Abstract
RNA-DNA differences (RDD) have previously been identified in the human mitochondrial RNA (mt-RNA) transcripts, yet their functional impact is poorly understood. By analyzing 4928 RNA-seq samples from 23 body sites, we found that mtDNA gene expression negatively correlated with the levels of both m1A 947 16 S rRNA modification (mtDNA position 2617) and the m1A 1812 ND5 mRNA modification (mtDNA position 13,710) in 15 and 14 body sites, respectively. Such correlation was not evident in all tested brain tissues, thus suggesting a tissue-specific impact of these modifications on mtDNA gene expression. To assess the response of the tested modifications to environmental cues, we analyzed pairs of skin samples that were either exposed to the sun or not. We found that the correlations of mtDNA gene expression with both mt-RNA modifications were compromised upon sun exposure. As a first step to explore the underlying mechanism, we analyzed RNA-seq data from keratinocytes that were exposed to increasing doses of UV irradiation. Similar to sun exposure, we found a significant decrease in mtDNA gene expression upon increase in UV dosage. In contrast, there was a significant increase in the m1A 947 16 S rRNA modification levels upon elevation in UV dose. Finally, we identified candidate modulators of such responses. Taken together, our results indicate that mt-RNA modifications functionally correlate with mtDNA gene expression, and responds to environmental cues, hence supporting their physiological importance.
Similar content being viewed by others
Introduction
Mitochondria are the major source of cellular energy (adenosine triphosphate, ATP) and pivotal players in metabolism, and hence are critical for the activities of most tissues. However, cell types and tissues differ in mitochondrial DNA (mtDNA) transcription [1], mitochondrial mass, morphology, and the ATP produced via the oxidative phosphorylation system (OXPHOS) [2]. Accordingly, tissue-dependent mitochondrial disorders have been reported [3], and mtDNA common variants associate with tendency to develop tissue-dependent phenotypes [4]. Hence, regulation of mitochondrial functions is likely tissue dependent.
Although most mitochondrial factors are encoded by the nuclear DNA (nDNA) [5], 37 essential genes are still encoded by the vertebrate mtDNA. Specifically, human mtDNA encodes 13 OXPHOS subunits, 22 tRNAs, and two rRNAs (12 S and 16 S), which are co-transcribed in 2-3 strand-specific polycistrons [1, 6]. During the past few years deep DNA sequence comparisons to corresponding RNA sequences revealed three mtDNA positions with RNA-DNA differences (RDDs) that are consistently altered in mitochondrial RNA transcripts (mt-RNAs) in various individuals and tested cell lines [7,8,9]. It has been shown that the RDD at mtDNA position 2617, reflects an m1A 947 16 S rRNA modification (or in short 16 S modification), introduced by the tRNA Methyltransferase 61B (TRMT61B) [10]. Similarly, it has been suggested that the second RDD, at mtDNA position 13,710, also reflects an m1A modification, which is likely introduced by a different tRNA methyltransferase, TRMT10C (tRNA Methyltransferase 10 C) [8]. The third RDD is within the non-coding D-loop position 295, which overlaps a SNP that defines a specific mtDNA genetic background, haplogroup J; thus, its underlying mechanism has been given less attention. Although these RDDs occur in all tested samples, their functional importance is unknown.
Recently, we demonstrated co-expression of mtDNA and nDNA-encoded OXPHOS genes, likely reflecting coregulation across many human body sites [11]. Although, in general, there was positive cross-tissue correlation in mito-nuclear OXPHOS genes’ co-expression, there was difference in the magnitude and nature of such correlations between tissues. One may thus ask whether such tissue differences also affect the levels of the above-mentioned mt-RNA modifications, especially in response to environmental cues. We hypothesize that assessing the impact of both physiological differences and environmental conditions on mt-RNA modifications will shed new light on their potential functional importance. Here, we assessed the correlation between mt-RNA modifications and altered gene expression patterns in a variety of body sites. Specifically, we analyzed the mtDNA-encoded transcript sequences of samples from the GTEx consortium and studied the correlation between mitochondrial RDD levels with altered gene expression. We found that the 16 S modification, negatively correlated with altered mtDNA gene expression across many human body sites. Similar correlation with mtDNA gene expression was observed while analyzing levels of the RDD at mtDNA position 13,710, indicative of an m1A 1812 ND5 mRNA modification (or in short, the ND5 modification). Such correlation was also assessed in samples from skin with or without sun exposure and in keratinocytes that were experimentally exposed to different UV irradiation dosage. The implications of these findings for the functionality of mt-RNA modifications are discussed.
