Abstract
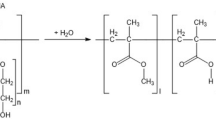
A copolymer of acrylamide (AA), acrylonitrile (AN) and sodium 2-acrylamido-2-methylpropanesulfonate (AMPSNa) was synthesized by radical polymerization. The effect of drag reduction of turbulent water flow by the synthesized acrylate copolymer was studied by capillary turbulent viscometry at temperatures up to 140 °C. The temperature dependence of the characteristic value of drag reduction (fDR), the increment in the volumetric flow rate (∆Q) and the drag coefficient (λ) were determined. With the use of IR spectroscopy and elemental analysis data, the chemical composition of the acrylate copolymer was ascertained to be dependent on thermohydrolysis temperatures of up to 180 °C. The colloidal and molecular weight characteristics of the initial and hydrolyzed acrylate copolymer were measured by dynamic light scattering and capillary viscometry. The temperature dependencies of the copolymer characteristics were determined. The optimal composition of the acrylate copolymer in terms of its thermohydrolysis resistance within the operating temperature range of up to 180 °C was revealed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Toms B. Some observations on the flow of linear polymer solutions through straight tubes at large reynolds numbers. In: Proceedings of the 1st International Congress on Rheology, North Holland, Amsterdam; 1948. p. 135–141
Xi L. Turbulent drag reduction by polymer additives: fundamentals and recent advances. Phys Fluids. 2019;31:121302 https://doi.org/10.1063/1.5129619
Han WJ, Dong YZ, Choi HJ. Applications of water-soluble polymers in turbulent drag reduction. Process 2017;5:24 https://doi.org/10.3390/pr5020024
Shanshool J, Abdul Jabbar MF, Slaiman IN. The influence of mechanical effects on degradation of polyisobutylenes as drag reducing agents. Pet Coal. 2011;53:218–222.
Pereira AS, Andrade RM, Soares EJ. Drag reduction induced by flexible and rigid molecules in a turbulent flow into a rotating cylindrical double gap device: comparison between poly (ethylene oxide), polyacrylamide, and xanthan gum. J Nonnewton Fluid Mech. 2013;202:72–87. https://doi.org/10.1016/j.jnnfm.2013.09.008
Asidin MA, Suali E, Jusnukin T, Lahin FA. Review on the applications and developments of drag reducing polymer in turbulent pipe flow. Chin J Chem Eng. 2019;27:1921–1932. https://doi.org/10.1016/j.cjche.2019.03.003
Zhang X, Duan X, Muzychka Y. Degradation of flow drag reduction with polymer additives—a new molecular view. J Mol Liq. 2019;292:111360 https://doi.org/10.1016/j.molliq.2019.111360
Habibpour M, Koteeswaran S, Clark PE. Drag reduction behavior of hydrolyzed polyacrylamide/polysaccharide mixed polymer solutions—effect of solution salinity and polymer concentration. Rheol Acta. 2017;56:683–694. https://doi.org/10.1007/s00397-017-1024-1
Le Brun N, Zadrazil I, Norman L, Bismarck A, Markides CN. On the drag reduction effect and shear stability of improved acrylamide copolymers for enhanced hydraulic fracturing. Chem Eng Sci. 2016;146:135–143. https://doi.org/10.1016/j.ces.2016.02.009
Shah SN, Vyas A. Temperature and salinity effects on drag-reduction characteristics of polymers in coiled tubing. SPE/ICoTA Coiled Tubing Well Inter Confer Exhib 2009;26:55–66. https://doi.org/10.2118/130685-MS
Yang S-Q. Drag reduction in turbulent flow with polymer additives. J Fluids Eng. 2009;131:051301 https://doi.org/10.1115/1.3111255
White CM, Mungal MG. Mechanics and prediction of turbulent drag reduction with polymer additives. Annu Rev Fluid Mech. 2008;40:235–256. https://doi.org/10.1146/annurev.fluid.40.111406.102156
MacAndrew R, Parry N, Prieur J-M, Wiggelman J, Diggins E, Guicheney P, et al. Drilling and testing hot, high-pressure wells. Oilf Rev 1993;5:15–32.
