Abstract
As one of the most intractable neurological diseases, spinal cord injury (SCI) often leads to permanent neurological impairment in patients. Unfortunately, due to the complex pathological mechanisms and unique postinjury microenvironment, there is currently no way to completely repair the injured spinal cord. In recent years, with the rapid development of tissue engineering technology, the combination of biomaterials and medicine has provided a new idea for treating SCI. Here, we systematically summarize representative biomaterials, including natural, synthetic, nano, and hybrid materials, and their applications in SCI treatment. In addition, we describe several state-of-the-art fabrication techniques for tissue engineering. Importantly, we provide novel insights for the use of biomaterial-based therapeutic strategies to reduce secondary damage and promote repair. Finally, we discuss several biomaterial clinical studies. This review aims to provide a reference and new insights for the future exploration of spinal cord regeneration strategies.
Similar content being viewed by others
Introduction
Spinal cord injury (SCI) refers to damage to the spinal cord caused by direct or indirect external factors that often results in sensory, motor, and autonomic dysfunctions below the level of injury1. According to the World Health Organization, between 250,000 and 500,000 people suffer from SCI each year2. In Canada, the total annual economic burden of SCI is estimated at $2.67 billion3. While advances in clinical management have reduced morbidity and improved patient outcomes, there is still a lack of effective repair strategies that promote SCI recovery.
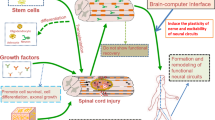
Developing new approaches to spinal cord restoration requires an in-depth comprehension of the drastic changes in the microenvironment following injuries. Dynamic alterations of cell number, phenotypes, and spatiotemporal distribution are found in the SCI microenvironment. Primary injuries refer to the compression, splitting, twisting or shearing of the spinal cord caused by direct or indirect external mechanical force, resulting in axonal breakage, nerve tissue destruction and neuronal death4. These primary injuries trigger a cascade of secondary pathophysiological events. Disruption of the blood‒spinal cord barrier (BSCB) triggers inflammatory cells to invade the afflicted region, releasing a large number of inflammatory factors5. Phagocytic inflammatory cells produce reactive oxygen species (ROS), further inducing cellular necrosis and apoptosis6. Subsequently, glial and fibrous scars form at the lesion site. These secondary injuries inhibit neuroregeneration and prolongs the duration of injury, impeding tissue regeneration and functional recovery7 (Fig. 1).
Adapted with permission240. Copyright 2023, Springer Nature. All figures in this review should be printed in color.
As tissue engineering progresses, biomaterials, predominantly nanoscale materials of various structures, have become pivotal. These materials are tailored to interact with the nervous system with high specificity. They can serve not only as spatial scaffolds or drug/cell delivery vehicles8 but also as artificial bioactive niches that resemble the extracellular matrix (ECM)9,10. In this review, we summarize representative biomaterials for injured spinal cord repair, advanced tissue engineering fabrication techniques, biomaterial-based therapeutic strategies, and several clinical studies of functional bioscaffolds. This review aims to provide a reference and novel insights for further exploration of spinal cord regeneration strategies.
Biomaterials for SCI repair
Why we use biomaterials for SCI repair
The most common approach to repair peripheral nerve injuries is surgical direct repair/direct coaptation11. When the gap in a peripheral injury exceeds 5 mm, autograft reconstruction is often used as the “gold standard” treatment12,13. However, central nervous system (CNS) injury is accompanied by the formation of an inhibitory milieu, which often restricts nerve regeneration14,15,16. Therefore, these two conventional strategies are not sufficient for treating CNS injuries. In recent decades, various natural and synthetic biomaterials have been tailored based on selective physical and chemical properties to promote a favorable microenvironment and CNS nerve regeneration between severed nerve stumps17,18.
Currently, biological approaches to restoring neural connectivity at the lesion site are focused on delivering biomaterials to the lesion to re-establish communication through new relay circuits or to stimulate endogenous axon regrowth. By manipulating the components, morphology and architecture of biomaterials, they can produce suitable topographical and biochemical cues that promote tissue regeneration. These biomaterials can also be modified to offer a permissive substrate for axonal penetration into the injured CNS region19,20.
To promote neural and nonneural cell differentiation as well as axonal growth, bioscaffolds should possess essential characteristics such as degradability and compatibility (Fig. 2). Therefore, the selection of suitable scaffold materials is crucial for the overall design strategy of neural tissue engineering. Moreover, advanced fabrication and tissue engineering techniques are required. In the following sections, we summarize the main scaffold materials and state-of-the-art fabrication techniques.
Reproduced with permission241. Copyright 2020, American Chemical Society.
Representative scaffolding materials
Natural Materials
Collagen
Collagen is a principal constituent of the ECM and exhibits high conservation across species, making it readily accessible. It stands out for its nontoxicity, biocompatibility, and biodegradability21. Furthermore, as a bioprinting material, collagen has the potential to carry targeted tissue, cells, and biomolecules to exert the desired pharmacokinetics and therapeutic effects22. Owing to these advantages, collagen has been extensively used for bioprinting23 and cell transplantation24 in SCI. Collagen biomaterials can be manufactured into different scaffolds, including hydrogels25, electrospun fibers26,27, linear-ordered collagen scaffolds (LOCS)28, aligned sponges29, and tubes30. Through cross-linking reactions and noncovalent interactions, collagen scaffolds can be further modified to control mechanical properties and biochemical functions31. However, due to the shortcomings of collagen scaffolds, including weak biomechanical properties, limited plasticity, and rapid degradation32, recent studies have focused on composite scaffolds, such as collagen/gelatin33 and collagen/chitosan scaffolds23.
Fibrin
Fibrin, composed of fibrinogen and thrombin, is one of the most important proteins involved in hemostasis and tissue regeneration. In recent years, fibrin-based biomaterials have been extensively investigated in spinal cord tissue engineering. Nazari et al. demonstrated that fibrin hydrogels could increase the viability of cells and trigger the differentiation of induced pluripotent stem cells (iPSCs) into preoligodendrocytes, thereby promoting injured spinal cord repair34. Yao et al. implanted a three-dimensional (3D) aligned fibrin hydrogel (AFG) scaffold into the spinal cords of dorsal hemi-transection model rats, which resulted in faster motor function recovery compared to that of the control group over a 2-week period35. Similar to collagen, its poor mechanical properties can be overcome by combining it with other materials, such as poly-ε-caprolactone (PCL)36.
Hyaluronic Acid
As the main component of the ECM, hyaluronic acid (HA) is highly concentrated in the nervous system, particularly in the CNS, and possesses excellent biocompatibility and biodegradability. Studies have shown that HA scaffolds can provide neuroprotection and promote injured spinal cord repair by alleviating secondary damage after SCI37. Nevertheless, HA materials exhibit disadvantages such as poor cell adhesion38. To overcome these drawbacks, HA can be modified, such as with the integrin-binding peptide arginine-glycine-aspartic acid (RGD)39, to enhance cell adhesion and migration to implants. Moreover, 3D hydrogel scaffolds prepared by blending HA with gelatin are effective in promoting endogenous neural stem cell (NSC) migration and neurogenesis40.
Chitosan
Chitosan is obtained by the deacetylation of chitin, which is widely found in the cell walls of crustaceans, bacilli, and fungi41,42. As a natural polymer with low immunogenicity, nontoxicity, biodegradability, and suitable cell adhesion, chitosan is an ideal scaffold material and has been extensively studied in the repair of SCI. When implanted into the rat spinal cord after bilateral dorsal hemisection, physical chitosan microhydrogels were shown to promote spinal tissue and vasculature reconstitution, diminish fibrous glial scarring, and modulate the inflammatory response43. However, chitosan-based scaffolds also have issues, such as rapid degradation and increased swelling. Compounding chitosan with other materials results in a scaffold with complementary properties, enhancing the overall performance of the scaffold. For example, a composite hydrogel scaffold composed of chitosan and alginate was used as a tissue-engineered spinal cord, which exhibited better mechanical properties, suitable swelling, negligible toxicity, and suitable biocompatibility with adipose-derived mesenchymal stem cells44.
Alginate
Alginate is a natural polysaccharide carbohydrate extracted from the cell walls of brown algae. As a tissue engineering biomaterial, in addition to its advantages of biocompatibility, low toxicity and low cost, alginate can be cross-linked with divalent cations (e.g., Ca2+) to form alginate hydrogels under mild conditions. Depending on the divalent cation used, the physical properties (e.g., mechanical strength, elasticity, or stability) vary45. The use of alginate-based hydrogels in regenerative medicine has grown exponentially. Nonfunctionalized soft alginate hydrogels showed potential in preventing fibrous scarring and promoting functional recovery after a severe SCI46; for chronic SCI, implants of alginate hydrogels showed the ability to restore the anatomical continuity of the spinal cord, slightly improve motor function, and restore partial electrophysiological conductivity47. Likewise, to enhance the repair effect, alginate is often combined with other natural materials, cells, and growth factors48,49,50.
Polysialic Acid
Polysialic acid (PSA) is an endogenous polysaccharide attached to nerve cell adhesion molecules that control CNS development by regulating cell adhesion and promoting axonal growth. Induction of endogenous PSA expression through cell- or gene-based approaches, such as broad or selected targeting at neurons51, astrocytes52, or transplanted Schwann cells53,54, is a strategy for the treatment of SCI. There is no solid evidence for the efficacy of exogenous PSA infusion in repairing injured spinal cord, but several PSA mimetics, such as PSA cyclic mimetic peptide (PR-21)55, linear mimetic peptide H-NTHTDPYIYPID-OH56, tegaserod57, and 5-nonyloxytryptamine oxalate58 have shown therapeutic effects in animal models of SCI. In recent years, drug-loaded PSA scaffolds or micelles have shown therapeutic effects on SCI59,60. The exact mechanism of exogenous PSA in the treatment of SCI remains to be elucidated.