Methods
GTEx RNA-seq and variant data
GTEx v6 [12] RNA-seq fastq files and whole genome VCF were obtained from dbGaP (phs000424.v6.p1). Analysis was limited to body sites with data from at least 100 individuals (except for ovary). Only the most recently collected samples were kept for replicate analysis.
Mapping RNA-seq reads to human mtDNA
RNA-seq reads were trimmed using trimmomatic [13] according to their fastQC and then mapped onto the entire human genome reference sequence (GRCh38) using STAR v2.5.3 [14]. The mapping process followed the ENCODE long mRNA protocol (https://github.com/ENCODE-DCC/long-rna-seq-pipeline), using the [--outFilterMismatchNoverLmax 0.05], [--alignSJoverhangMin 8], [--alignSJDBoverhangMin 1], [--alignSJDBoverhangMax 999] [--alignIntronMin 20], [--alignIntronMax 1000000], and [--alignMatesGapMax 1000000], with the modification of [--outFilterMultimapNmax 1] mapping parameters to achieve unique mapping. Since mtDNA sequence variability can impact the number of mapped RNA-seq reads, reads were re-mapped against a personalized genome, as previously described [15]. During the secondary mapping stage, most of the parameters of the first-tier mapping were retained while utilizing the 2-pass mode ([--twoPassMode Basic] and [--outFilterType BySJout]). Mapping accuracy was further increased by allowing fewer mismatches [--outFilterMismatchNmax 8]. To maintain sufficient mtDNA coverage (i.e., 10X across most mtDNA positions), samples with mtDNA read coverage of less than 165,690 (Supplementary Fig. S1) were excluded.
Reconstruction of complete mtDNA sequences from RNA-seq data and assessing the levels of RNA-DNA differences at positions 2617 and 13,710
Taking advantage of the polycistronic transcription of the mtDNA [6], mtDNA sequences were reconstructed from each of the RNA-seq samples using an in-house script as previously described [15]. Each reconstructed mtDNA sequence underwent haplogroup assignment using HaploGrep [16]. Subsequent analyses included comparisons of mtDNA gene expression between individuals according to their mitochondrial haplogroups, thereby eliminating potential population stratification. To calculate RDD levels at mtDNA positions 2,617 or 13,710, we focused only on samples with a minimum coverage of 500X considering these positions, followed by calculation of read fractions harboring either thymidine or guanine.
Expression pattern analysis considering mtDNA SNPs and RNA modification levels
RNA-seq reads were counted using HTSeq-count [17] and normalized to library size using DESeq [18]. Samples with mtDNA expression higher than average plus two standard deviations (SD) were considered outliers and were excluded from further analyses. mtDNA sequences of all individuals were aligned to identify polymorphic positions, as previously described [15]. Next, using the linear model in Matrix eQTL R package [19], eQTL mapping was calculated, while considering age, gender, cause-of-death (Hardy index), postmortem interval (PMI), and RNA integrity number (RIN) as covariates. Bonferroni correction for multiple testing was employed. To reduce false positive discovery rates, we used SNPs shared by at least five individuals [20]. R built-in linear regression function (lm) enabled calculating correlation between mtDNA gene expression and each of two RDDs (i.e., the 16 S and ND5 modifications, separately). To identify additional candidate factors that might introduce the 16 S modification, correlation tests of gene expression with the modification levels were performed against the entire MitoCarta [5]. Following Bonferroni correction for multiple testing, the results with significant values were extracted and filtered to generate a list of genes with known activity of RNA methylation (RNA methyltransferases), extracted from AmiGO [21].
Promoter analysis for candidate regulators of mt-RNA methyltransferases
To identify candidate regulators of TRMT61B and TRMT10C, the putative promoters of these genes were identified using “ENCODE cCREs” in the UCSC Genome Browser [22]. Specifically, an upstream candidate cis-regulatory element (cCRE) with a promoter-like signature was chosen for further analysis. Using the ENCODE [23] accession number of the putative promoter, we listed all available ChIP-seq signals from the top hit in descending order based on signal consistency among the different ChIP-seq experiments in Supplementary Table S1 (for TRMT61B) and Supplementary Table S2 (for TRMT10C).
