Abstract
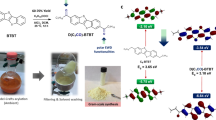
In this study, we first demonstrate the synthesis of recyclable polymer beads for Cu2+ sensing based on radially aligned liquid crystal (LC) assistance. To detect metal ions in water, sensing probe monomers and polymers derived from rhodamine B were synthesized. Recyclable polymeric LC beads were prepared from LC monomers RM257 and RM105, a nonreactive mesogen of 5CB and a rhodamine B-derived monomer. Due to the assistance of radially aligned LCs, highly sterically hindered spirocyclic terminal groups of rhodamine B-derived monomers were aligned and fixed at the outer surface of liquid crystal beads. The color of the polymeric LC beads changed from light pink to deep pink after the beads were dropped into an aqueous Cu2+ solution. The results were ascribed to the spiro ring-opening mechanism. The addition of a nonreactive mesogen resulted in the porous structure of the polymeric LC beads. The high sensitivity of the Cu2+ solution using polymeric LC beads was confirmed. The fabricated polymeric LC beads were recycled by putting the polymeric LC beads into aqueous ammonia. The removal of Cu2+ from polymeric LC beads was due to the formation of [Cu(NH3)4]2+. This recyclable LC bead sensor is an easy method for the detection of metal ions in aqueous solutions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout












Similar content being viewed by others
References
Jiao Z, Zhang P, Chen H, Li C, Chen L, Fan H, et al. Differentiation of heavy metal ions by fluorescent quantum dot sensor array in complicated samples. Sens Actuators B Chem. 2019;295:110–6.
Araoka F, Shin KC, Takanishi Y, Ishikawa K, Takezoe H, Zhu Z, et al. How doping a cholesteric liquid crystal with polymeric dye improves an order parameter and makes possible low threshold lasing. J Appl Phys. 2003;94:279–83.
Raghunandhan R, Chen LH, Long HY, Leam LL, So PL, Ning X, et al. Chitosan/PAA based fiber-optic interferometric sensor for heavy metal ions detection. Sens Actuators B Chem. 2016;233:31–8.
Denis M, Pancholi J, Jobe K, Watkinson M, Goldup SM. Chelating rotaxane ligands as fluorescent sensors for metal ions. Angew Chem Int Ed. 2018;57:5310–4.
Lee SJ, Lee JE, Seo J, Jeong IY, Lee SS, Jung JH. Optical sensor based on nanomaterial for the selective detection of toxic metal ions. Adv Funct Mater. 2007;17:3441–6.
Molina P, Tárraga A, Caballero A. Ferrocene‐based small molecules for multichannel molecular recognition of cations and anions. Eur J Inorg Chem. 2008;2008:3401–17.
Sugunan A, Thanachayanont C, Dutta J, Hilborn JG. Heavy-metal ion sensors using chitosan-capped gold nanoparticles. Sci Tech Adv Mater. 2005;6:335–40.
Harzat A, Ezzat K, Ikram I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem. 2019;2019:6730305.
Mehrandish R, Rahimian A, Shahriary A. Heavy metals detoxification: a review of herbal compounds for chelation therapy in heavy metals toxicity. J Herbmed Pharm. 2019;8:69–77.
Baatrup E. Structural and functional effects of heavy metals on the nervous system, including sense organs, of fish. Comp Biochem Physiol. 1991;100:253–7.
Bost M, Houdart S, Oberli M, Kalonji E, Huneau JF, Margaritits I. Dietary copper and human health: current evidence and unresolved issues. J Trace Elem Med Biol. 2016;35:107–15.
Gaetke LM, Chow-Johnson HS, Chow CK. Copper: toxicological relevance and mechanisms. Arch Toxicol. 2014;88:1929–38.
Valfredo AL, Elenir SS, Ednilton MG. A comparative study of two sorbents for copper in a flow injection preconcentration system. Sep Purif Technol. 2007;56:212–9.