Gelatin
Gelatin is a macromolecular hydrophilic colloid partially hydrolyzed from collagen that promotes cell attachment and proliferation due to the presence of abundant RGD sequences and integrin-binding motifs61. Using gelatin-based biomaterial scaffolds for the treatment of SCI has been extensively studied. For example, a photosensitive gelatin hydrogel scaffold was loaded with collagen-binding stromal cell-derived factor-1α and Taxol liposomes to promote their sustained release, thereby synergistically promoting neurovascular unit regeneration and motor function improvement62. Moreover, 3D gelatin methacrylate (GelMA) hydrogel combined with iPSC-derived NSCs reduced the cavity areas, inhibited glial scar formation, and promoted axonal regeneration63. Furthermore, gelatin hydrogels can be modified with enzymes. For example, a dual-enzymatically cross-linked gelatin hydrogel by hydrogen horseradish peroxidase and galactose oxidase could enhance the survival, proliferation, and neural differentiation of human umbilical cord MSCs (hUC-MSCs)64. In conclusion, gelatin-based scaffolds have great therapeutic potential.
Agarose
Agarose, a linear polymer derived from seaweed, has been shown in a previous in vitro study to promote cell attachment and neurite outgrowth65. Further studies found that after the implantation of agarose scaffolds with uniaxial channels into the injured rat spinal cord, the scaffolds could be well integrated with host tissue, where axons grew in a strikingly linear fashion66. Combining materials is a common strategy. The experimental results of Han et al. demonstrated that an agarose scaffold containing Matrigel could enhance linear axon regeneration and promote the reconnection of functional axons after SCI67. An agarose/gelatin/polypyrrole (PPy) hydrogel with similar conductivity and modulus as the spinal cord can fill the cavity, mimicking the physiological properties of the spinal cord to promote injured spinal cord repair68. Agarose can also be used for cell delivery69,70,71, drug delivery72, and bioink preparation73.
Decellularized extracellular matrix scaffolds
Decellularized extracellular matrix (dECM) scaffolds are constructed by removing cells from the matrix (namely, decellularization)74. Injectable hydrogels, electrospun scaffolds and bioprinted scaffolds are three common types of dECM scaffolds75 (Fig. 3). In addition to the advantages of suitable histocompatibility, low immunogenicity, suitable degradability and nontoxicity of degradation products, dECM scaffolds can also simulate an optimal nonimmune environment with native 3D structures and various bioactive components76. For the treatment of SCI, acellular spinal cord (ASC) scaffolds have been widely studied. They are commonly used as carriers for stem cells, drugs, and neurotrophic factors77,78. To enhance mechanical properties, ASC scaffolds are often modified or compounded with other materials79,80. In addition to ASC scaffolds, dECM scaffolds derived from other tissue have also been reported, such as decellularized meningeal scaffolds81 and decellularized optic nerve scaffolds82.
Reproduced with permission75. Copyright 2022, Elsevier.
Synthetic materials
Compared with natural scaffolds, synthetic materials show the advantages of high raw material availability, controllable biodegradability and customizable physical and mechanical properties and have been widely used in scientific research.
Synthetic polymeric materials
There are many kinds of synthetic polymeric materials, including degradable polymers (e.g., PCL) and nondegradable polymers (e.g., poly(2-hydroxyethyl methacrylate)). In Table 1, we summarize some commonly used synthetic polymeric materials in the construction of bioscaffolds.
In addition to the materials summarized in Table 1, studies on conductive polymers such as polyaniline and PPy are emerging. After SCI, the electrical properties of tissue cells at the injury site are altered, generating an extracellular endogenous electric field with the ability to guide cell migration and promote tissue repair. However, in the case of severe SCI, current conduction is blocked, inhibiting self-repair of the injured spinal cord83, which is the theoretical basis for the application of conductive polymers in spinal cord tissue engineering. Wang et al. assessed an electroactive polyaniline-polylactic acid scaffold that promoted the adhesion, survival and proliferation of olfactory ensheathing cells84. However, further in vitro experiments are needed to observe the various behavioral changes of cells on the scaffold surface under different electrical stimuli. A soft conducting polymer hydrogel (CPH) prepared by mixing tannic acid (TA), pyrrole (Py), and Fe3+ has been shown to promote NSC differentiation into neurons rather than astrocytes in vitro and to activate new endogenous neurogenesis in vivo85 (Fig. 4). These studies reveal the unlimited potential of conductive biomaterials in the treatment of SCI.
a Schematic illustration of the preparation of a cross-linked CPH. b The possible therapeutic mechanism of CPH. Reproduced with permission85. Copyright 2018, American Chemical Society.
Synthetic Peptide Materials
Consisting of short and repeating amino acid units, self-assembling peptides (SAPs) have been widely applied to SCI regeneration due to their suitable mechanical strength, suitable water solubility and excellent biocompatibility86. Four main SAP systems and representative examples are shown in Fig. 5. SAP scaffolds can function as carriers. SAP hydrogels loaded with chondroitinase ABC (ChABC) facilitated host neural regeneration and behavioral recovery in chronic SCI model rats, since the stable and sustained release of ChABC attenuated the inhibitory effect of chondroitin sulfate proteoglycans (CSPGs)87. To further improve the properties of SAP scaffolds, they can be modified. For example, by mixing RADA16 (the SAP) and FGL (the motif from a neural cell adhesion molecule), researchers prepared an FGLmx peptide hydrogel scaffold with the function of promoting NSC proliferation and migration88. Hong et al. showed that RADA16 modified with substance P stimulated angiogenesis and enhanced functional improvement in rats with spinal cord contusion injury89.
Reproduced under terms of the CC-BY license242. Copyright 2022, Wiley‐VCH GmbH.
Nanomaterials
Some nanomaterials have electronic and catalytic properties that positively affect neural regeneration. GO and carbon nanotubes are two typical examples. By genipin crosslinking and lyophilization, Yang and coworkers fabricated a conductive GO-composited chitosan scaffold90. It was shown to promote the oriented growth of nerve cells, angiogenesis, neuron migration and neural tissue regeneration. However, there is still debate regarding the toxicity of graphene-derived materials91. Bendable microfibers composed of reduced graphene oxide have been shown to promote cell adhesion without evident signs of subacute local toxicity92. In addition, to GO, Usmani et al. reported an artificial carbon nanotube-based scaffold that could improve motor function recovery in SCI model rats, possibly owing to regenerating fibers successfully crossing the lesion site93. Some nanoparticles (NPs) have oxidation and reduction catalytic capabilities to relieve oxidative stress following SCI. For example, by dispersing manganese dioxide (MnO2) NPs in a PPFLMLLKGSTR peptide-modified hydrogel, Li et al. fabricated an MnO2 NP-dotted HA hydrogel94. In addition to promoting stem cell adhesion, growth and neural tissue bridging, it also alleviated the oxidative environment to effectively improve MSC viability (Fig. 6).
Reproduced with permission94. Copyright 2019, American Chemical Society.
Hybrid Materials
Hybrid materials are mixtures of two nano- or molecular-scale components. They possess superior spinal cord tissue engineering properties with the advantages of synthetic, natural, and nanomaterials and have become a research hotspot in recent years. The RADA16 hybrid hydrogel scaffold constructed by Zhai et al. possessed enhanced mechanical properties and slower degradation while retaining the nanostructure of the peptide and achieved remarkable results in animal experiments95. By using Fmoc-grafted chitosan and Fmoc peptide, an injectable and self-healing hybrid hydrogel was developed with the ability to release curcumin slowly and persistently96. It could facilitate functional recovery after SCI by locally reassembling the ECM, modulating the local inflammatory response and recruiting Schwann cells to remyelinate the regenerated nerves. By constructing porous PLGA microcapsules loaded with neurotrophin-3 (NT-3) within GelMA hydrogel, Chiang et al. established a hierarchical hybrid GelMA-microcapsule hydrogel (HGMH)97. As shown in Fig. 7, the HGMH platform provided a four-dimensional (4D) spatiotemporal distribution of NT-3, which induced NSC differentiation and migration, thereby promoting the full recovery after SCI in rats.
Reproduced with permission97. Copyright 2021, Elsevier.
Fabrication Techniques
Hydrogel
Hydrogel is a functional polymeric material with 3D network structures that can swell and retain plenty of water in its structure98. Many natural materials that are widely available and inexpensive can be prepared into hydrogels. Recently, the research and application of hydrogels have become increasingly abundant. However, simplex hydrogel scaffold materials often have difficulty accurately matching the size and shape of the defect in SCI, and the invasive implantation process can easily lead to secondary injuries99. Injectable hydrogels offer a solution, as they can fill pathological defects with minimal surgical trauma by injecting flowable hydrophilic materials in situ. In addition, as suitable carriers for sustained drug release, injectable hydrogels have high drug loading efficiency, as well as the ability to uniformly and continuously release the loaded substances100,101. They can also carry stem cells, such as NSCs63 or MSCs102, to promote nerve regeneration and local microenvironment repair. Stimulus-responsive smart hydrogels, such as conductive hydrogels68,103 and thermosensitive hydrogels104, are another promising class of hydrogels that have provided new directions for SCI treatment. With multiple controllable properties in terms of time or space, these smart hydrogels can quickly respond to changes in the external environment to achieve the controlled release of drugs105. Nevertheless, there are still many issues to be solved, including relatively weak mechanical properties (especially natural polymer hydrogels) and excessively high swelling properties. Walsh et al. summarized the ideal physical properties of hydrogels for injured spinal cord repair106 (Fig. 8).