Assessing differential expression of potential mtDNA gene expression regulators
To identify candidate regulators of skin response to sun or UV exposure, differential expression analysis of nDNA genes was performed using DESeq2 bioconductor R package [24]. Differential expression results were then narrowed and corrected for a specified list of genes with mitochondrial regulatory function. The list contains genes with known mitochondrial RNA-binding activity [15, 25], transcription factors that were recently identified in human mitochondria but are not included in MitoCarta (i.e., c-Jun, JunD, C/EBPB, Mef2D, KAT8, NFATC1, THRA) [26,27,28,29,30], OXPHOS and mitochondrial ribosome subunits (Supplementary Table S3). Genes with FDR adjusted p-value of <0.05 and log fold change >0.2 or < −0.2 were considered differentially expressed.
Results
Certain mtDNA SNPs associate with altered mtDNA gene expression in a tissue-dependent manner
We analyzed 5079 GTEx RNA-seq samples from 23 body sites, each surveyed in at least 100 individuals (Supplementary Fig. S2). Since the entire human mtDNA sequence is transcribed, we used RNA-seq data to reconstruct personal mtDNAs of each sample, call variants, and assign each individual to mtDNA genetic backgrounds (i.e., haplogroups) (Supplementary Table S4) [16]. To avoid sample size bias, we excluded Asian mtDNA haplogroups from further analyses (haplogroups A, B, C, D, and N1a9), due to their overall small representation (N = 11). Notably, although the sequencing read coverage across the mtDNA differed among the tested body sites, it did not preferentially impact any certain mtDNA haplogroup (Supplementary Fig. S1). Following mtDNA-based outlier removal, a total of 4928 samples were left and the analyzed sample sizes ranged from N = 94 (ovary) to N = 405 (skeletal muscle) (Supplementary Table S5). As previously performed, we restricted our analyses to uniquely mapped reads, to avoid potential bias introduced by NUMTs (i.e., nuclear mitochondrial DNA pseudogenes, Supplementary Table S6) [15, 31]. We then screened for possible association of mtDNA haplogroups with altered mtDNA gene expression in the available tissues. To this end, we assessed the association of mtDNA SNPs with mtDNA-encoded gene expression in eight body sites with sample sizes of >250. Notably, the 22 tRNA genes were too short to be detected using the long reads RNA-seq libraries. Therefore, our analyses focused on the 13 protein coding genes and the two rRNA genes. Although we found significant associations of certain mtDNA SNPs with altered mtDNA gene expression (Supplementary Table S7), only one SNP (A10,398 G) represented mtDNA haplogroups exceeding 5% of the samples. A10,398 G is a recurrent variant, shared by haplogroups I, J, L, K1 [20], and it associated with lower ND3 expression in the tibial nerve (Supplementary Fig. S3).
m1A RNA modifications correlate with altered mtDNA gene expression in multiple body sites
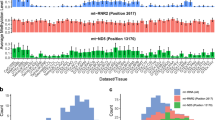
Next, we assessed whether the levels of recently-discovered mitochondrial RDDs in position 2617 (indicative of the m1A 947 16 S rRNA modification) and in mtDNA position 13,710 (indicative of the 1812 m1A ND5 mRNA modification) [7] associate with altered mtDNA gene expression. To this end, we took advantage of an indicative RNA sequence signatures of these modifications – a mixture of A, T, and G in the relevant mtDNA positions. Firstly, we found considerable variation among different body sites in the levels of the sequence signatures of the two tested RDDs (Fig. 1). Notably, the modification levels displayed a continuous distribution across tissues in both sites. Firstly, the levels of the m1A 947 16 S rRNA modification (in short, the 16 S modification) ranged from the lowest in testis to the highest in the heart atrial appendage; the 1812 m1A ND5 modification levels (in short, the ND5 modification) ranged from the lowest in the skeletal muscle, to the highest in the ovary. Second, we found negative correlation between 16 S modification levels and mtDNA gene expression in 14 body sites, as well as positive correlation with the expression of certain mtDNA transcripts in 3 body sites (Fig. 2A, and Supplementary Fig. S4). Like the 16 S modification, ND5 modification levels showed negative correlation with mtDNA gene expression in 11 body sites, and positive correlation in four body sites (Fig. 2B, and Supplementary Fig. S5). Notably, no significant correlation with both tested RNA modifications with mtDNA gene expression was found in the brain.
mtDNA gene expression patterns correlate with the levels of the m1A RNA modifications at mtDNA positions 2617 (A) and 13,710 (B). mtDNA gene expression either positively (orange) or negatively (blue) correlate with the levels of m1A 947 16 S rRNA modification, in sixteen body sites, while seven body sites lack such correlations (A), and with the m1A ND5 RNA modification in 14 body sites (B).