Santos EM, Ball JS, Williams TD, Wu H, Ortega F, Aerle RV, et al. Identifying health impacts of exposure to copper using transcriptomics and metabolomics in a dish model. Environ Sci Technol. 2010;44:820–6.
Skibniewski M, Skibniewska EM, Kosia T, Olbrych K. The content of copper and molybdenum in the liver, kidneys, and skeletal muscles of elk (Alces alces) from North-Eastern Poland. Biol Trace Elem Res. 2016;169:204–10.
Marwa AI, Mai DI. Acrylamide‐induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ Toxicol. 2020;35:300–8.
Li Q, Liu H, Alattar M, Jiang S, Han J, Ma Y. et al. The preferential accumulation of heavy metals in different tissues following frequent respiratory exposure to PM2.5 in rats. Sci Rep.2015;5:16936.
Iamsaard S, Anger E, Aßhoff SJ, Depauw A, Fletcher SP, Katsonis N. Fluorinated azobenzenes for shape‐persistent liquid crystal polymer networks. Angew Chem Int Ed. 2016;55:9908–12.
Keith JM, King JA, Miller MG, Tomson AM. Thermal conductivity of carbon fiber/liquid crystal polymer composites. J Appl Poly Sci. 2006;102:5456–62.
Cattle J, Bao P, Bramble JP, Bushby RJ, Evans SD, Lydon JE, et al. Controlled planar alignment of discotic liquid crystals in microchannels made using SU8 photoresist. Adv Funct Mater. 2013;23:5997–6006.
Serra F, Eaton SM, Cerbino R, Buscaglia M, Cerullo G, Osellame R, et al. Nematic liquid crystals embedded in cubic microlattices: memory effects and bistable pixels. Adv Funct Mater. 2013;23:3990–4.
Yu H, Szilvási T, Rai P, Twieg RJ, Mavrikakis M, Abbott NL. Computational chemistry‐guided design of selective chemoresponsive liquid crystals using pyridine and pyrimidine functional groups. Adv Funct Mater. 2018;28:1703581.
Bögels GM, Lugger JAM, Goor OJGM, Sijbesma RP. Size‐selective binding of sodium and potassium ions in nanoporous thin films of polymerized liquid crystals. Adv Funct Mater. 2016;26:8023–30.
Jung YD, Khan M, Park SY. Fabrication of temperature- and pH-sensitive liquid-crystal droplets with PNIPAM-b-LCP and SDS coatings by microfluidics. J Mater Chem B. 2014;2:4922–8.
Wang N, Evans JS, Liu Q, Wang S, Khoo IC, He S. Electrically controllable self-assembly for radial alignment of gold nanorods in liquid crystal droplets. Opt Mat Express. 2015;5:1065–70.
Lee JH, Kamel T, Roth SV, Zhang P, Park SY. Structures and alignment of anisotropic liquid crystal particles in a liquid crystal cell. RSC Adv. 2014;4:40617.
Wang Y, Chang H, Wu W, Peng W, Yan Y, He C, et al. Rhodamine 6 G hydrazone bearing pyrrole unit: ratiometric and selective fluorescent sensor for Cu2+ based on two different approaches. Sens Actuators B Chem. 2016;228:395–400.
Doumani N, Maroun EB, Maalouly J, Tueni M, Dubois A, Bernhard C, et al. A new pH-dependent macrocyclic rhodamine B-based fluorescent probe for copper detection in white wine. Sensors. 2019;19:4514.
Maji A, Lohar S, Pal S, Chattopadhyay P. A New rhodamine based ‘turn-on’ Cu2+ ion selective chemosensor in aqueous system applicable in bioimaging. J Chem Sci. 2017;129:1423–30.
Li XM, Zhao R, Yang Y, Lv XW, Wei YL, Tan R, et al. A Rhodamine-based fluorescent sensor for chromium ions and its application in bioimaging. Chin Chem Lett. 2017;28:1258–61.