Reproduced under terms of the CC-BY license106. Copyright 2022, Elsevier.
Electrospinning
The electrospinning technique refers to the process in which polymers in a molten or dissolved state are formed into fibers under the action of a high-voltage electrostatic field. As a technique for preparing fibers with nano- to micron-scale diameters26, it is widely used to create nerve scaffolds not only for its simple experimental equipment but also for its feasibility in controlling the morphological characteristics of fibers and porosity of fiber surfaces83,107. The previously described AFG scaffold is a typical example35, suggesting that oriented scaffold structures can promote cell migration and tissue regeneration, which is exactly the advantage of electrospinning. In addition, the electrospinning process enables the incorporation of all kinds of substances into nanofibers, such as proteins, low-molecular lipophilic drugs and nucleic acids.
3D Printing
Because of the highly spatially ordered cellular distribution and 3D structure of native spinal cord tissue, conventional 3D scaffolds have limitations in adequately and accurately controlling the external shape and internal architecture108,109,110. An emerging technology, 3D printing, offers a solution because it enables the fabrication of individualized scaffolds that match the precise structure of SCI and provide a suitable microenvironment to stimulate and guide axon regeneration. Koffler et al. used the 3D printing technique to precisely construct the transverse architecture of gray and white matter of the spinal cord in rats and then grafted neural progenitor cells (NPCs) within the 3D scaffold111 (Fig. 9). In this study, the combination of 3D biomimetic scaffolds and NSC grafts significantly enhanced host axonal regeneration into the lesion site and supported partial motor functional recovery, as assessed by the Basso, Beattie and Bresnahan (BBB) locomotor scale112,113. In addition, astrocytic processes were strikingly rearranged, penetrating into scaffold channels rather than forming a “wall” at the host/scaffold interface.
a Schematic diagram of the 3D-printed scaffold. Fluorescence images of b) NPCs, c Corticospinal axons, d Corticospinal axons converging on a neuron, e NPC-derived axons and f Excitatory contacts (VGlut2) forming between NPC-derived axons and host neurons. g BBB motor scores. h Schematic diagram of the electrophysiology study. i Motor evoked potential (MEP) responses. j Mean MEP amplitude. Reproduced with permission111. Copyright 2019, Springer Nature.
Decellularization Techniques
A variety of dECM scaffolds have been introduced above and have many advantages, such as suitable histocompatibility and low immune rejection. Furthermore, decellularization can remove inhibitory components to restore the balance of the SCI immune microenvironment114. The preparation methods of decellularized scaffolds include physical, chemical, and biological methods. In general, two or more methods are used in combination to preserve more of the physiological structure and biological activity of the ECM under the premise of maximizing decellularization115. In addition, the preparation method can be further improved. For instance, Xing et al. combined 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide crosslinking with chemical extraction methods, significantly improving the preparation efficiency of ASC scaffolds116. It is worth noting that appropriate and sufficient biological tissue sources are the biggest dilemmas limiting the development of this technology. The cryopreservation technique of meningeal tissues studied by Vishwakarma et al. solved this problem to a certain extent117. The different challenges that can be encountered during decellularization are summarized in Fig. 10.
Reproduced under terms of the CC-BY license243. Copyright 2022, Frontiers.
Biomaterial-based therapeutic strategies for SCI
Strategies to limit the impact of secondary events
As mentioned above, the initial SCI triggers various secondary cascades that result in an inhibitory neuroregenerative microenvironment as well as an expanded lesion area. To restore normal function, managing these secondary cascades is an important first step118. Below, we identify four broad targets for intervention.
Managing inflammation
After SCI, activated immune cells infiltrate the lesion site, releasing inflammatory factors and ROS. These prolonged immune and inflammatory cascades lead to secondary damage. Hence, it is beneficial to deliver anti-inflammatory treatments to modulate the immune microenvironment of SCI119.
Biomaterials commonly function as carriers for the local delivery of anti-inflammatory drugs. For example, by using PLGA NPs embedded in agarose gel, low doses of 17-β estradiol were focally delivered to the contused spinal cord, eliciting rapid anti-inflammatory effects120. An injectable PLGA-PEG-PLGA thermosensitive hydrogel designed by Zheng et al. controllably released the JAK1/2 inhibitor baricitinib, which led to decreased expression levels of inflammatory cytokines and improved functional recovery121. Intriguingly, local delivery of flavopiridol, a proliferation inhibitor originally developed to treat chronic lymphocytic leukemia122, in PLGA NPs was also proven to improve recovery from SCI by inhibiting inflammatory factor synthesis123. In addition to conventional drugs, biomaterials can deliver endogenous biological signaling molecules to suppress inflammation. By activating nuclear factor-erythroid 2-related factor 2 protein, H2S exerted a series of neuroprotective effects on SCI model rats, including reducing the secretion of inflammatory factors, oxidative stress, and nerve cell apoptosis124. Jiang et al. reported a near-infrared light-triggered NO-releasing system, which was constructed with upconversion NPs coated by zeolitic imidazolate framework–8 and an NO photochemical donor125. In vivo experiments proved that NO can effectively inhibit gliosis and inflammation, promote neural regeneration and protect neurons from apoptosis.
Biomaterials can have innate anti-inflammatory properties. Chitosan, for example, can control inflammation by inhibiting the secretion of TNF-α and IL-6126. Ferulic acid-modified glycol chitosan NPs can not only suppress astrogliosis and inflammation but also preserve axons and myelin127. Partially reduced GO scaffolds were able to attenuate the inflammatory response while supporting angiogenesis128. Polylaminin, a polymeric form of laminin, also exerts an unsuspected anti-inflammatory effect, which may involve interfering with macrophage recruitment to the lesion site129. Austin et al. proved that intrathecal injection of a hyaluronan and methyl cellulose hydrogel could dampen SCI-related arachnoiditis and improve functional recovery130.
Reducing oxidative stress
Superoxide, hydrogen peroxide, and hydroxyl radicals are three physiologically relevant ROS, the production and elimination of which are tightly controlled. After SCI, excess ROS are released into the extracellular microenvironment, disrupting this equilibrium. Reducing ROS is often a byproduct of reducing inflammation, but it equally deserves attention, given that inflammation is not the only source of ROS118. The intake of antioxidant molecules is a countermeasure to reduce ROS, but they have limitations such as easy degradation, poor water solubility, and low bioavailability131. Fortunately, the antioxidant properties of natural and synthetic compounds have been extensively studied, leading to the development of new therapeutic strategies.
Some materials can reduce ROS levels as a result of their intrinsic properties or ROS-responsive functional groups. Taking PEG as an example, without the ability to scavenge free radicals by itself, it can still inhibit injury-induced ROS production by mediating plasma membrane repair132. Further studies have shown that PEG may also interact with mitochondria directly to inhibit abnormal oxidative metabolism, which plays a dominant role in the later stage of repair133. However, the application of PEG has limitations in terms of concentration and molecular weight. Cho et al. thus developed PEG-decorated silica NPs (PSiNPs), which showed better membrane-sealing properties than uncoated particles or PEG alone. Further in vivo tests demonstrated that PSiNPs significantly reduced ROS production134. Unlike PEG, some polymers have the ability to scavenge ROS. In general, these chemical functional groups can be present in biomaterials, and the mechanisms by which they can reduce ROS levels mainly involve solubility or chemical bond changes135. For instance, in the presence of ROS, thioether can be converted to hydrophilic sulfoxide and sulfone groups. Zhang et al. synthesized a high-density thioether-containing polymer to construct ROS-scavenging lipid-polymer NPs, denoted as PELPNPs136. The results showed that PELPNPs could readily scavenge overproduced ROS, thereby reducing secondary injury and promoting functional recovery in a rat spinal cord contusion model (Fig. 11).
a Transmission electron microscope image of PELPNPs. b Cellular uptake of PELPNPs (red). c An SCI model of T10 contusion. d BBB scores of rats injected with 5% glucose solution (control group), 10 mg kg-1 PELPNPs (NPs (10) group), 5 mg kg-1 PELPNPs (NPs (5) group), and 30 mg kg-1 methylprednisolone (MP (30) group). e Immunohistochemistry images of GFAP and ionized calcium binding adapter molecule 1 (Iba1) in the damaged spinal cord on day 28 after injury. Quantitative analysis of the mean immunointensity of f) GFAP and g) Iba1. Reproduced with permission136. Copyright 2021, Elsevier.
Developing delivery systems for antioxidant drugs is an effective strategy. For example, as the main active ingredients of the traditional Chinese medicines Huangqi and Danshen, Astragalus polysaccharide (APS) and tanshinone IIA (TSIIA) have suitable antioxidant activities. Rao et al. utilized selenium NPs as delivery vehicles to improve the bioavailability of APS and TSIIA, demonstrating a positive therapeutic antioxidative effect on SCI137. Resveratrol (RES) is a nonflavonoid polyphenol compound with important functions, such as antioxidation and anti-free radicals. Based on this, researchers prepared functional resveratrol-biodegradable manganese-doped silica NPs, referred to as PMMSN-RES. As a sustained-release formulation, PMMSN-RES effectively delivered resveratrol to the spinal cord, mitigated oxidative stress, reduced neuronal apoptosis and inhibited inflammation, thus promoting the recovery of mouse motor function138.