Although both the 16 S modification and the ND5 modification displayed negative correlation with mtDNA gene expression in multiple overlapping body sites (Fig. 2B), there were several differences: firstly, in whole blood, in contrast to all other body sites, the ND5 modification positively correlated with gene expression. Secondly, the ovary which largely showed negative correlation of 16 S modification levels with gene expression, did not reveal such correlation with the ND5 modification. Thus, although the correlation of mtDNA gene expression with both mt-RNA modifications largely overlapped, differences likely reflect variation in the underlying mechanism.
TRMT61B is involved in the correlation of m1A 947 16 S rRNA modification with altered mtDNA gene expression
Previously we found that TRMT61B, a mitochondrial RNA methyltransferase [32], is the best candidate to introduce the 16 S modification [10]. We, therefore, asked whether TRMT61B contributes to the observed correlation between mtDNA gene expression and the level of 16 S modification. To address this, we first assessed the expression level of TRMT61B across body sites (Supplementary Fig. S6A). Next, we calculated the correlation between the expression levels of TRMT61B and the modification levels across body sites. We found significant correlation in the tibial artery, tibial nerve, transformed fibroblasts, skin that was not exposed to sun, atrial appendage of the heart, mammary tissue, and in the colon (Fig. 3, Supplementary Fig. S7). Notably, such correlation was mostly positive (tibial artery, tibial nerve, transformed fibroblasts, unexposed skin, mammary tissue, and colon) except for negative correlation in atrial heart appendage. This indicates positive contribution of TRMT61B to the 16 S modification levels. While analyzing the upstream putative TRMT61B promoter region for transcription factor binding signals in the ENCODE database, our attention was drawn to strong signals of JunD, CCAAT enhancer binding protein beta (C/EBPB), and a weaker signal for c-Jun (Supplementary Table S1). These factors are the only ones in the list that were experimentally localized in human mitochondria and bind the mtDNA [26]. Hence, the same factors likely modulate both the levels of 16 S modification and mtDNA genes expression. Notably, although MitoCarta contains 19 mitochondrial RNA methyltransferases (including TRMT61B), none could explain the lack of association between TRMT61B and the 16 S modification levels in certain tissues, including all tested brain tissues (Supplementary Fig. S8, Supplementary Table S8). This lack of association also includes HSD17B10, the only additional known mitochondrial m1A RNA methyltransferase in this list. Regardless, we cannot exclude that in addition to TRMT61B, alternative factors modulate the correlation of the 16 S modification with mtDNA gene expression in these body sites.
Correlation analysis was performed between the expression of TRMT61B and the m1A 947 16 S rRNA modification in body sites which displayed correlation between the modification and mtDNA gene expression. Shown are the body sites which displayed a significant correlation (p < 0.05) (A–G). A representative body site which did not show any significant correlation (H). Y axes – m1A 16 S rRNA modification level. X axes – TRMT61B normalized read counts. Notably, the remaining analyzed correlations between TRMT61B expression and m1A 947 16 S rRNA modification levels are shown in Supplementary Fig. S7.
Although both the 16 S and ND5 RNA modifications are m1A modifications, they are likely introduced by different RNA methyltransferases. Most intriguingly, the expression of mitochondrial RNA methyltransferase TRMT10C (Supplementary Fig. S6B), who likely introduces the ND5 modification [8], was negatively correlated with ND5 modification levels across the tested body sites (Fig. 4, Fig. S9), thus questioning its direct involvement in introducing this modification. Unlike TRMT61B, analysis of the putative TRMT10C promoter region did not reveal binding sites of any known mtDNA regulator (Supplementary Table S2).
Correlation analysis was performed between the expression of TRMT10C and the m1A 1812 ND5 mRNA modification in body sites which displayed correlation between the modification and mtDNA gene expression. Shown are representative body sites which displayed a significant correlation (p < 0.05) (A–J). The one body site (unexposed skin) which did not show any significant correlation (K). Y axes – m1A 1812 ND5 mRNA modification level. X axes – TRMT10C normalized read counts. Notably, the remaining analyzed correlations between TRMT10C expression and m1A 1812 ND5 mRNA modification levels are shown in Supplementary Fig. S9.