Elkatatny S. Mitigation of barite sagging during the drilling of high-pressure high-temperature wells using an invert emulsion drilling fluid. Powder Technol. 2019;352:325–330. https://doi.org/10.1016/j.powtec.2019.04.037
Wenjun S, Shixian T, Fan F, Weimin Y, Zhitao Z. Research on the drilling fluid technology for high temperature over 240 °C. Procedia Eng. 2014;73:218–229. https://doi.org/10.1016/j.proeng.2014.06.191
Mao H, Qiu Z, Shen Z, Huang W. Hydrophobic associated polymer based silica nanoparticles composite with core–shell structure as a filtrate reducer for drilling fluid at utra-high temperature. J Pet Sci Eng. 2015;129:1–14. https://doi.org/10.1016/j.petrol.2015.03.003
Kamel A, Shah SN. Effects of salinity and temperature on drag reduction characteristics of polymers in straight circular pipes. J Pet Sci Eng. 2009;67:23–33. https://doi.org/10.1016/J.PETROL.2009.02.004
Zhang K, Lim GH, Choi HJ. Mechanical degradation of water-soluble acrylamide copolymer under a turbulent flow: Effect of molecular weight and temperature. J Ind Eng Chem. 2016;33:156–161. https://doi.org/10.1016/J.JIEC.2015.09.031
Levitt DB, Pope GA, Jouenne S. Chemical degradation of polyacrylamide polymers under alkaline conditions. SPE Reserv Eval Eng. 2011;14:281–286. https://doi.org/10.2118/129879-PA
Seright RS, Campbell AR, Mozley PS, Han P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. SPE J. 2009;15:341–348. https://doi.org/10.2118/121460-PA
Sifferman TR, Greenkorn RA. Drag reduction in three distinctly different fluid systems. Soc Pet Eng J. 1981;21:663–669. https://doi.org/10.2118/8722-PA
Ma Q, Shuler PJ, Aften CW, Tang Y. Theoretical studies of hydrolysis and stability of polyacrylamide polymers. Polym Degrad Stab. 2015;121:69–77. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2015.08.012
Zhang K, Choi HJ, Jang CH. Turbulent drag reduction characteristics of poly(acrylamide-co-acrylic acid) in a rotating disk apparatus. Colloid Polym Sci. 2011;289:1821–1827. https://doi.org/10.1007/s00396-011-2502-0
Limparyoon N, Seetapan N, Kiatkamjornwong S. Acrylamide/2-acrylamido-2-methylpropane sulfonic acid and associated sodium salt superabsorbent copolymer nanocomposites with mica as fire retardants. Polym Degrad Stab. 2011;96:1054–1063. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2011.03.012
Jouenne S. Polymer flooding in high temperature, high salinity conditions: selection of polymer type and polymer chemistry, thermal stability. J Pet Sci Eng. 2020;195:107545 https://doi.org/10.1016/J.PETROL.2020.107545
Rashidi M, Blokhus AM, Skauge A. Viscosity and retention of sulfonated polyacrylamide polymers at high temperature. J Appl Polym Sci. 2011;119:3623–3629. https://doi.org/10.1002/app.33056
Kelland MA. Production chemicals for the oil and gas industry. 2nd ed. CRC Press; 2014. https://doi.org/10.1201/B16648
Kamal MS, Sultan AS, Al-Mubaiyedh UA, Hussein IA. Review on polymer flooding: rheology, adsorption, stability, and field applications of various polymer systems. Polym Rev. 2015;55:491–530. https://doi.org/10.1080/15583724.2014.982821
Nechaev AI, Gorbunova MN, Lebedeva II, Val’tsifer VA, Strel’nikov VN. Synthesis by radical polymerization and structure of drag reducing terpolymers based on acrylamide, acrylonitrile, and 2-acrylamido-2-methylpropanesulfonic acid. Russ J Appl Chem. 2017;90:1524–1531. https://doi.org/10.1134/S1070427217090233
Nechaev AI, Lebedeva II, Val’tsifer VA, Strel’nikov VN. Influence of the composition of acrylamide–acrylonitrile–2-acrylamido-2-methylpropanesulfonic acid terpolymer on its resistance to high temperatures and salts. Russ J Appl Chem. 2016;89:1296–1301. https://doi.org/10.1134/S1070427216080139
Kurenkov VF, Safin AG, Yanushkevich EA, Chernyaeva EE. Kinetics of radical polymerization of 2-acrylamido-2-methylpropanesulfonic acid and its salts in reverse emulsions. Russ J Appl Chem. 1999;72:282–286.
Nieuwstadt FTM, den Toonder JMJ. Drag reduction by additives: a review. In: Soldati A, Monti R, editors. Turbulence structure and modulation. Springer Vienna; 2001. p. 269–316. https://doi.org/10.1007/978-3-7091-2574-8_10
Kolek E, Simko P, Simon P. Confirmation of polymerisation effects of sodium chloride and its additives on acrylamide by infrared spectrometry. J Food Nutr Res. 2007;46:39–44.
Rosa F, Bordado J, Casquilho M. Hydrosoluble copolymers of acrylamide-(2-acrylamido-2-methylpropanesulfonic acid). Synthesis and characterization by spectroscopy and viscometry. J Appl Polym Sci. 2003;87:192–198. https://doi.org/10.1002/app.11325
Aggour YA, Bekhat G, Atia A. Copolymerization and thermal investigation of 2-acrylamido-2-methylpropanesulfonic acid with acrylonitrile. J Polym Mater. 2000;17:193–197.
Aggour YA. Investigation of the thermal degradation and stability of copolymers of 2-acrylamido-2-methylpropanesulphonic acid and methyl methacrylate. Polym Degrad Stab. 1998;60:317–320. https://doi.org/10.1016/S0141-3910(97)00085-2
Parker WO, Lezzi A. Hydrolysis of sodium-2-acrylamido-2-methylpropanesulfonate copolymers at elevated temperature in aqueous solution via 13C n.m.r. spectroscopy. Polymer. 1993;34:4913–4918. https://doi.org/10.1016/0032-3861(93)90018-6
Acknowledgements
The work was carried out using the equipment of The Core Facilities Center «Research of materials and matter» at the PFRC UB RAS.
Funding
The reported study was funded by RFBR and Perm Territory, project number 20-43-596014.
Author information
Authors and Affiliations
Contributions
AIN: Investigation, formal analysis, writing - original draft. NSV: Validation, writing - review & editing, visualization. VNS: Project administration, funding acquisition. VAV: Methodology, conceptualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nechaev, A.I., Voronina, N.S., Strelnikov, V.N. et al. Drag reduction by acrylate copolymers under thermohydrolysis. Polym J 54, 1029–1038 (2022). https://doi.org/10.1038/s41428-022-00649-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-022-00649-5