Nanozymes refer to nanomaterials with enzyme-like characteristics that can mimic natural antioxidant enzymes to react with ROS139. A type of well-known nanozyme is cerium oxide NPs (CONPs), which can be either a superoxide dismutase mimic or a catalase mimic depending on the Ce3+/Ce4+ ratio140. Administration of CONPs to SCI model rats can improve their motor function and attenuate inflammation and apoptosis141. It is noteworthy that there is an optimal therapeutic dose range, above which CONPs might be cytotoxic and adversely affecting functional recovery. MnO2, another type of nanozyme, can effectively improve the viability of MSCs by alleviating the oxidative environment94, which reveals the synergistic role of cell-based therapy and microenvironment regulation in SCI treatment. Iron oxide NPs (IONPs) also have the ability to scavenge free radicals142. The combination of IONP implantation and magnetic field exposure has been shown to significantly promote functional recovery and reduce lesion volume in SCI model rats.
Restoring the Blood‒Spinal Cord Barrier
The BSCB is a blood‒brain barrier-like structure that exists in spinal cord tissue. Normally, the diffusion of molecules and cells across the BSCB is highly regulated by tight junctions (TJs), allowing the CNS to function as an isolated system143. Structural disruption and increased permeability of the BSCB are universal consequences of SCI, leading to the infiltration of immune cells and inflammatory factors into the stroma144. Moreover, BSCB damage is long-lasting. Dynamic contrast-enhanced MRI showed that the BSCB remained impaired 56 days post-SCI145. Given that BSCB disruption mediates secondary and long-term damage, its restoration appears particularly important.
Biomaterials can restore the BSCB by delivering drugs to certain targets. For example, curcumin has been proven to improve the integrity of the BSCB by attenuating inflammatory factors and upregulating TJ protein expression146. Incorporating curcumin into a polyacetal increased its bioavailability and stability, thereby enhancing neuroprotection and improving functional recovery in acute SCI147. Joshi et al. incorporated carbon monoxide–releasing molecule-2 (CORM-2) into a solid lipid NP (CORM-2-SLN) to improve its short carbon monoxide release half-life148. In vivo experiments showed that compared with CORM-2 alone, CORM-2-SLN effectively rescued endothelial cell damage and prevented BSCB disruption, but the exact mechanism remains to be clarified. In another study, a thermosensitive heparin-poloxamer hydrogel was developed to load and deliver acidic fibroblast growth factor, which has an SCI-protective effect104. This composite hydrogel reduced the loss of junction proteins and thus prominently attenuated BSCB disruption, providing a successful SCI protection strategy.
Cell transplantation is another way biomaterials can restore the BSCB. For instance, a two-component polymer implant seeded with NPCs and endothelial cells promoted the formation of stable, functional blood vessels, half of which stained for endothelial barrier antigen, indicating that BSCB was being re-established149. After transplantation of microvascular cells within the self-assembling polypeptide scaffold RADA-16I, microvessels with BSCB integrity were successfully formed, alleviating secondary SCI events such as inflammation and scar formation150. MSCs also exert a positive effect on BSCB recovery. Intravenous delivery of MSCs reduced the permeability and leakage of the BSCB, resulting in its stabilization151,152. Providing a carrier material for MSCs may enhance this effect. A unique PLGA scaffold can augment the stemness, engraftment, and function of hMSCs153. These scaffolds of nontransdifferentiated hMSCs exhibited multimodal effects, including angiogenesis-promoting effects. Nevertheless, this study did not explore BSCB recovery.
Reducing Glial Scarring
After SCI, astrocytes proliferate, become hypertrophic and form glial scars154. Glial scars not only act as a physical barrier to axon regeneration but also contain inhibitory chemical components represented by CSPGs155,156,157. In 2002, a study demonstrated that ChABC could reduce glial scar formation to promote SCI recovery158. Since then, numerous SCI studies have concentrated on regulating glial scarring, either by altering astrocyte behavior or targeting CSPGs118. Therefore, our subsequent discussion will thoroughly explore into these two aspects.
Glial scar formation can be reduced by alleviating astrocyte activation or infiltration, which is mainly applicable to acute SCI. Biomaterials can function through inherent properties. For example, alginate/chitosan/genipin hydrogel composites reduced astrocyte reactivity in vitro while sequestering extracellular Ca2+ to prevent Ca2+-induced secondary injury159. A new aligned hydrogel microfiber scaffold constructed using photocrosslinked gelatin methacryloyl and electrospinning technology significantly reduced astrocyte activity in vivo, thus inhibiting glial scar formation160. For biomaterial-based drug delivery systems, targeting the injury site could improve efficacy and avoid systemic side effects of high-dose therapy. A peptide CAQK identified via in vivo phage display screening can specifically target brain and nerve injury sites161,162. Accordingly, Sun et al. grafted the FITC fluorescent molecule and CAQK peptides onto the surface of arctigenin-loaded mesoporous silica NPs to target the SCI site163. Arctigenin is an anti-inflammatory agent isolated from the Chinese medicinal herb Arctium lappa. In addition, the size of this nanocarrier, approximately 100 nm, enabled it to penetrate the BSCB. Experiments showed that this nano‑drug platform can inhibit the activation of astrocytes, a process in which the molecular mechanisms involve IL-17. Compared to targeting damaged sites, directly targeting astrocytes is apparently a more attractive strategy. A nanogel consisting of copolymers of PEG and PEI with high selectivity toward astrocytes was developed164. Functionalization with both NH2 and Cy5 groups limited uptake by macrophages, allowing it to be exclusively internalized in astrocytes. After endocytosis, nanogels undergo degradation by lysosomes to release compounds with potential therapeutic effects, such as rolipram.
Existing scars can be degraded by targeting CSPGs, which is mainly suitable for chronic SCI. As the most commonly used scar-degrading biomolecule, ChABC can specifically and efficiently cleave glycosaminoglycan (GAG) chains inside CSPGs165. However, its low bioavailability and poor thermal stability may limit its applications, for which biomaterials could provide a solution. For example, ChABC was encapsulated in a hydrogel based on SAPs named FAQ for long-term release with high activity for up to 42 days in vitro87. Injection of this hydrogel resulted in the degradation of gliotic scars and locomotor recovery improvement in chronic SCI model rats. PLGA NPs were also able to protect enzymes from decomposition while controlling their release166. The immunohistochemistry results demonstrated glial scar degradation in the ChABC-treated particle group, with the BBB score indicating remarkable functional improvement compared with the other groups. However, this study did not include a direct ChABC-treated control group, so it was not possible to specifically assess the improvement brought by NP administration. For greater therapeutic efficacy in vivo, combination with other drugs or neurotrophic factors is appealing. Sustained and combinatorial delivery of ChABC and NT-3 facilitated by a hydrogel-microtube scaffold system can enhance axonal sprouting and functional recovery after SCI167. Notably, the chondroitin sulfate GAG levels remained markedly decreased for up to 6 weeks.
However, a study demonstrated that the lack of reactive astrocytes after SCI led to an obvious inflammatory response, BSCB repair failure, and pronounced motor defects168. Anderson et al. used a gene-targeted knockout approach in adult mouse SCI models to show that none of preventing astrocyte scar formation, attenuating scar-forming astrocytes or deleting chronic astrocytic scars resulted in the spontaneous regrowth of transected corticospinal, sensory or serotonergic axons20. Hence, the hypothesis that axonal regeneration can be promoted by only reducing glial scars may be oversimplified, as it ignores that early glial scars can limit the spread of inflammation. Transplantation of peripheral blood-derived MSCs may provide a solution. In this study, the levels of the astrocyte biomarker glial fibrillary acidic protein (GFAP) were initially increased followed by a decrease, suggesting that PB-MSC transplantation could promote glial scar formation in the early stage but inhibit it in the later stage169. Furthermore, simply targeting CSPG or astrocytes to modulate glial scar formation may lead to inaccurate conclusions because other cells and extracellular components are also involved in the formation of the glial scar154. Taken together, there is still a long way to go to fully and correctly understand the effects of reducing glial scars in SCI.
Strategies to enhance neurorestoration
On the basis of managing secondary inflammatory cascades, neurorestoration should also be strengthened, as it is indispensable to achieve functional recovery, the ultimate goal of injured spinal cord repair. The following sections outline three biomaterial-based strategies to enhance neural repair.
Stimulation of axonal regeneration
The overwhelming cell death in SCI causes the formation of multiloculated, perilesional microcysts, which eventually combine to form larger cavities that are detrimental to axonal regeneration170. Therefore, bioscaffolds that can act as a bridge and guide axon regeneration are attractive. Among them, injectable hydrogels have been extensively studied because they can conform to the irregular shape of the lesions, providing ideal contact between the severed spinal cord stumps and the biomaterial171. Porous scaffolds, a more advanced biomaterial implant, are effective in regenerating axons because they not only exhibit higher mechanical stability and longer degradation periods than hydrogels but also support and guide the organized and linear growth of injured axons172,173. However, the geometry of porous scaffolds is determined before implantation, while injury sites tend to be quite irregular, making it difficult to find an optimal design for each unique spinal lesion171,174. Additionally, scaffolds of synthetic molecules that mimic proteins can transmit cellular signals to promote tissue regeneration. Recently, a study published in Science reported the development of a supramolecular scaffold of nanoscale fibrils that integrates two different orthogonal biological signals to activate the transmembrane receptor β-1 integrin, the basic fibroblast growth factor 2 receptor and their respective downstream effectors175. Intensifying the motions of molecules within scaffold fibrils could optimize cell signaling, leading to significant improvements in axonal regeneration, myelination, and functional recovery.