Sun exposure impacts the correlation of mtDNA gene expression in human skin with the m1A 947 16 S modification, and to a lesser extent the m1A 1812 ND5 mRNA modification
UV irradiation was previously shown to alter mitochondrial gene expression in keratinocytes [33]. With this in mind, we considered GTEx skin samples collected from two body sites of the same individuals, one sun-exposed and the other not exposed to sun (N = 170 of each condition). The two types of skin samples showed stark differences in terms of mtDNA gene expression, with skin not exposed to sun displaying significantly higher mtDNA transcript levels than matched sun-exposed samples (Fig. 5). Strikingly, whereas 16 S modification levels correlated with gene expression in the skin not exposed to sun, such correlation was largely lost in sun-exposed skin (Table 1). A similar trend, yet to a lesser extent, was observed while analyzing the association of the ND5 modification with mtDNA gene expression in sun-exposed versus sun-unexposed skin (Table 1). Since TRMT61B could explain the correlation between 16 S modification levels with mitochondrial gene expression, we asked whether its expression differed between the two environmental conditions. Indeed, sun-exposed skin showed significantly lower TRMT61B expression as compared to skin that was not exposed to sun (paired t-test, p = 5.1 × 10−7, Fig. 6). Taken together, sun exposure largely compromised the correlation between mtDNA gene expression and the two tested mt-RNA modifications. Thus, certain sunlight component interfered with modulating the connection between mtDNA gene expression and the tested modifications.
Differential expression analysis of nDNA genes offer candidates that could explain mtDNA differential expression
Since mtDNA gene expression is governed by nDNA-encoded regulators, we asked whether sun exposure affected such genes, and therefore, may provide an explanation for mtDNA gene expression differences. Therefore, we performed differential expression analysis of genes that are known to participate in mitochondrial transcription and posttranscriptional regulation, regulation of mtDNA replication as well as nDNA-encoded members of the OXPHOS machinery, and the mitochondrial ribosome (Supplementary Table S3). Such analysis revealed twelve genes with mostly reduced expression upon sun exposure: two OXPHOS complex IV subunits (CYCS, COX6B2), an OXPHOS complex III subunit (UQCRB), 3 factors of the large mitochondrial ribosome subunit (MRPL1, MRPL32, MRPL48), 3 known regulators of mtDNA gene expression (MTERF2, HEMK1, and COMTD1) and 3 nDNA transcription factors which bind the mtDNA in vivo (cJun, JUND, C/EBPB) [26] (Supplementary Fig. S10).
UV irradiation affected the levels of both mtDNA gene expression and of the m1A 947 16 S modification in keratinocytes
Previously it has been shown that ultraviolet (UV) irradiation affected gene expression [34], and mitochondrial activity [35]. We, therefore, hypothesized that UV irradiation played a role in the effect of sunlight on mtDNA gene expression and RNA modifications in human skin. As a first step to test this hypothesis, we analyzed RNA-seq experimental data from keratinocytes that were exposed to UV irradiation at different times and doses [34]. While considering the mtDNA gene expression levels, principal component analysis (Supplementary Fig. S11) and hierarchical clustering (Fig. 7A) revealed two groups. Specifically, one of these groups comprised all control samples and most of the lower-level UV-irradiated samples (Group 1), whereas the second group included most of the samples that were irradiated with higher UV levels (Group 2). Analysis of mtDNA gene expression levels revealed significant expression reduction in group 2 as compared to group 1 (Fig. 7B). This resembled the impact of sunlight on mtDNA gene expression in the skin. Secondly, we noticed a significant increase in the level of the 16 S modification in group 2 as compared to group 1 (Fig. 7C). The level of the ND5 modification was very low in these cells, thus excluding it from further analysis. Finally, to get insight into the regulatory mechanism that underlies the observed changes in mtDNA transcripts, we performed differential expression analysis of nDNA-encoded genes involved in mtDNA gene expression, mtDNA replication, the OXPHOS and the mitochondrial ribosome (Supplementary Table S3). As the kertainocyte dataset contained few samples, we focused on genes that proved significant while analyzing GTEx skin samples (Supplementary Fig. S10). None of those genes displayed significant difference between the two groups.
Hierarchical clustering of the UV-irradiated samples (A). Sample annotation of three keratinocyte cell line replicates (N0, N1, and N2) that were UV-irradiated in three doses (10, 20, or 30 mJ/cm2) and were collected after one or three days for RNA-sequencing (1d, 3d). mtDNA gene expression levels were higher in Group 1 (blue), as compared to Group 2 (orange) (B). X axis- mtDNA genes. Y axis- normalized read counts. Mann–Whitney U-test, significance values are as follows: (*) < 3.3 × 10−3, (**) < 1 × 10−4, (***) < 1 × 10−5. The m1A 947 16 S modification level differs between the two groups (C). X axis- group assignment. Y axis- m1A 947 16 S rRNA modification level. Mann–Whitney U-test p = 3.2 × 10−3.
