Abstract
Fibrin is an optimal scaffold for tissue-engineering applications because it mimics the extracellular matrix. Despite this interesting feature, fibrin gel owns only poor mechanical properties that limit its applications. Different approaches have been used for fibrin electrospinning, however all the methods investigated required washing steps, cross-linking agent treatment or immersion. The aim of this work was to produce a bilayered fibrin/polyurethane scaffold by combination of the electrospun method and the spray, phase-inversion method for the preparation of a fibrin nanostructured layer to be attached onto a poly(ether)urethane microporous support layer. The synthetic layer was obtained by the spray, phase-inversion technique onto a rotating metallic collector, while fibrinogen was processed to obtain a nanofibrous structure by electrospinning. Finally, fibrin polymerization was obtained by thrombin solution spraying onto the electrospun nanofibers. SEM analysis showed the formation of filamentous structure with diameter in the range of μm attached onto the synthetic layer. This scaffold could be applied in soft tissue regeneration such as wound healing or as drug delivery system.
Similar content being viewed by others
Introduction
Fibrin is a naturally occurring plasma protein that functions as a major element in the coagulation cascade, contributing to clot formation, cell interaction and wound healing. For these biological properties, fibrin gel was widely investigated as a hemostatic agent in surgery1, as a scaffold in tissue-engineering applications2, as an angiogenic promoter in vascular graft endothelialisation3, and as a drug delivery system in wound healing4.
Fibrin is an excellent scaffold for tissue-engineering applications because it mimics the extracellular matrix and improves cellular interaction with the scaffold. Despite these features, the poor mechanical properties of fibrin gel render it unsuitable for clinical applications in which it is necessary to handle fibrin gel or to provide mechanical strength, such as in wound healing. For these reasons, many researchers tried to combine fibrin with synthetic scaffolds5,6,7.
Electrospinning has been successfully employed to obtain nanostructured scaffold fibrin, however all the investigated methods required washing steps for water soluble polymer removal8, or thrombin and CaCl2 bath9 or cross-linking agent use such as glutaraldehyde vapour10,11.
The aim of this work was to produce a bilayered fibrin/polyurethane scaffold, by combination of the electrospun method and the spray, phase-inversion method for the preparation of a composite scaffold constituted by a fibrin nanostructured layer attached onto a polyurethane microporous support layer.
Results
Fibrin-based electrospun/sprayed scaffold characterization
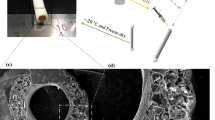
SEM images of poly(ether)urethane scaffold showed a microporous surface with pores of diameter 41.8 ± 21.5 μm (Fig. 1A,a), while the electrospun fibrinogen on the spray layer loaded with thrombin fibrin fiber resulted in a diameter on the micron order (4.8 ± 3.5 μm) (Fig. 1B,b). The final treatment with thrombin solution, through the spray technique induces a modification of the morphology of the electrospun filament fibrin layer. In particular, SEM images showed a flattened and fused fiber structure with diameter of 4.2 ± 2.9 μm (Fig. 1C,c). The scaffold final thickness was 230 ± 23 µm.
Peeling test
Manual peeling test revealed that the nanostructured fibrin layer was adherent onto the synthetic microporous surface of the bilayered scaffold.The SEM analysis of morphological structure of poly(ether)urethane spray layer after peeling of electrospun fibrin layer, revealed an adhesion of the layer onto the spray surface of the graft indicated by the presence of fused fibrin fiber on the porous synthetic support layer (Fig. 2).
Discussion
In a previous study we showed that the combination of spray and electrospinning techniques was successfully used for the fabrication of bilayered synthetic graft12. The material choice for the fabrication of two different layers, fibrin and polyurethane, was based on our previous experience in manufacturing scaffolds for wound healing applications4,5. These studies had shown the capability of fibrin as a delivery system for growth factors, platelet lysate, and of polyurethane as handling support either in vitro or in vivo experiments.
In this study we demonstrated the possibility of combining two different consolidated techniques to manufacture an electrospun fibrin layer firmly attached onto a polyurethane sprayed layer to enhance fibrin handling with the aim of application in soft tissue regeneration, such as in wound healing application. Moreover, since this manufacturing process does not require washing or immersion steps, it can be useful in the preparation of drug delivery devices.
Materials and Methods
Fibrin-based electrospun/sprayed scaffold fabrication
The Fibrin-based scaffold was fabricated by a combined spray/electrospinning apparatus equipped with metallic rotating cylindrical collectors. The support and the microporous layers were obtained using a biocompatible aromatic poly(ether)urethane (Estane 5714F1, Lubrizol, Oevel-Westerlo, Belgium) by a spray, phase-inversion method as previously described13. Polyurethane solutions (2 and 0.2%) were prepared by dissolving polyurethane grain in a solvent mixture of tetrahydrofuran and 1,4-dioxane (1:1).The 0.2% solution was brought close to the point of precipitation by the addition of 17% (v/v) of distilled water as a non-solvent. The support layer was obtained by co-spraying the 2% polyurethane solution and distilled water, while the microporous layer by the 0.2% polyurethane solution and thrombin solution at 25 U/mL in 275 mM CaCl2 on a collector of 5 cm in diameter at 88 rpm and flow rate of 2 mL/min for both solutions.