Scaffolds can incorporate growth-promoting molecules to accelerate axonal growth through the lesion. For instance, as a class of small, well-conserved noncoding RNAs, microRNAs (miRs) play a vital role in neural development and function176. The delivery of a cocktail of miR-132/miR-222/miR-431 along with glial cell-derived neurotrophic factor (GDNF) using a 3D fiber-hydrogel scaffold significantly promoted and preserved mature axon regeneration and survival177. Additionally, when combined with methylprednisolone (MP), functional recovery was further enhanced without alteration in axon regeneration (Fig. 12). Courtine and Sofroniew demonstrated that a combination of temporally controlled and spatially targeted delivery of neuronal intrinsic growth factors, growth-supportive substrates, and chemoattractant factors could stimulate robust propriospinal axon regrowth178. Unfortunately, little improvement in locomotor function was detected. Another common strategy is to seed scaffolds with cells that produce neurotrophic factors, such as bone marrow stromal cells69,71 and genetically altered fibroblasts179,180,181. Optimized scaffolds with porous structures may facilitate the long-term survival of these transplanted cells174.
a Schematic diagram of the fabrication of the 3D fiber-hydrogel scaffold. b Cumulative release of negative miR (Neg miR) and GDNF. c Representative fluorescence images of neurofilament 200 (NF200) staining. d Quantification analysis of NF200+ area. e Representative fluorescence images of 5-hydroxytryptamine (5-HT) staining. f Quantification analysis of 5-HT+ area. g Representative fluorescence images of calcitonin gene-related peptide (CGRP) staining. h Quantification analysis of CGRP+ area. i BBB scores in the presence of MP. j Von Frey Hair test in the presence of MP. NF200 is a marker of mature axons; 5-HT and CGRP are markers for different types of axons, labeling serotonergic and sensory axons, respectively. Reproduced under terms of the CC-BY license177. Copyright 2021, Wiley‐VCH GmbH.
Formation of a neuronal relay network
Although axon regeneration after SCI has been extensively studied, it remains difficult to stimulate the regeneration of brain-derived descending axons, such as the corticospinal tract (CST), across the SCI area to innervate target neurons182. Providing an interneuronal network to relay neural information through the defect area may be a feasible solution. This neuronal relay repair strategy has been detailed in many reviews182,183,184,185,186,187. This article only provides a brief overview from the perspective of biomaterials.
The endogenous neuronal relay strategy refers to activating and inducing endogenous stem cells to differentiate into neurons to form a neural network at the SCI site. Based on this concept, scientists have developed a series of functionalized bioscaffolds to specifically induce adhesion, migration, proliferation, and neuronal differentiation of endogenous NSCs. For example, a combined treatment of photocrosslinked hydrogel transplantation and PLX3397, a colony-stimulating factor 1 receptor inhibitor, suppressed inflammation mediated by microglia/macrophage, thereby promoting the neurogenesis of endogenous neural stem/progenitor cells and improving functional recovery in complete transection SCI model mice188. Recently, a novel biodegradable material called “Bio-C” was constructed by combining collagen with high molecular weight HA. This material has been shown to enhance neural regeneration and motor-functional restorations in SCI model mice189. Further in vitro experiments showed that Bio-C promoted the proliferation of NSCs and their differentiation toward neuronal lineages. These studies suggest that programming endogenous NSCs might be a feasible strategy for motor function recovery rather than exclusively pursuing CST regeneration. However, the complexity and diversity of endogenous spinal cord NSCs cannot be ignored. Different endogenous NSCs postinjury might have distinct activation-inducing factors, signalling pathways, time courses, and differentiation capacities and profiles183. Further research is needed to improve and optimize this repair strategy.
In cases of severe SCI (e.g., completely transected SCI), depletion or insufficient mobilization of endogenous NSCs may occur, necessitating supplementation with exogenous NSCs. This supplementation is a part of the exogenous neuronal relay repair strategy. For example, researchers developed a functional collagen scaffold by loading liposomes encapsulated with the microtubule-stabling agent paclitaxel into a collagen microchannel scaffold190. Animal experiments proved that this scaffold provided a suitable microenvironment for mature neuronal differentiation of grafted NSCs. Recently, a highly permeable DNA supramolecular hydrogel was designed to repair completely transected SCI in rats191. This DNA hydrogel was found to facilitate both implanted and recruited NSCs to migrate, proliferate, and differentiate sufficiently, thereby promoting the formation of a continuous regenerative neural network. Within 8 weeks, the rats recovered basic hindlimb function with detectable motor-evoked potentials. However, challenges persist with exogenous neuronal relay strategies, including abnormal proliferation, slow differentiation and maturation, and long-distance migration of transplanted NSCs in vivo182. In terms of clinical translation, there are also enormous challenges to face, such as immune rejection, the risk of neoplasm and teratoma formation183. In short, a considerable amount of work remains to be done before exogenous NSCs can be applied in SCI treatment.
Recently, tissue engineering strategies for nerve repair and regeneration have been rapidly developed. For complete SCI, this strategy can meet the diverse needs of spinal cord structural and functional repair through a delicate combinatorial design of biomaterials, seed cells, and bioactive factors. Based on this, Zeng’s team constructed exogenous neural network tissue192,193,194,195, proposing the hypothesis of tissue-engineered neuron relays to repair spinal cord defects196. By combining NSCs, a collagen scaffold, and neurotrophic factors and their receptors, they further constructed spinal cord-like tissue (SCLT) in vitro for the first time197. Transplantation of this SCLT into a 2 mm completely transected spinal cord defective area significantly promoted the regeneration of brain-derived descending axons and the neurons derived from NSCs established synaptic connections with descending axons to transmit brain-derived excitatory neural information and promote motor function recovery. However, nascent neurons serving as relays typically require months to functionally integrate into the host spinal cord neural networks187. Before that, functional silencing or disuse atrophy can occur. Therefore, the stem cell-derived neuronal relay strategy should be combined with rehabilitation physiotherapy, such as functional electrical stimulation, to restore the innervation of spinal cord motor neurons to target muscles as soon as possible182.
Promotion of remyelination
Another pathway to functional recovery after SCI is to promote axonal remyelination, which depends on the health and availability of oligodendrocyte progenitor cells (OPCs)174. However, the highly inhibitory postinjury microenvironment not only hinders the migration of remaining endogenous OPCs to the demyelinating site but also strongly influences the fate of transplanted exogenous OPCs198,199,200. A possible solution is to use biomaterial systems that can create supportive niches for transplanted cells, thus enhancing remyelination.
Some biomaterials can serve as candidates for OPC transplantation. ECM-mimicking hydrogels are useful biomaterials for regeneration For example, a highly biocompatible HA/gelatin hydrogel crosslinked by poly-(ethylene glycol) diacrylate was developed to provide a permissive microenvironment for the survival, oligodendrogenic differentiation, and remyelination of transplanted OPCs201. This study also found that hydrogels of medium stiffness (at an elastic modulus of approximately 120 Pa) can best support the natural oligo-morphology and proliferation of OPCs. Similarly, Führmann et al. synthesized a hyaluronan and methylcellulose (HAMC) hydrogel modified with an RGD peptide and platelet-derived growth factor (PDGF-A)202. In addition to promoting early survival and integration of transplanted OPCs, this hydrogel could also promote their differentiation to attenuate teratoma formation, which is one of the major challenges in the transplantation of pluripotent stem cells and their differentiated counterparts (Fig. 13). Electrospun fibers are another instructive biomaterial. For instance, PCL electrospun nanofibers have been shown to support the growth and differentiation of OPCs, and copolymerization with gelatin could further enhance myelination203. In another study, electrospun fibers were utilized as artificial axons to investigate how fiber (axon) diameter affects OPC myelination204. Researchers determined the minimum fiber diameter threshold to be 0.4 µm, above which the fiber diameter was sufficient to initiate concentric wrapping by rat primary oligodendrocytes. In summary, OPCs differ from other CNS cell types in many ways and thus may have different requirements for biomaterial properties200.
a, b Representative fluorescence images of SOX10 (the OPC marker) and Ki67 (the proliferation marker) staining after cells were transplanted in media. c, d Representative fluorescence images of SOX10 and Ki67 staining after cells were transplanted in HAMC-RGD/PDGF-A. e Count of green fluorescent protein-positive (GFP+) cells. f Proportion of GFP+ cells to initial cells. g Count of Ki67+ cells. h, i Migration of injected cells in media and HAMC-RGD/PDGF-A. j Quantification of migration degree. k, l Nonspinal cord structures in the OPC-media group and OPC-HAMC-RGD/PDGF-A group. m Quantification of the four structures. Reproduced with permission202. Copyright 2016, Elsevier.
NSCs also have the potential to differentiate into oligodendrocytes, providing new ideas for promoting remyelination. For instance, a soft methacrylamide chitosan hydrogel with a Young’s elastic modulus (E(Y)) <1000 Pa promoted NSC differentiation into neurons and astrocytes, while stiffer scaffolds (E(Y) > 7000 Pa) drove oligodendrocyte differentiation205. By providing instructive physical cues, a unique graphene-nanofiber hybrid scaffold could lead to selective differentiation of NSCs into mature oligodendrocytes without requiring culture media differentiation inducers206. Another combinatorial therapy to enhance remyelination is using novel material to carry Schwann cells. For example, Chen et al. seeded Schwann cells, which highly expressed GDNF, into multichannel positively charged oligo [poly (ethylene glycol) fumarate] scaffolds and implanted them into transected rat spinal cords207. Experimental results demonstrated that these GDNF-Schwann cells enhanced remyelination of regenerating axons. However, it should be noted that remyelination was most likely a result of endogenous Schwann cell recruitment, as myelinating cell and axon complexes were not formed by implanted cells but by host Schwann cells. Recently, a porcine decellularized optic nerve scaffold loaded with NT-3-overexpressing Schwann cells was also proven to enhance remyelination in the injured dorsal white matter of the rat spinal cord82.
