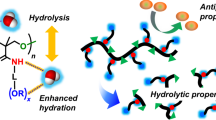
Abstract
Although biomedical devices have greatly evolved, none of the materials that have been used to date are able to meet all the hemocompatibility criteria. The rapid accumulation of proteins at the implant surface and the subsequent physiological response are the main causes of failure. Thus, the appropriate design of antithrombotic materials is of the utmost importance. In this work, we employed Carbothane® electrospun matrices (PCU) for lysine surface modification, using oligomers obtained from allyl glycidyl ether (AGE) reaction as spacers. This technique enables the binding of several lysine molecules per urethane linkage, which, along with the large surface-to-volume ratio of the electrospun membranes, leads to high ε-amino free lysine grafting (29 ± 2 nmol cm−2). The incorporation of AGE oligomers significantly reduced the nonspecific protein adsorption, while further modification with lysine led to a more pronounced decrease (25% for BSA, 35% for fibrinogen, and 30% for PNP proteins, with respect to PCU membranes). The lysine-modified matrices presented increased plasminogen adsorption capacity and in vitro clot lysis ability after incubation in pooled normal human plasma and tissue plasminogen activator, confirming the plasminogen adsorption selectivity and thus improving the hemocompatibility behavior of these matrices. Therefore, the obtained electrospun membranes are promising coatings for biomedical devices with fibrinolytic activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mc Namara K, Alzubaidi H, Jackson JK. Cardiovascular disease as a leading cause of death: how are pharmacists getting involved? Integr Pharm Res Pract. 2019;8:1–11. https://doi.org/10.2147/iprp.s133088.
Fischer M, Maitz MF, Werner C Coatings for biomaterials to improve hemocompatibility. In: Siedlecki CA, ed. Hemocompatibility of biomaterials for clinical applications: blood-biomaterials interactions. Cambridge: Woodhead Publishing; 2018:163−90.
Williams DF. There is no such thing as a biocompatible material. Biomaterials. 2014;35:10009–14. https://doi.org/10.1016/j.biomaterials.2014.08.035.
Chen H, Yuan L, Song W, Wu Z, Li D. Biocompatible polymer materials: role of protein-surface interactions. Prog Polym Sci (Oxf). 2008;33:1059–87. https://doi.org/10.1016/j.progpolymsci.2008.07.006.
Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thrombosis Haemost. 2015;13:S72–S81. https://doi.org/10.1111/jth.12961.
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials science: an introduction to materials in medicine. 3rd ed. New York, NY: Academic Press.; 2013.
Zdrahala RJ, Zdrahala IJ. Biomedical applications of polyurethanes: a review of past promises, present realities, and a vibrant future. J Biomater Appl. 1999;14:67–90. https://doi.org/10.1177/088532829901400104.
De Bartolo L, Morelli S, Piscioneri A, Lopez LC, Favia P, d’Agostino R, et al. Novel membranes and surface modification able to activate specific cellular responses. Biomolecular Eng. 2007;24:23–26. https://doi.org/10.1016/j.bioeng.2006.07.001. 1 SPEC. ISS.
Solouk A, Solati-Hashjin M, Najarian S, Mirzadeh H, Seifalian AM. Optimization of acrylic acid grafting onto POSS-PCU nanocomposite using response surface methodology. Iran Polym J. 2011;20:91–107.
Caracciolo PC, Rial-Hermida MI, Montini-Ballarin F, Abraham GA, Concheiro A, Alvarez-Lorenzo C. Surface-modified bioresorbable electrospun scaffolds for improving hemocompatibility of vascular grafts. Mater Sci Eng C. 2017;75:1115–27. https://doi.org/10.1016/j.msec.2017.02.151.
Abraham GA, De Queiroz AAA, Román JS. Immobilization of a nonsteroidal antiinflammatory drug onto commercial segmented polyurethane surface to improve haemocompatibility properties. Biomaterials. 2002;23:1625–38. https://doi.org/10.1016/S0142-9612(01)00289-7.
Guan YQ, Zheng Z, Li Z, Liu JM. Cell death in HeLa mediated by thermoplastic polyurethane with co-immobilized IFN-γ plus TNF-α. Acta Biomaterialia. 2012;8:1348–56. https://doi.org/10.1016/j.actbio.2011.11.023.
Zhang J, Yang B, Jia Q, Xiao M, Hou Z. Preparation, physicochemical properties, and hemocompatibility of the composites based on biodegradable poly(ether-ester-urethane) and phosphorylcholine-containing copolymer. Polymers. 2019;11:860 https://doi.org/10.3390/polym11050860.
Hou Z, Xu J, Teng J, Jia Q, Wang X. Facile preparation of medical segmented poly(ester-urethane) containing uniformly sized hard segments and phosphorylcholine groups for improved hemocompatibility. Mater Sci Eng C 2020;109:110571 https://doi.org/10.1016/j.msec.2019.110571.