Then, a fibrinogen solution for electrospinning was prepared by dissolving the fibrinogen (80 mg/mL) in a solvent mixture of 1,1,1,3,3,3-heaxafluoro-2-propanol and distilled water (9:1, v/v). The fibrinogen layer was electrospun on the poly(ether)urethane microporous layer. Electrospinning was performed using needle-to-collector distance of 10 cm, voltage of 22 kV, flow rate of 0.5 mL/h and rotation speed of 125 rpm. The process was prolonged for 3 h to obtain an about 100 μm thick electrospun network.
Finally, thrombin solution (25 U/mL) was sprayed on the electrospun layer at a flow rate of 0.5 mL/min for 5 min and the scaffolds was incubated at 37 °C for 30 min to allow fibrin polymerization and scaffold drying.
Human fibrinogen and thrombin were supplied by Sigma-Aldrich (S. Louis, MO, USA).
Fibrin-based electrospun/sprayed scaffold characterization
The structure of the layers of fibrin-based scaffolds was evaluated by a scanning electron microscope (FlexSEM 1000, Hitachi, Tokyo, Japan). Scaffold samples (n = 3) were dry after the electrospinning process; therefore they did not require other treatments before SEM analysis. SEM microphotographs were taken at 100, 300, 700 and 1000x magnifications with a 5 or 10 kV acceleration voltage. The images (n = 5) acquired at 1000x were analyzed by a free, open source image processing program (ImageJ) to quantitatively determine the fibrin nanofibers mean diameter before and after reaction with the thrombin solution. Six random measurements were performed for each image.
Peeling test
Fibrin layer adhesion onto the synthetic surface of the scaffolds (n = 3) was qualitatively verified by pulling the sample edge with forceps and observing the presence of fibrin nanofibers on the synthetic material. Scaffold sampleswere analyzed by SEM to evaluate the morphological structure of the surface sprayed layer after peeling of electrospun layer12.
References
Spotnitz, W. D. Fibrin sealant: past, present, and future: a brief review. World J. Surg. 34, 632–4 (2010).
Ahmed, T. A., Dare, E. V. & Hincke, M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 14, 199–215 (2008).
Prasad, C. K. & Krishnan, L. K. Regulation of endothelial cell phenotype by biomimetic matrix coated on biomaterials for cardiovascular tissue engineering. Acta Biomater. 4, 182–91 (2018).
Losi, P. et al. Healing Effect of a fibrin-based scaffold loaded with platelet lysate in full-thikness skin wounds. J. Bioact. Compat. Polym. 30, 222–237 (2015).
Briganti, E. et al. A composite fibrin-based scaffold for controlled delivery of bioactive pro-angiogenetic growth factors. J. Control. Release 25, 14–21 (2010).
Hausner, T. et al. Nerve regeneration using tubular scaffolds from biodegradable polyurethane. Acta Neurochir. Suppl. 100, 69–72 (2007).
Hasanzadeh, E. et al. Preparation of fibrin gel scaffolds containing MWCNT/PU nanofibers for neural tissue engineering. J. Biomed. Mater. Res. A 107, 802–814 (2019).
Perumcherry, S. R., Chennazhi, K. P., Nair, S. V., Menon, D. & Afeesh, R. A.Novel method for the fabrication of fibrin-based electrospun nanofibrous scaffold for tissue-engineering applications. Tissue Eng. Part C Methods. 17, 1121–30 (2011).
Du, J. et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 55, 296–309 (2017).
McManus, M. C. et al. Mechanical properties of electrospun fibrinogen structures. Acta Biomater. 2, 19–28 (2006).
McManus, M. C. et al. Electrospun fibrinogen: feasibility as a tissue engineering scaffold in a rat cell culture model. J. Biomed. Mater. Res. A. 81, 299–309 (2017).
Al Kayal, T. et al. Combined method for bilayered vascular graft fabrication. J. Mater. Sci. Mater. Med. 26, 96 (2015).
Briganti, E. et al. Silicone based polyurethane materials: a promising biocompatible elastomeric formulation for cardiovascular applications. J. Mater. Sci. Mater. Med. 17, 259–66 (2006).
Acknowledgements
This work was supported through NanoCube Project by Tuscany Region (POR FESR 2014–2020).
Author information
Authors and Affiliations
Contributions
T.A.K., P.L. and S.P. carry out the experimental part and wrote the manuscript text and G.S. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al Kayal, T., Losi, P., Pierozzi, S. et al. A New Method for Fibrin-Based Electrospun/Sprayed Scaffold Fabrication. Sci Rep 10, 5111 (2020). https://doi.org/10.1038/s41598-020-61933-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61933-z
This article is cited by
-
Electrospun silk fibroin/fibrin vascular scaffold with superior mechanical properties and biocompatibility for applications in tissue engineering
Scientific Reports (2024)
-
Reinforced levan-based electrospun nanofibers for application as adhesive scaffolds for tissue engineering
Biotechnology and Bioprocess Engineering (2024)
-
Antimicrobial agents for biomaterial application
Emergent Materials (2023)
-
Cod Gelatin as an Alternative to Cod Collagen in Hybrid Materials for Regenerative Medicine
Macromolecular Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.