In summary, cell transplantation strategies based on biomaterials can enhance remyelination and achieve some reparative effects. However, there is still a considerable amount of work to be done. In terms of mechanisms, oligodendrogenesis is an understudied process, and the regulatory mechanisms of OPCs and differentiated oligodendrocytes remain elusive200. The pathophysiological process and regulatory mechanism of Schwann cells involved in myelin repair after SCI also need to be further elucidated208. In the aspect of clinical translation, a study has questioned whether remyelination is a validated target after moderate spinal cord contusion because experimental data demonstrated that oligodendrocyte remyelination was not necessary for the spontaneous recovery of walking209.
Combination biomaterial and cell transplantation strategies
With the ongoing advancement in cell culture technology, cell transplantation has emerged as an innovative approach in SCI treatment, serving not only to replace or protect damaged tissue but also to promote axonal regrowth through both physical and trophic support210. Several candidate cell types have been studied in clinical trials in SCI patients, such as autologous Schwann cells211, olfactory ensheathing cells212,213, BMSCs214,215, and neural stem/progenitor cells216. However, the efficacy of cell transplantation is often compromised by the flow of cerebrospinal fluid and the hostile microenvironment at the injury site, resulting in ineffective colonization, a low cell survival rate, and uncertain differentiation. To better address these issues, researchers often employ a combination of biomaterials and cell transplantation. In the preceding sections, we have listed numerous research examples of biomaterial and cell transplantation strategies. Below, we concisely explore this combination strategy in depth.
Biomaterial scaffolds can benefit cell transplantation in a variety of ways: (1) Specific 3D microstructures can be engineered into small “chambers” or aligned channels/fibers suitable for cell seeding and directional linear growth of axons69. (2) They can serve as a physical matrix for cell adhesion, thus enhancing the survival and retention of transplanted cells at the lesion site217,218. (3) They can separate the transplanted cells from the host tissue, thereby providing an independent microenvironment for cell differentiation and proliferation. (4) They can effectively fill cavities and bridge lesions, significantly reducing the number of cells for transplantation. This is particularly appealing for clinical applications, as the availability of autologous cells from patients is limited210. Biomaterials dictate the fate of transplanted cells through complex physical properties. The morphology (internal structure, pore size, etc.) and mechanical properties (stiffness, viscoelasticity, and rheological properties) of biomaterials influence the survival, proliferation, and adhesion of transplanted cells via different signalling pathways219. For example, the crosslinking of hydrogels typically improves the long-term stability of biomaterials, yet it also results in increased stiffness. This balance between stiffness and stability is delicate for cell adhesion, migration and nerve regeneration220,221. Injectable in situ polymerized hydrogels facilitate the direct delivery of cells and factors to the lesion site, thereby reducing the invasiveness of surgical intervention. They form a homogeneous 3D matrix that mimics the natural ECM microstructure to modulate cell fate102,202. Appropriate modifications to biomaterials can enhance the therapeutic effect of cell transplantation. For example, surface modification with ECM components can create a less hostile molecular microenvironment within biomaterials, thereby improving cell adhesion and survival222,223. In summary, biomaterials that possess excellent biocompatibility, appropriate mechanical parameters, microenvironment manipulation or neuroprotective capability all have the potential to enhance the efficacy of cell transplantation in SCI treatment.
Cell transplantation based on biomaterials can be categorized into four approaches. The first is the transplantation matrix, referring to the in vitro mixture of cells with biomaterials to form a tissue-like matrix, which is then implanted into the injury site. This technique has been widely employed as a delivery system to confine transplanted cells to the site of injury. The second, injection and in situ gelling, entails the coinjection of self-assembling biomaterials and cells into the injury site to assemble seeded scaffolds in vivo. This technique has gained popularity for filling irregular lesion cavities formed post-SCI. The first two approaches are primarily applied to gel materials. The third approach uses preseeded scaffolds, which refer to cells seeded onto prepared bioactive scaffolds before implantation. This technique is predominantly utilized for solid scaffolds with a predetermined shape. The fourth approach aims to promote the integration of the scaffold into the host tissue to increase axonal bridging. This is achieved by implanting a prefabricated scaffold into the injury site and then injecting cells around it. The latter two approaches are primarily applied to solid biomaterials210,224.
Clinical trials of functional bioscaffolds
Although many biomaterials have been applied in SCI research, few have actually entered clinical trials. Current clinical trials mainly focus on the implantation of two functional bioscaffolds: the Neuro-Spinal scaffold and NeuroRegen scaffold (Table 2).
Neuro-spinal scaffold
The Neuro-Spinal scaffold made of PLGA and poly (L-lysine) has been shown to promote functional recovery in acute SCI model rats and incomplete SCI model African green monkeys225,226. Based on these studies, InVivo Therapeutics initiated a series of clinical trials to evaluate the efficacy of this scaffold in treating acute traumatic thoracic SCI, collectively known as the INSPIRE trials.
The first INSPIRE trial started in 2014 and is estimated to be completed in 2024 (NCT02138110). Selected participants in this trial must have complete nonpenetrating SCI defined as an American Spinal Injury Association Impairment Scale (AIS) grade A at T2-T12. The researchers first reported a case study of one patient with a T11 AIS grade A traumatic SCI227. They observed an improvement in AIS grade from A to C at 3 months after implantation. However, it remains uncertain whether the improvement could be attributed solely to the implanted scaffold and not related to surgical decompression. Recently, published 6-month follow-up data from the INSPIRE 1 trial reported no serious adverse events (SAEs) related to the scaffold or the implantation procedure228. AIS grade improvements were observed in 7 of 16 patients (43.8%). These results illustrate the efficacy and safety of the Neuro-Spinal scaffold in the short term. To further evaluate the safety and probable benefits of the Neuro-Spinal scaffold, the INSPIRE 2 trial was initiated in May 2019 and estimated for completion in July 2028 (NCT03762655).
NeuroRegen Scaffold
Since 2009, several animal experiments have demonstrated that LOCSs linked to multiple functional molecules are effective in the treatment of SCI229,230,231,232. To further examine the therapeutic effect of LOCSs, they have been applied in five clinical trials under the product name NeuroRegen.
On January 16, 2015, a phase I trial was carried out to examine the safety and efficacy of bone marrow mononuclear cell (BMMC)- and MSC-loaded NeuroRegen scaffolds in chronic SCI patients (NCT02352077). Five patients who received transplantation of the NeuroRegen Scaffold with BMMCs showed autonomic and electrophysiological improvements during a 12-month follow-up233. Unfortunately, no other meaningful motor or sensory improvements were reported. In contrast, some positive results in motor function and sensation level were observed in eight patients after implantation of the same scaffold loaded with hUC-MSCs234. A summary of 2-5 years of follow-up data involving this trial has been recently published235. Among the 51 patients enrolled, no SAEs associated with functional scaffold transplantation were observed. Data analysis suggested that younger patients might be more likely to regain sensation. Another phase I trial was conducted to examine the use of the NeuroRegen scaffold in patients with acute SCI (NCT02510365). Two patients with acute SCI at T11 and C4 were followed up for one year after receiving a transplantation of the NeuroRegen scaffold with hUC-MSCs236. Both patients experienced significant improvements in sensory and motor functions, especially an improvement in injury status from AIS grade A to C. Similarly, seven patients with acute thoracic SCI of AIS grade A underwent the implantation of NeuroRegen scaffolds loaded with autologous BMMCs237. During the 3-year follow-up, some patients exhibited improvements in sensation and autonomic nervous functions. Unfortunately, no recovery of motor function was observed, nor was there obvious improvement in the AIS grade. During the 2-5 year follow-up period, none of the 15 enrolled patients experienced SAEs related to scaffold transplantation235. More than one-third of the patients, particularly those with shorter injury lengths, showed significant recovery of sensory and motor functions. Notably, four patients even recovered their voluntary walking ability.
While some therapeutic effects of biomaterial implantation have been observed in these clinical trials of SCI, there is currently a lack of large-scale clinical studies supporting their routine clinical use. Additionally, it is essential to properly assess and monitor the safety of biomaterial implants before and during clinical trials to avoid premature human studies, a scenario that has occurred in the past238. Undeniably, with the initiation of these clinical trials, the blueprint for SCI treatment is unfolding. In the near future, biomaterials are expected to be part of combinatorial treatments alongside other interventions, such as electrical stimulation, stem cell treatment and growth factor administration, to facilitate neurological recovery in SCI patients.
Conclusions and outlook
A series of complex pathophysiological processes post-SCI result in the formation of microenvironments that inhibit nerve regeneration, while the role of many cells remains controversial. For instance, as a natural source of myelinating cells, OPCs can ameliorate pathological deterioration in SCI31. However, there is evidence that OPCs may also participate in glial scar formation239. In addition to OPCs, astrocytes have complex functions. Recent findings indicated that astrocyte scar formation may actually promote, rather than inhibit, CNS axon regeneration20. Elucidating the mechanisms underlying the actions of these cells will advance the development of novel therapeutic strategies for SCI. Furthermore, components within the cellular microenvironment do not act in isolation. In the future, researchers should elucidate the regulatory relationships between the components to better aid neural repair following SCI.
Tissue engineering scaffolds for SCI treatment are rapidly evolving. After years of research and exploration, biocompatibility, low toxicity, and degradability have become the basic properties of tissue engineering scaffolds. In addition to serving as a bridge to reconnect the lesion gap, bioscaffolds can carry various bioactive molecules, cells, and drugs to promote the reconstruction of the microenvironment. However, numerous issues remain unresolved. For example, the detailed mechanism through which bioscaffolds regulate cell behavior and microenvironments is unclear. Concerning the safety of scaffold materials, unified international evaluation standards are still lacking. Moreover, the timing of transplantation and the location and degree of SCI require careful consideration.
In the future, research should focus on the development and application of novel regenerative biomaterials74. Building on this, exploring the optimal combination of scaffold materials, seed cells, cytokines and drugs is also essential. In short, further optimization of biomaterial-based combinatorial strategies will be the core of future injured spinal cord repair research. Tissue engineering scaffolds are a topic with enormous research potential and application value. As research deepens, it is believed that more novel scaffolds will be transitioned into clinical applications, promoting the treatment of SCI patients.