Discussion
Here, we studied correlation between mtDNA gene expression patterns across different body sites in the GTEx dataset and mt-RNA modifications as well as with mtDNA SNPs. Previously, we found association between mtDNA SNPs and mtDNA gene expression in lymphoblastoid cells [15]. Additionally, mtDNA gene expression differs among mitochondrial cytoplasmic hybrids – cybrids [35, 36]. Our analysis of the GTEx RNA-seq dataset revealed association between the A10,398 G variant and reduced expression of the mtDNA-encoded ND3 gene. Although position 10,398 is within ND3, one cannot dispose this finding as a mapping artifact, as our personalized mapping of each sample against their own mtDNA genomes [15] retained the same effect, hence favoring biological relevance. The A10,398 G variant is an ancient recurrent mutation, shared by the I, J, L, and K1 haplogroups, which are included in the GTEx cohort. Moreover, this recurrent mutation has been previously associated with altered tendency to develop a variety of phenotypes [4]. Since mtDNA gene expression has not been systematically studied in the context of mitochondrial and complex disorders, our results offer a previously unexplored view on the underlying mechanism of such phenotypes as well as tissue-specific mitochondrial phenotypes [3]. As association was observed only between A10,398 G and ND3, which is co-transcribed along with 11 additional OXPHOS subunits, it might reflect the impact of A10,398 G on posttranscriptional regulation of ND3 gene expression in the tibial nerve.
We identified mostly negative correlations between the levels of either 16 S or ND5 RNA modifications and mtDNA transcript levels across multiple body sites. Previously, we employed a bacterial model system to assess the importance of the 16 S modification for translation and cell life [10]. Our current observation extends the role of the 16 S modification to correlation with mtDNA transcript levels in a variety of body sites. As mtDNA gene expression and the levels of both mt-RNA modifications negatively correlate, it is of interest to investigate the physiological importance underlying not only the 16 S modification, but also that of the ND5 modification.
Close inspection of the body sites for which correlation was observed between the levels of either of the tested RNA modifications and mtDNA gene expression, revealed lack of correlation at all tested brain sites. Indeed, the nervous system is preferentially affected in mitochondrial diseases and common disorders [4]. Our results offer a previously unexplored view on the underlying mechanism of such phenotypes.
We previously discovered that the best candidate enzyme to introduce the 16 S modification is the RNA methyltransferase TRMT61B [10]. Indeed, we observed positive correlation between TRMT61B expression levels with the level of 16 S modification. Intriguingly, analysis of TRMT61B promoter revealed experimentally supported binding sites of transcription factors that were previously shown to bind the human mtDNA (JUND, C/EBPB, and to a lesser extent c-Jun) [26]. Apparently, this result implies putative coregulation of TRMT61B and mtDNA gene expression. However, our results indicate negative correlation between the levels of the 16 S modification and mtDNA gene expression, namely elevated modification levels were accompanied by reduced mtDNA gene expression. Therefore, if coordinated regulation of mtDNA genes and TRMT61B is considered, the factors involved should oppositely regulate these two parties. This interpretation should be tested in the future. In contrast, the expression of TRMT10C, which likely introduces the ND5 modification [8], was negatively correlated with its corresponding modification levels. This is counterintuitive and urge to reassess the role of TRMT10C in introducing the ND5 modification. In case TRMT10C indeed introduces the m1A 1,812 ND5 mRNA modification, one avenue to pursue, would be to assess the timing of such introduction. Notably, the 16 S rRNA modification is introduced right after transcription, already at the precursor polycistronic mitochondrial RNA [7]. If the m1A 1812 ND5 modification is introduced into the mt-RNA as early as at the polycistronic stage, it would be interesting to assess in the future whether higher levels of this modification interfere with the steady state levels of mtDNA transcripts.
