R, Müller PL, et al. Choroidal changes associated with Bruch membrane pathology in pseudoxanthoma elasticum. Am J Ophthalmol. 2014;158:198–207.
Marchese A, Parravano M, Rabiolo A, Carnevali A, Corbelli E, Cicinelli MV, et al. Optical coherence tomography analysis of evolution of Bruch’s membrane features in angioid streaks. Eye (Lond). 2017. https://doi.org/10.1038/eye.2017.112.
Grisanti S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405.
Kim JW, Kang KH, Burrola P, et al. Retinal degeneration triggered by inactivation of PTEN in the retinal pigment epithelium. Genes Dev. 2008;22:3147–57.
Sennlaub F, Auvynet C, Calippe B, et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol Med. 2013;5:1775–93.
Abri Aghdam K, Pielen A, Framme C, Junker B. Correlation between hyperreflective foci and clinical outcomes in neovascular age-related macular degeneration after switching to aflibercept. Invest Ophthalmol Vis Sci. 2015;56:6448–55.
Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82:971–3.
Acknowledgements
MBP has the following disclosures: Bausch & Lomb Inc (C). FB has the following disclosures: Alcon (C), Allergan Plc.(C), Famila-Thea (C), Bayer Schering Pharma AG (C), Bausch & Lomb (C), Hoffmann-La-Roche (C), Novartis (C), Sanofi-Aventis (C), and Carl Zeiss AG (C). FR, SM, AA, AM, MVC, and GCA have no financial disclosures.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Romano, F., Mercuri, S., Arrigo, A. et al. Identification of hyperreflective foci in angioid streaks. Eye 33, 1916–1923 (2019). https://doi.org/10.1038/s41433-019-0483-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-019-0483-2