References
McDonald, J. W. & Sadowsky, C. Spinal-cord injury. Lancet 359, 417–425 (2002).
Courtine, G. & Sofroniew, M. V. Spinal cord repair: advances in biology and technology. Nat. Med. 25, 898–908 (2019).
Fehlings, M. G. et al. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 7, 84s–94s (2017).
Li, J. P., He, L. M. & Wu, W. T. Pathological changes and repair strategies for spinal cord injury (in Chinese). Sci Sin Vitae. 51 (2021).
Ulndreaj, A., Chio, J. C., Ahuja, C. S. & Fehlings, M. G. Modulating the immune response in spinal cord injury. Expert Rev. Neurother. 16, 1127–1129 (2016).
Ahuja, C. S. et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 80, S9–s22 (2017).
Anwar, M. A., Al Shehabi, T. S. & Eid, A. H. Inflammogenesis of secondary spinal cord injury. Front. Cell. Neurosci. 10, 98 (2016).
Hollister, S. J. Scaffold design and manufacturing: from concept to clinic. Adv. Mater. 21, 3330–3342 (2009).
Cho, A. N. et al. Aligned brain extracellular matrix promotes differentiation and myelination of human-induced pluripotent stem cell-derived oligodendrocytes. ACS Appl Mater. Interfaces 11, 15344–15353 (2019).
Singh, D., Singh, D., Zo, S. & Han, S. S. Nano-biomimetics for nano/micro tissue regeneration. J. Biomed. Nanotechnol. 10, 3141–3161 (2014).
Gezercan, Y. et al. The outcomes of late term surgical treatment of penetrating peripheral nerve injuries. Turk. Neurosurg. 26, 146–152 (2016).
Whitworth, I. H., Doré, C. J., Green, C. J. & Terenghi, G. Increased axonal regeneration over long nerve gaps using autologous nerve-muscle sandwich grafts. Microsurgery 16, 772–778 (1995).
Cunha, C., Panseri, S. & Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. : Nanotechnol., Biol., Med. 7, 50–59 (2011).
Fawcett, J. W. & Asher, R. A. The glial scar and central nervous system repair. Brain Res. Bull. 49, 377–391 (1999).
Milich, L. M., Ryan, C. B. & Lee, J. K. The origin, fate, and contribution of macrophages to spinal cord injury pathology. Acta Neuropathol. 137, 785–797 (2019).
Simon, D. W. et al. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191 (2017).
Ahuja, C. S., Martin, A. R. & Fehlings, M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res 5, 1017 (2016).
Yang, L. et al. Effective modulation of CNS inhibitory microenvironment using bioinspired hybrid-nanoscaffold-based therapeutic interventions. Adv. Mater. 32, e2002578 (2020).
Han, Q. et al. Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metab. 31, 623–641.e628 (2020).
Anderson, M. A. et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200 (2016).
Shi, Q. et al. Collagen scaffolds modified with collagen-binding bFGF promotes the neural regeneration in a rat hemisected spinal cord injury model. Sci. China Life Sci. 57, 232–240 (2014).
Moncal, K. K., Ozbolat, V., Datta, P., Heo, D. N. & Ozbolat, I. T. Thermally-controlled extrusion-based bioprinting of collagen. Journal of materials science. Mater. Med. 30, 55 (2019).
Sun, Y. et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res. A 107, 1898–1908 (2019).
Zou, Y. et al. Aligned collagen scaffold combination with human spinal cord-derived neural stem cells to improve spinal cord injury repair. Biomater. Sci. 8, 5145–5156 (2020).
Yang, Y. et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 269, 120479 (2021).
Schaub, N. J., Johnson, C. D., Cooper, B. & Gilbert, R. J. Electrospun fibers for spinal cord injury research and regeneration. J. Neurotrauma 33, 1405–1415 (2016).
Li, X. et al. Electrospun collagen fibers with spatial patterning of SDF1α for the guidance of neural stem cells. Adv. Healtc. Mater. 4, 1869–1876 (2015).
Han, S. et al. Human placenta-derived mesenchymal stem cells loaded on linear ordered collagen scaffold improves functional recovery after completely transected spinal cord injury in canine. Sci China Life Sci. 61, 2–13 (2018).
Zou, Y. et al. Comparison of regenerative effects of transplanting three-dimensional longitudinal scaffold loaded-human mesenchymal stem cells and human neural stem cells on spinal cord completely transected rats. ACS Biomater. Sci. Eng. 6, 1671–1680 (2020).
Ma, F. et al. Use of natural neural scaffolds consisting of engineered vascular endothelial growth factor immobilized on ordered collagen fibers filled in a collagen tube for peripheral nerve regeneration in rats. Int J. Mol. Sci. 15, 18593–18609 (2014).
Shen, H. et al. Advances in biomaterial-based spinal cord injury repair. Adv. Funct. Mater. 32, 2110628 (2022).
Lu, Q., Feng, Q., Hu, K. & Cui, F. Preparation of three-dimensional fibroin/collagen scaffolds in various pH conditions. Journal of materials science. Mater. Med. 19, 629–634 (2008).
Ghorbani, F., Zamanian, A., Kermanian, F. & Shamoosi, A. A bioinspired 3D shape olibanum-collagen-gelatin scaffolds with tunable porous microstructure for efficient neural tissue regeneration. Biotechnol. Prog. 36, e2918 (2020).
Nazari, B. et al. Fibrin hydrogel as a scaffold for differentiation of induced pluripotent stem cells into oligodendrocytes. J. Biomed. Mater. Res B Appl Biomater. 108, 192–200 (2020).
Yao, S. et al. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int J. Nanomed. 13, 2883–2895 (2018).
Yang, L. et al. Improved mechanical properties by modifying fibrin scaffold with PCL and its biocompatibility evaluation. J. Biomater. Sci. Polym. Ed. 31, 658–678 (2020).
Kushchayev, S. V. et al. Hyaluronic acid scaffold has a neuroprotective effect in hemisection spinal cord injury. J. Neurosurg. Spine 25, 114–124 (2016).
Wang, X., He, J., Wang, Y. & Cui, F. Z. Hyaluronic acid-based scaffold for central neural tissue engineering. Interface focus 2, 278–291 (2012).
Zaviskova, K. et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. Part A 106, 1129–1140 (2018).
Zhao, X. et al. Optimized, visible light-induced crosslinkable hybrid gelatin/hyaluronic acid scaffold promotes complete spinal cord injury repair. Biomed. Mater. 17, 024104 (2022).
Patel, H., Bonde, M. & Srinivasan, G. Biodegradable polymer scaffold for tissue engineering. Trends Biomater. Artif. Organs 25, 20–29 (2011).
Ikeda, T. et al. Fabrication and characteristics of chitosan sponge as a tissue engineering scaffold. Biomed. Res Int 2014, 786892 (2014).
Chedly, J. et al. Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 138, 91–107 (2017).
Hua-bin, Z., Lin, L. & Lu, C. Tissue-engineered spinal cord construction by chitosan alginate scaffold and adipose-derived mesenchymal stem cells in the treatment of acute spinal cord injury. Chin. J. Tissue Eng. Res. 21, 4199 (2017).
Grijalvo, S., Nieto-Diaz, M., Maza, R. M., Eritja, R. & Diaz, D. D. Alginate hydrogels as scaffolds and delivery systems to repair the damaged spinal cord. Biotechnol. J. 14, e1900275 (2019).
Sitoci-Ficici, K. H. et al. Non-functionalized soft alginate hydrogel promotes locomotor recovery after spinal cord injury in a rat hemimyelonectomy model. Acta Neurochir. (Wien.) 160, 449–457 (2018).
Huang, L. et al. Anisotropic alginate hydrogels promote axonal growth across chronic spinal cord transections after scar removal. ACS Biomater. Sci. Eng. 6, 2274–2286 (2020).
Schackel, T. et al. Peptides and Astroglia improve the regenerative capacity of alginate gels in the injured spinal cord. Tissue Eng. Part A 25, 522–537 (2019).
Liu, S. et al. Regulated viral BDNF delivery in combination with Schwann cells promotes axonal regeneration through capillary alginate hydrogels after spinal cord injury. Acta Biomater. 60, 167–180 (2017).
Gholami, M., Gilanpour, H., Sadeghinezhad, J. & Asghari, A. Facile fabrication of an erythropoietin-alginate/chitosan hydrogel and evaluation of its local therapeutic effects on spinal cord injury in rats. Daru 29, 255–265 (2021).
Zhang, Y. et al. Lentiviral-mediated expression of polysialic acid in spinal cord and conditioning lesion promote regeneration of sensory axons into spinal cord. Mol. Ther. : J. Am. Soc. Gene Ther. 15, 1796–1804 (2007).
El Maarouf, A., Petridis, A. K. & Rutishauser, U. Use of polysialic acid in repair of the central nervous system. Proc. Natl Acad. Sci. USA 103, 16989–16994 (2006).
Ghosh, M. et al. Extensive cell migration, axon regeneration, and improved function with polysialic acid-modified Schwann cells after spinal cord injury. GLIA 60, 979–992 (2012).
Papastefanaki, F. et al. Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury. Brain. 130, 2159–2174 (2007).
Marino, P., Norreel, J. C., Schachner, M., Rougon, G. & Amoureux, M. C. A polysialic acid mimetic peptide promotes functional recovery in a mouse model of spinal cord injury. Exp. Neurol. 219, 163–174 (2009).
Mehanna, A. et al. Polysialic acid glycomimetic promotes functional recovery and plasticity after spinal cord injury in mice. Mol. Ther. 18, 34–43 (2010).