Our results suggest that the negative correlation of both the 16 S and the ND5 RNA modifications with mtDNA transcript levels in skin not exposed to the sun was largely compromised upon sun exposure. Previously, it was shown that UV irradiation changes mitochondrial morphology, reduces mitochondrial oxygen consumption rate in keratinocytes, and altered expression of mitochondria-related genes [33]. Accordingly, we found reduced mtDNA gene expression both upon exposure of keratinocytes to increasing UV dosage, and in skin that was exposed to sunlight. However, whereas the reduction in mtDNA gene expression levels led to apparent loss of correlation with the levels of the mt-RNA modifications in skin samples, such mtDNA gene expression reduction in UV-exposed keratinocytes was accompanied with rather increased levels of the 16 S modification. The apparent opposite response of the 16 S modification and mtDNA gene expression to UV irradiation in keratinocytes likely reflects a more prominent negative correlation, in contrast to the impact of sunlight on skin samples. Therefore, whereas sunlight and UV affect mtDNA gene expression in a similar manner, these two conditions differentially affect mt-RNA modification levels. Hence, although the impact of sunlight on mtDNA gene expression could be largely attributed to UV, the regulatory link between mt-RNA modifications and mtDNA gene expression is likely more complex, thus involving other additional wavelengths.
Identification of novel insights into mtDNA gene expression is of great interest. Indeed, by screening for nDNA-encoded candidates that could explain the differences in mtDNA gene expression upon skin exposure to sunlight, several genes were highlighted. We found significantly reduced expression of several OXPHOS subunits, the mitochondrial ribosome and of several known (MTERF2, COMTD1, and HEMK1) and candidate (c-Jun, JunD) regulators of mtDNA gene expression. Additionally, the expression of the candidate regulatory factor C/EBPB was increased in response to sunlight. Firstly, this suggests that although both sunlight and UV led to reduced mtDNA gene expression, they likely did not interfere with the known co-expression regulation of nDNA and mtDNA-encoded OXPHOS subunits. Secondly, elevated C/EBPB levels upon UV treatment is consistent with the negative correlation observed between C/EBPB expression and the expression of both mtDNA and nDNA-encoded OXPHOS subunits [11]. Hence, our findings underline C/EBPB as a candidate negative regulator of mtDNA gene expression. Taken together, association of mt-RNA modifications with altered mtDNA gene expression, unearth a previously unexplored view on the impact of mt-RNA functional variation on phenotypes, and lends new insights into the mechanisms underlying the regulation of mtDNA gene expression.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. Specifically, this study constitutes re-analyses of publicly available data (mainly the GTex and a dataset from citation 34). The supplementary information which accompanies this study consists of 11 supplemental figures and 8 supplementary tables.
References
Blumberg A, Rice EJ, Kundaje A, Danko CG, Mishmar D. Initiation of mtDNA transcription is followed by pausing, and diverges across human cell types and during evolution. Genome Res. 2017;27:362–73.
Fernandez-Vizarra E, Enriquez JA, Perez-Martos A, Montoya J, Fernandez-Silva P. Tissue-specific differences in mitochondrial activity and biogenesis. Mitochondrion. 2011;11:207–13.
Boczonadi V, Ricci G, Horvath R. Mitochondrial DNA transcription and translation: clinical syndromes. Essays Biochem. 2018;62:321–40.
Marom S, Friger M, Mishmar D. MtDNA meta-analysis reveals both phenotype specificity and allele heterogeneity: a model for differential association. Sci Rep. 2017;7:43449.
Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res. 2016;44:D1251–7.
Aloni Y, Attardi G. Symmetrical in vivo transcription of mitochondrial DNA in HeLa cells. Proc Natl Acad Sci USA. 1971;68:1757–61.
Bar-Yaacov D, Avital G, Levin L, Richards AL, Hachen N, Rebolledo Jaramillo B, et al. RNA-DNA differences in human mitochondria restore ancestral form of 16S ribosomal RNA. Genome Res. 2013;23:1789–96.
Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, Bar-Yaacov D, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–5.
Hodgkinson A, Idaghdour Y, Gbeha E, Grenier JC, Hip-Ki E, Bruat V, et al. High-resolution genomic analysis of human mitochondrial RNA sequence variation. Science. 2014;344:413–5.
Bar-Yaacov D, Frumkin I, Yashiro Y, Chujo T, Ishigami Y, Chemla Y, et al. Mitochondrial 16S rRNA Is Methylated by tRNA Methyltransferase TRMT61B in All Vertebrates. PLoS Biol. 2016;14:e1002557.
Barshad G, Blumberg A, Cohen T, Mishmar D. Human primitive brain displays negative mitochondrial-nuclear expression correlation of respiratory genes. Genome Res. 2018;28:952–67.
Consortium GT. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Cohen T, Levin L, Mishmar D. Ancient out-of-africa mitochondrial DNA variants associate with distinct mitochondrial gene expression patterns. PLoS Genet. 2016;12:e1006407.