Ontaneda A, Annich GM. Novel surfaces in extracorporeal membrane oxygenation circuits. Front Med. 2018;5(NOV):321.
Park KD, Okano T, Nojiri C, Kim SW. Polyurethaneurea surfaces - effect of hydrophilic spacers. J Biomed Mater Res. 1988;22:977–92.
Chen H, Zhang Y, Li D, Hu X, Wang L, McClung WG, et al. Surfaces having dual fibrinolytic and protein resistant properties by immobilization of lysine on polyurethane through a PEG spacer. J Biomed Mater Res. 2009;90:940–6. https://doi.org/10.1002/jbm.a.32152.
Gu H, Chen X, Yu Q, Liu X, Zhan W, Chen H, et al. A multifunctional surface for blood contact with fibrinolytic activity, ability to promote endothelial cell adhesion and inhibit smooth muscle cell adhesion. J Mater Chem B. 2017;5:604–11. https://doi.org/10.1039/c6tb02808j.
Li D, Chen H, Glenn McClung W, Brash JL. Lysine-PEG-modified polyurethane as a fibrinolytic surface: Effect of PEG chain length on protein interactions, platelet interactions and clot lysis. Acta Biomaterialia. 2009;5:1864–71. https://doi.org/10.1016/j.actbio.2009.03.001.
Wu Z, Chen H, Li D, Brash JL. Tissue plasminogen activator-containing polyurethane surfaces for fibrinolytic activity. Acta Biomaterialia. 2011;7:1993–8. https://doi.org/10.1016/j.actbio.2011.01.026.
Li D, Chen H, Wang S, Wu Z, Brash JL. Lysine–poly(2-hydroxyethyl methacrylate) modified polyurethane surface with high lysine density and fibrinolytic activity. Acta Biomaterialia. 2011;7:954–8. https://doi.org/10.1016/j.actbio.2010.10.021.
Anglés-Cano E. Overview on fibrinolysis: plasminogen activation pathways on fibrin and cell surfaces. Chem Phys Lipids. 1994;67–68:353–62. https://doi.org/10.1016/0009-3084(94)90157-0.
Klement P, Du YJ, Berry L, Andrew M, Chan AKC. Blood-compatible biomaterials by surface coating with a novel antithrombin-heparin covalent complex. Biomaterials. 2002;23:527–35. https://doi.org/10.1016/S0142-9612(01)00135-1.
Sheppeck IIJE, Kar H, Hong H. A convenient and scaleable procedure for removing the Fmoc group in solution. Tetrahedron Lett. 2000;41:5329–33.
Moon JH, Shin JW, Park JW. Self-assembly of aminosilane layers: determination of surface density of the amine group through a reversible chemical reaction. Mol Cryst Liq Cryst Sci Technol Sect A Mol Cryst Liq Cryst. 1997;295:185–8. https://doi.org/10.1080/10587259708042826.
Laemmli UK. Cleavage of structural proteins during the assembly of head of bacteriophage T4. Nat Publ Group. 1970;227:680–5.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–75. https://doi.org/10.1038/nmeth.2089. Accessed 9 July 2014.
R Core Team. R: A language and environment for statistical computing. 2019.
Meng F, Qiao Z, Yao Y, Luo J. Synthesis of polyurethanes with pendant azide groups attached on the soft segments and the surface modification with mPEG by click chemistry for antifouling applications. RSC Adv. 2018;8:19642–50. https://doi.org/10.1039/c8ra02912a.
Xie D, Howard L, Almousa R. Surface modification of polyurethane with a hydrophilic, antibacterial polymer for improved antifouling and antibacterial function. J Biomater Appl. 2018;33:340–51. https://doi.org/10.1177/0885328218792687.
Feng Y, Tian H, Tan M, Zhang P, Chen Q, Liu J. Surface modification of polycarbonate urethane by covalent linkage of heparin with a PEG spacer. Trans Tianjin Univ. 2013;19:58–65. https://doi.org/10.1007/s12209-013-1894-y.
Zhou Z, Meyerhoff ME. Preparation and characterization of polymeric coatings with combined nitric oxide release and immobilized active heparin. Biomaterials. 2005;26:6506–17. https://doi.org/10.1016/j.biomaterials.2005.04.046.
Joung YK, Hwang IK, Park KD, Lee CW. CD34 monoclonal antibody-immobilized electrospun polyurethane for the endothelialization of vascular grafts. Macromol Res. 2010;18:904–12. https://doi.org/10.1007/s13233-010-0908-z.
Lin H‐B, Sun W, Mosher DF, García-Echeverría C, Schaufelberger K, Lelkes PI, et al. Synthesis, surface, and cell‐adhesion properties of polyurethanes containing covalently grafted RGD‐peptides. J Biomed Mater Res. 1994;28:329–42. https://doi.org/10.1002/jbm.820280307.
