Abstract
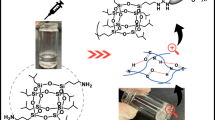
Mannich-type polycondensation with 4,4’-diaminodiphenylmethane (DDM), 2,2-bis(4-hydroxyphenol) propane (BPA) and paraformaldehyde (PF) in different solvents was carried out to prepare main-chain benzoxazines. When pure CHCl3 was used as the solvent, the decomposition rate of PF in CHCl3 was very slow, thus preventing gelation caused by the formation of triazine in the early reaction, producing P(B-D)1 composed of partially closed and unclosed structures. The addition of alkaline triethylamine into CHCl3 (CHCl3/triethylamine = 6:1) greatly enhanced the decomposition rate of PF but gave a large triazine gel. The introduction of triethanolamine into CHCl3 was found to promote the decomposition of PF, enhance the polymerization rate, and prevent gelation caused either by the formation of triazine or by the ring-opening of oxazine ring through a solvation effect. Polymers P(B-D) 6~P(B-D)8 obtained in CHCl3/triethanolamine had a high-oxazine content and a high number molecular weight (Mn) near 8000. Further optimization of Mannich-type polycondensation in CHCl3/triethanolamine gave preferred conditions: a CHCl3/triethanolamine ratio of 4:1, a PF of 1.2 eq and a reaction time of 24 h. Compared with P(B-D)1 and P(B-D)3 (obtained in toluene/ethanol), P(B-D)9 obtained under the optimized conditions showed a higher Mn (10,000) and a higher yield (97.0%). The optimized conditions were also applicable for other kinds of diamines and bisphenols.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Liu J, Agag T, Ishida H. Main-chain type benzoxazine oligomers: a new concept for easy processable high performance polybenzoxazines. In: Ishida H, Agag T, editors. Handbook of benzoxazine resins. Amsterdam: Elsevier B.V.; 2011. p. 355–62.
Ghosh NN, Kiskan B, Yagci Y. Polybenzoxazines—new high performance thermosetting resins: synthesis and properties. Prog Polym Sci. 2007;32:1344–91.
Takeichi T, Kawauchi T, Agag T. High performance poly-benzoxazines as a novel type of phenolic resin. Polym J. 2008;40:1121–31.
Demir KD, Kiskan B, Aydogan B, Yagci Y. Thermally curable main-chain benzoxazine prepolymers via polycondensation route. React Funct Polym. 2013;73:346–59.
Kobayashi T, Muraoka M, Goto M, Minami M, Sogawa H, Sanda F. Main-chain type benzoxazine polymers consisting of polypropylene glycol and phenyleneethynylene units: spacer effect on curing behavior and thermomechanical properties. Polym J. 2022;54:133–41.
Murai Y, Uemura T, Chen Y, Kawauchi T, Takeichi T. Synthesis of high-molecular-weight benzoxazines from various combinations of bisphenols and diamines via Mannich condensation and properties of their thermosets. Polym J. 2021;53:439–47.
Dogan Demir K, Kiskan B, Yagci Y. Thermally curable acetylene-containing main-chain benzoxazine polymers via sonogashira coupling reaction. Macromolecules. 2011;44:1801–7.
Kobayashi T, Goto M, Minami M, Sanda F. Synthesis and crosslinking reaction of a novel polymer containing benzoxazine and phenyleneethynylene moieties in the main chain. J Polym Sci Part A Polym Chem. 2019;57:2581–9.
Ohara M, Yoshimoto K, Kawauchi T, Takeichi T. Synthesis of high-molecular-weight benzoxazines having azomethine linkages in the main-chain and the properties of their thermosetting resins. Polymer. 2020;202:122668.
Zhang K, Liu Y, Ishida H. Polymerization of an AB-type benzoxazine monomer toward different polybenzoxazine networks: when Diels-Alder reaction meets benzoxazine chemistry in a single-component resin. Macromolecules. 2019;52:7386–95.
Kiskan B, Aydogan B, Yagci Y. Synthesis, characterization, and thermally activated curing of oligosiloxanes containing benzoxazine moieties in the main chain. J Polym Sci Part A Polym Chem. 2009;47:804–11.
Aydogan B, Sureka D, Kiskan B, Yagci Y. Polysiloxane-containing benzoxazine moieties in the main chain. J Polym Sci Part A Polym Chem. 2010;48:5156–62.
Zhang K, Yu X, Kuo S-W. Outstanding dielectric and thermal properties of main chain-type poly(benzoxazine-co-imide-co-siloxane)-based cross-linked networks. Polym Chem. 2019;10:2387–96.
Nagai A, Kamei Y, Wang X-S, Omura M, Sudo A, Nishida H, et al. Synthesis and crosslinking behavior of a novel linear polymer bearing 1,2,3-triazol and benzoxazine groups in the main chain by a step-growth click-coupling reaction. J Polym Sci Part A Polym Chem. 2008;46:2316–25.
Chernykh A, Agag T, Ishida H. Synthesis of linear polymers containing benzoxazine moieties in the main chain with high molecular design versatility via click reaction. Polymer. 2009;50:382–90.
Kiskan B, Yagci Y, Ishida H. Synthesis, characterization, and properties of new thermally curable polyetheresters containing benzoxazine moieties in the main chain. J Polym Sci Part A Polym Chem. 2008;46:414–20.