Wang J, Long L, Xiao G, Fang F. Reversible fluorescent turn-on sensors for Fe3+ based on a receptor composed of tri-oxygen atoms of amide groups in water. Open Chem. 2018;16:1268–74.
Cui P, Jiang X, Sun J, Zhang Q, Gao F. A water-soluble rhodamine B-derived fluorescent probe for pH monitoring and imaging in acidic regions. Methods Appl Fluoresc. 2017;5:024009.
Singh SK, Nandi R, Mishra K, Singh HK, Singh RK, Singh B. Liquid crystal based sensor system for the real time detection of mercuric ions in water using amphiphilic dithiocarbamate. Sens Actuators B Chem. 2016;226:381–7.
Han GR, Jang CH. Detection of heavy-metal ions using liquid crystal droplet patterns modulated by interaction between negatively charged carboxylate and heavy-metal cations. Talanta. 2014;128:44–50.
Xu Z, Zhang L, Guo R, Xiang T, Wu C, Zheng Z, et al. A highly sensitive and selective colorimetric and off–on fluorescent chemosensor for Cu2+ based on rhodamine B derivative. Sens Actuators B Chem. 2011;156:546–52.
Xu L, Xu Y, Zhu W, Zeng B, Yang C, Wu B, et al. Versatile trifunctional chemosensor of rhodamine derivative for Zn2+, Cu2+ and His/Cys in aqueous solution and living cell. Org Biomol Chem. 2011;9:8284–7.
Jun Y, Péter S, Daniel AP, John MDS, Corrie TI, Antal J, et al. Spherical-cap droplets of a photo-responsive bent liquid crystal dimer. Soft Matter. 2019;15:989–98.
Poon CK, Tang O, Chen XM, Kim B, Hartlieb M, Pollock CA. et al. Fluorescent labeling and biodistribution of latex nanoparticles formed by surfactant-free RAFT emulsion polymerization. Macromol Biosci. 2017;17:1600366.
Kumar D, Talreja N. Nickel nanoparticles-doped rhodamine grafted carbon nanofibers as colorimetric probe: naked eye detection and highly sensitive measurement of aqueous Cr3+ and Pb2+. Korean J Chem Eng. 2019;36:126–35.
Tseng MH, Hu CC, Chiu TC. A fluorescence turn-on probe for sensing thiodicarb using rhodamine B functionalized gold nanoparticles. Dyes Pigm. 2019;171:107674.
Saha S, Chhatbar MU, Mahato P, Praveen L, Siddhanta AK, Das A. Rhodamine–alginate conjugate as self indicating gel beads for efficient detection and scavenging of Hg2+ and Cr3+ in aqueous media. Chem Commun. 2012;48:1659–61.
Wnag D, Park SY, Kang IK. Liquid crystals: emerging materials for use in real-time detection applications. J Mater Chem C. 2015;3:9038–47.
Kim HN, Lee MH, Kim HJ, Kim JS, Yoon J. A new trend in rhodamine-based chemosensors: application of spirolactam ring-opening to sensing ions. Chem Soc Rev. 2008;37:1465–72.
Niu X, Luo D, Chen R, Wang F. Optical biosensor based on liquid crystal droplets for detection of cholic acid. Opt Commun. 2016;381:286–91.
Acknowledgements
The authors would like to thank the Ministry of Science and Technology (MOST) of the Republic of China (Taiwan) for financially supporting this research under contract MOST 107-2923-E-006-001 and MOST 108-2218-E-006-049. This research was also supported in part by the Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University (NCKU).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, JH., Hung, YH., Lin, SN. et al. Recyclable liquid crystal polymeric sensor beads based on the assistance of radially aligned liquid crystals. Polym J 53, 373–383 (2021). https://doi.org/10.1038/s41428-020-00428-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-00428-0