Kang IK, Kwon OH, Kim MK, Lee YM, Sung YK. In vitro blood compatibility of functional group-grafted and heparin-immobilized polyurethanes prepared by plasma glow discharge. Biomaterials. 1997;18:1099–107. https://doi.org/10.1016/S0142-9612(97)00035-5.
Hörl H-H, Nussbaumer D, Wünn E. Process for grafting of nitrogen containing polymers and polymers obtained thereby. Sartorius AG. US5556708, 09/17/1996. https://patentscope.wipo.int/search/en/detail.jsf?docId=US38574050&_fid=WO1991003506.
McClung WG, Clapper DL, Hu SP, Brash JL. Adsorption of plasminogen from human plasma to lysine-containing surfaces. J Biomed Mater Res. 2000;49:409–14. https://doi.org/10.1002/(SICI)1097-4636(20000305)49:3<409::AID-JBM14>3.0.CO;2-0.
Arrua RD, Moya C, Bernardi E, Zarzur J, Strumia M, Igarzabal CIA. Preparation of macroporous monoliths based on epoxy-bearing hydrophilic terpolymers and applied for affinity separations. Eur Polym J. 2010;46:663–72. https://doi.org/10.1016/j.eurpolymj.2010.01.009.
Fields GB Methods for removing the Fmoc Group. In: Pennington MW, Dunn BM, eds. Methods in molecular biology (Clifton, N.J.), 35. Totowa, NJ: Humana Press; 1994:17–27.
Moon JH, Kim JH, Kim KJ, Kang T-H, Kim B, Kim C-H, et al. Absolute surface density of the amine group of the aminosilylated thin layers: ultraviolet-visible spectroscopy, second harmonic generation, and synchrotron-radiation photoelectron spectroscopy study. Langmuir. 1997;13:4305–10. https://doi.org/10.1021/la9705118.
Xu H, Luan Y, Wu Z, Li X, Yuan Y, Liu X, et al. Incorporation of lysine-containing copolymer with polyurethane affording biomaterial with specific adsorption of plasminogen. Chin J Chem. 2014;32:44–50. https://doi.org/10.1002/cjoc.201300735.
McClung WG, Clapper DL, Hu SP, Brash JL. Lysine-derivatized polyurethane as a clot lysing surface: conversion of adsorbed plasminogen to plasmin and clot lysis in vitro. Biomaterials. 2001;22:1919–24. https://doi.org/10.1016/S0142-9612(00)00378-1.
Tang Z, Liu X, Luan Y, Liu W, Wu Z, Li D, et al. Regulation of fibrinolytic protein adsorption on polyurethane surfaces by modification with lysine-containing copolymers. Polym Chem. 2013;4:5597–602. https://doi.org/10.1039/c3py00710c.
Yang W, Tang Z, Luan Y, Liu W, Li D, Chen H. Thermoresponsive copolymer decorated surface enables controlling the adsorption of a target protein in plasma. ACS Appl Mater Interfaces. 2014;6:10146–52. https://doi.org/10.1021/am501193b.
Wang H, Feng Y, Fang Z, Yuan W, Khan M. Co-electrospun blends of PU and PEG as potential biocompatible scaffolds for small-diameter vascular tissue engineering. Mater Sci Eng C. 2012;32:2306–15. https://doi.org/10.1016/j.msec.2012.07.001.
Luan Y, Li D, Wei T, Wang M, Tang Z, Brash JL, et al. “Hearing Loss” in QCM measurement of protein adsorption to protein resistant polymer brush layers. Anal Chem. 2017;89:4184–91. https://doi.org/10.1021/acs.analchem.7b00198.
Vroman L, Adams AL, Fischer GC, Munoz PC. Interaction of high molecular weight kininogen, factor XII, and fibrinogen in plasma at interfaces. Blood. 1980;55:156–9. Accessed 12 Jan 2021 http://www.bloodjournal.org.
Acknowledgements
This work was supported by the Argentinian National Agency of Scientific and Technological Promotion (PICT 2015-153 and PICT 2018–2334 grants), National Research Council (PIP 2017-0153 and PIP 2015-0596 grants) and Universidad Nacional de Mar del Plata (EXA 832/17 and PI2Ba 2020-08 grants). AP thanks the Argentinian National Scientific and Technical Research Council (CONICET) for the postdoctoral fellowship awarded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pepe, A., Guevara, M.G., Abraham, G.A. et al. Lysine-oligoether-modified electrospun poly(carbonate urethane) matrices for improving hemocompatibility response. Polym J 53, 1393–1402 (2021). https://doi.org/10.1038/s41428-021-00534-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-021-00534-7