Kloss-Brandstatter A, Pacher D, Schonherr S, Weissensteiner H, Binna R, Specht G, et al. HaploGrep: a fast and reliable algorithm for automatic classification of mitochondrial DNA haplogroups. Hum Mutat. 2011;32:25–32.
Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 2015;31:166–9.
Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106.
Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–8.
Levin L, Zhidkov I, Gurman Y, Hawlena H, Mishmar D. Functional recurrent mutations in the human mitochondrial phylogeny: dual roles in evolution and disease. Genome Biol Evol. 2013;5:876–90.
Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S, et al. AmiGO: online access to ontology and annotation data. Bioinformatics. 2009;25:288–9.
Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006.
Consortium EP, Moore JE, Purcaro MJ, Pratt HE, Epstein CB, Shoresh N, et al. Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583:699–710.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Wolf AR, Mootha VK. Functional genomic analysis of human mitochondrial RNA processing. Cell Rep. 2014;7:918–31.
Blumberg A, Sri Sailaja B, Kundaje A, Levin L, Dadon S, Shmorak S, et al. Transcription factors bind negatively selected sites within human mtDNA genes. Genome Biol Evol. 2014;6:2634–46.
She H, Yang Q, Shepherd K, Smith Y, Miller G, Testa C, et al. Direct regulation of complex I by mitochondrial MEF2D is disrupted in a mouse model of Parkinson disease and in human patients. J Clin Invest. 2011;121:930–40.
Chatterjee A, Seyfferth J, Lucci J, Gilsbach R, Preissl S, Bottinger L, et al. MOF acetyl transferase regulates transcription and respiration in mitochondria. Cell. 2016;167:722–38.
Lambertini E, Penolazzi L, Morganti C, Lisignoli G, Zini N, Angelozzi M, et al. Osteogenic differentiation of human MSCs: Specific occupancy of the mitochondrial DNA by NFATc1 transcription factor. Int J Biochem Cell Biol. 2015;64:212–9.
Fernandez-Vizarra E, Enriquez JA, Perez-Martos A, Montoya J, Fernandez-Silva P. Mitochondrial gene expression is regulated at multiple levels and differentially in the heart and liver by thyroid hormones. Curr Genet. 2008;54:13–22.
Mishmar D, Ruiz-Pesini E, Brandon M, Wallace DC. Mitochondrial DNA-like sequences in the nucleus (NUMTs): insights into our African origins and the mechanism of foreign DNA integration. Hum Mutat. 2004;23:125–33.
Chujo T, Suzuki T. Trmt61B is a methyltransferase responsible for 1-methyladenosine at position 58 of human mitochondrial tRNAs. RNA. 2012;18:2269–76.
Kelly J, Murphy JE. Mitochondrial gene expression changes in cultured human skin cells following simulated sunlight irradiation. J Photochem Photobio B. 2018;179:167–74.
Shen Y, Kim AL, Du R, Liu L. Transcriptome analysis identifies the dysregulation of ultraviolet target genes in human skin cancers. PLoS One. 2016;11:e0163054.
Malik D, Hsu T, Falatoonzadeh P, Caceres-del-Carpio J, Tarek M, Chwa M, et al. Human retinal transmitochondrial cybrids with J or H mtDNA haplogroups respond differently to ultraviolet radiation: implications for retinal diseases. PLoS One. 2014;9:e99003.
Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–53.
Acknowledgements
We thank the Negev Foundation for a scholarship of excellence awarded to TC. We thank Dr. Liron Levin, BGU, for critical discussion, and Prof. Ofer Ovadia for critical consultation of statistical methods.
Funding
This study was funded by research grants from the Israel Science Foundation (ISF, 372/17), US-Israel BSF (2013060) and the US Army Life Sciences division (LS67993) awarded to DM. The funding agencies were not involved in study design, analysis, interpretation of data, and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
TC analyzed all the data and particiapted in writing the manuscript; HM participated in data analysis, especially while considering the UV exposed cells; CM participated in the initial QC of the GTex data; AE supervised the GTex QC and assisted in critical editing of the manuscript; DM concieved the study, supervised the analysis and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study consists analyses of publicly available data with no new samples added. Hence, no Helsinki approval was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cohen, T., Medini, H., Mordechai, C. et al. Human mitochondrial RNA modifications associate with tissue-specific changes in gene expression, and are affected by sunlight and UV exposure. Eur J Hum Genet 30, 1363–1372 (2022). https://doi.org/10.1038/s41431-022-01072-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-022-01072-3