Tuzun A, Kiskan B, AleDDMr N, Erciyes AT, Yagci Y. Benzoxazine containing polyester thermosets with improved adhesion and flexibility. J Polym Sci Part A Polym Chem. 2010;48:4279–84.
Demir KD, Kiskan B, Latthe SS, Demirel AL, Yagci Y. Thermally curable fluorinated main chain benzoxazine polyethers via Ullmann coupling. Polym Chem. 2013;4:2106–14.
Wang MW, Jeng RJ, Lin CH. The robustness of a thermoset of a main-chain type polybenzoxazine precursor prepared through a strategy of A-A and B-B polycondensation. RSC Adv. 2016;6:18678–84.
Lin CH, Chang SL, Shen TY, Shih YS, Lin HT, Wang CF. Flexible polybenzoxazine thermosets with high glass transition temperatures and low surface free energies. Polym Chem. 2012;3:935–45.
Takeichi T, Kano T, Agag T. Synthesis and thermal cure of high molecular weight polybenzoxazine precursors and the properties of the thermosets. Polymer. 2005;46:12172–80.
Chernykh A, Liu J, Ishida H. Synthesis and properties of a new crosslinkable polymer containing benzoxazine moiety in the main chain. Polymer. 2006;47:7664–69.
Chen J, Feng Z, Gu Y, Huang Y, Zeng M, Xu Q. A facile method for the preparation of aliphatic main-chain benzoxazine copolymers with high-frequency low dielectric constants. Polym Chem. 2018;9:2913–25.
Parveen S, Kim H. Synthesis and properties of main-chain polybenzoxazines based on bisphenol-S. Polym Eng Sci. 2018;58:1766–73.
Han M, You S, Wang Y, Zhang K, Yang S. Synthesis of highly thermally stable daidzein-based main-chain-type benzoxazine resins. Polymers. 2019;11:1–11.
Chen J, Zeng M, Feng Z, Pang T, Huang Y, Xu Q. Design and preparation of benzoxazine resin with high-frequency low dielectric constants and ultralow dielectric losses. ACS Appl Polym Mater. 2019;1:625–30.
Xu Q, Zeng M, Chen J, Zeng S, Huang Y, Feng Z, et al. Synthesis, polymerization kinetics, and high-frequency dielectric properties of novel main-chain benzoxazine copolymers. React Funct Polym. 2018;122:158–66.
Deliballi Z, Kiskan B, Yagci Y. Main-chain benzoxazine precursor block copolymers. Polym Chem. 2018;9:178–83.
Chen CH, Lin CH, Hon JM, Wang MW, Juang TY. First halogen and phosphorus-free, flame-retardant benzoxazine thermosets derived from main-chain type bishydroxydeoxybenzoin -based benzoxazine polymers. Polymer. 2018;154:35–41.
Wang MW, Jeng RJ, Lin CH. Study on the ring-opening polymerization of benzoxazine through multisubstituted polybenzoxazine precursors. Macromolecules. 2015;48:530–35.
Huang CH, Liu YL. A self-protection effect of monomers on preventing gelation in synthesis of main-chain polybenzoxazines with high molecular weights. Macromolecules. 2021;54:7434–40.
Ručigaj A, Štirn Ž, Šebenik U, Krajnc M. Main-chain benzoxazine oligomers: Effects of molecular weight on the thermal, mechanical, and viscoelastic properties. J Appl Polym Sci. 2018;135:1–11.
Shen L, Gan J, Wu K, Liang L, Zhang Y, Lu M, et al. Synthesis and characterization of a lateral phthalonitrile functionalized main-chain polybenzoxazine precursor. Macromol Res. 2016;24:409–14.
Lin CH, Chang SL, Hsieh CW, Lee HH. Aromatic diamine-based benzoxazines and their high performance thermosets. Polymer. 2008;49:1220–9.
Deng Y, Zhang Q, Zhang H, Zhang C, Wang W, Gu Y. Kinetics of 3,4-dihydro-2H-3-phenyl-1,3- benzoxazine synthesis from Mannich base and formaldehyde. Ind Eng Chem Res. 2014;53:1933–9.
Yang P, Gu Y. A novel benzimidazole moiety-containing benzoxazine: synthesis, polymerization, and thermal properties. J Polym Sci Part A Polym Chem. 2012;50:1261–71.
Zhang C-X, Deng Y-Y, Zhang Y-Y, Yang P, Gu Y. Study on products and reaction paths for synthesis of 3,4-dihydro-2H-3-phenyl-1,3-benzoxazine from phenol, aniline and formaldehyde. Chin Chem Lett. 2015;26:348–52.
Arslan M. Synthesis and characterization of novel bio-based benzoxazines from gallic acid with latent catalytic characteristics. React Funct Polym. 2019;139:9–16.
Acknowledgements
The authors are grateful for financial support from the Hunan Provincial Natural Science Foundation of China (Nos. 2019JJ60021 and 2021JJ50073), the Scientific Research Fund of Hunan Provincial Education Department (No. 20A075), and the Engineering Technology Center for Advanced Thermal Protection and Flame Retardant Functional Materials (HYNU).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, P., Liu, X., Lai, H. et al. CHCl3/triethanolamine: a new mixed solvent for preparing high-molecular-weight main-chain benzoxazines through Mannich-type polycondensation. Polym J 54, 1071–1079 (2022). https://doi.org/10.1038/s41428-022-00664-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00664-6