Pan, H. C., Shen, Y. Q., Loers, G., Jakovcevski, I. & Schachner, M. Tegaserod, a small compound mimetic of polysialic acid, promotes functional recovery after spinal cord injury in mice. Neuroscience 277, 356–366 (2014).
Loers, G. et al. Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture. J. neurochemistry 128, 88–100 (2014).
Zhang, S. et al. Polycaprolactone/polysialic acid hybrid, multifunctional nanofiber scaffolds for treatment of spinal cord injury. Acta Biomater. 77, 15–27 (2018).
Wang, X. J. et al. Polysialic-acid-based micelles promote neural regeneration in spinal cord injury therapy. Nano Lett. 19, 829–838 (2019).
Echave, M. C., Saenz del Burgo, L., Pedraz, J. L. & Orive, G. Gelatin as biomaterial for tissue engineering. Curr. Pharm. Des. 23, 3567–3584 (2017).
Liu, D. et al. Dual-Cues laden scaffold facilitates neurovascular regeneration and motor functional recovery after complete spinal cord injury. Adv. Health. Mater. 10, e2100089 (2021).
Fan, L. et al. Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Appl Mater. Interfaces 10, 17742–17755 (2018).
Yao, M. et al. Dual-enzymatically cross-linked gelatin hydrogel enhances neural differentiation of human umbilical cord mesenchymal stem cells and functional recovery in experimental murine spinal cord injury. J. Mater. Chem. B. 9, 440–452 (2021).
Luo, Y. & Shoichet, M. S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 3, 249–253 (2004).
Stokols, S. & Tuszynski, M. H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 27, 443–451 (2006).
Han, S. et al. Implantation of a Matrigel-loaded agarose scaffold promotes functional regeneration of axons after spinal cord injury in rat. Biochem. Biophys. Res. Commun. 496, 785–791 (2018).
Yang, B. et al. A conductive supramolecular hydrogel creates ideal endogenous niches to promote spinal cord injury repair. Bioact. Mater. 15, 103–119 (2022).
Gros, T., Sakamoto, J. S., Blesch, A., Havton, L. A. & Tuszynski, M. H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 31, 6719–6729 (2010).
An, H., Li, Q. & Wen, J. Bone marrow mesenchymal stem cells encapsulated thermal-responsive hydrogel network bridges combined photo-plasmonic nanoparticulate system for the treatment of urinary bladder dysfunction after spinal cord injury. J. Photochem. Photobiol. B, Biol. 203, 111741 (2020).
Gao, M. et al. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 34, 1529–1536 (2013).
Cox, A. et al. Nanoparticle-based estrogen delivery to spinal cord injury site reduces local parenchymal destruction and improves functional recovery. J. Neurotrauma 38, 342–352 (2021).
Gu, Q. et al. Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells. Adv. Health. Mater. 5, 1429–1438 (2016).
Liu, S., Xie, Y. Y. & Wang, B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen. Res 14, 1352–1363 (2019).
Brown, M., Li, J., Moraes, C., Tabrizian, M. & Li-Jessen, N. Y. K. Decellularized extracellular matrix: New promising and challenging biomaterials for regenerative medicine. Biomaterials 289, 121786 (2022).
Zhang, X. et al. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater.10, 15–31 (2022).
Wang, Y. H. et al. Reduced inflammatory cell recruitment and tissue damage in spinal cord injury by acellular spinal cord scaffold seeded with mesenchymal stem cells. Exp. therapeutic Med. 13, 203–207 (2017).
Xing, H., Ren, X., Yin, H., Sun, C. & Jiang, T. Construction of a NT-3 sustained-release system cross-linked with an acellular spinal cord scaffold and its effects on differentiation of cultured bone marrow mesenchymal stem cells. Mater. Sci. Eng. C. Mater. Biol. Appl. 104, 109902 (2019).
Jiang, T. et al. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater. Sci. Eng. C., Mater. Biol. Appl. 33, 3514–3521 (2013).
Ma, Y. H. et al. Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix-based neural repair of spinal cord injury. Biomaterials 279, 121192 (2021).
Vishwakarma, S. K., Bardia, A., Lakkireddy, C., Paspala, S. A. B. & Khan, A. A. Bioengineering human neurological constructs using decellularized meningeal scaffolds for application in spinal cord injury. Front. Bioeng. Biotechnol. 6, 150 (2018).
Bai, Y. R. et al. Decellularized optic nerve functional scaffold transplant facilitates directional axon regeneration and remyelination in the injured white matter of the rat spinal cord. Neural Regen. Res. 16, 2276–2283 (2021).
Shu, B. et al. Restoring electrical connection using a conductive biomaterial provides a new therapeutic strategy for rats with spinal cord injury. Neurosci. Lett. 692, 33–40 (2019).
Ling-ling, W. et al. Preparation and biocompatibility of an electroactive polyaniline/poly (lactic-acid) scaffold. Chin. J. Tissue Eng. Res. 22, 3557 (2018).
Zhou, L. et al. Soft conducting polymer hydrogels cross-linked and doped by tannic acid for spinal cord injury repair. ACS Nano 12, 10957–10967 (2018).
Wu, E. C., Zhang, S. & Hauser, C. A. Self‐assembling peptides as cell‐interactive scaffolds. Adv. Funct. Mater. 22, 456–468 (2012).
Raspa, A., Carminati, L., Pugliese, R., Fontana, F. & Gelain, F. Self-assembling peptide hydrogels for the stabilization and sustained release of active Chondroitinase ABC in vitro and in spinal cord injuries. J. Control. Rel. 330, 1208–1219 (2021).
Wang, J. et al. FGL-functionalized self-assembling nanofiber hydrogel as a scaffold for spinal cord-derived neural stem cells. Mater. Sci. Eng. C., Mater. Biol. Appl. 46, 140–147 (2015).
Hong, J. Y. et al. Self-assembling peptide gels promote angiogenesis and functional recovery after spinal cord injury in rats. J. Tissue Eng. 13, 20417314221086491 (2022).
Yang, B. et al. Graphene oxide-composited chitosan scaffold contributes to functional recovery of injured spinal cord in rats. Neural Regen. Res 16, 1829–1835 (2021).
Bussy, C., Ali-Boucetta, H. & Kostarelos, K. Safety considerations for graphene: lessons learnt from carbon nanotubes. Acc. Chem. Res. 46, 692–701 (2013).
González-Mayorga, A. et al. Favorable biological responses of neural cells and tissue interacting with graphene oxide microfibers. ACS Omega 2, 8253–8263 (2017).
Usmani, S. et al. Functional rewiring across spinal injuries via biomimetic nanofiber scaffolds. Proc. Natl Acad. Sci. USA 117, 25212–25218 (2020).
Li, L. et al. A MnO(2) nanoparticle-dotted hydrogel promotes spinal cord repair via regulating reactive oxygen species microenvironment and synergizing with mesenchymal stem cells. ACS nano 13, 14283–14293 (2019).
Zhai, H. et al. Mechanically strengthened hybrid peptide-polyester hydrogel and potential applications in spinal cord injury repair. Biomed. Mater. 15, 055031 (2020).
Luo, J. et al. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 6, 4816–4829 (2021).
Chiang, M. Y. et al. 4D spatiotemporal modulation of biomolecules distribution in anisotropic corrugated microwrinkles via electrically manipulated microcapsules within hierarchical hydrogel for spinal cord regeneration. Biomaterials 271, 120762 (2021).
Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 6, 105–121 (2015).
Abdulghani, S. & Mitchell, G. R. Biomaterials for in situ tissue regeneration: a review. Biomolecules 9, 750 (2019).
Ehsanipour, A. et al. Injectable, macroporous scaffolds for delivery of therapeutic genes to the injured spinal cord. APL Bioeng. 5, 016104 (2021).
Breen, B. A. et al. Therapeutic effect of Neurotrophin-3 treatment in an injectable collagen scaffold following rat spinal cord hemisection injury. ACS Biomater. Sci. Eng. 3, 1287–1295 (2017).
Boido, M. et al. Chitosan-based hydrogel to support the paracrine activity of mesenchymal stem cells in spinal cord injury treatment. Sci. Rep. 9, 6402 (2019).
Huang, F. et al. A conductive dual-network hydrogel composed of oxidized dextran and hyaluronic-hydrazide as BDNF delivery systems for potential spinal cord injury repair. Int J. Biol. Macromol. 167, 434–445 (2021).
Wang, Q. et al. A thermosensitive heparin-poloxamer hydrogel bridges aFGF to treat spinal cord injury. ACS Appl Mater. Interfaces 9, 6725–6745 (2017).
Fanz, P., Cheng, P. & Zhang, D. Progress on stimulus responsive smart hydrogels based on natural polymers. Mater. Rep. 34, 21012–21025 (2020).
Walsh, C. M., Wychowaniec, J. K., Brougham, D. F. & Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 234, 108043 (2022).
Reis, K. P. et al. Application of PLGA/FGF-2 coaxial microfibers in spinal cord tissue engineering: an in vitro and in vivo investigation. Regen. Med. 13, 785–801 (2018).
Liu, X. et al. 3D bioprinted neural tissue constructs for spinal cord injury repair. Biomaterials 272, 120771 (2021).
Hsieh, F. Y. & Hsu, S. H. 3D bioprinting: A new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 11, 153–158 (2015).
Mandrycky, C., Wang, Z., Kim, K. & Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 34, 422–434 (2016).
Koffler, J. et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 25, 263–269 (2019).
Basso, D. M., Beattie, M. S. & Bresnahan, J. C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 12, 1–21 (1995).
Basso, D. M., Beattie, M. S. & Bresnahan, J. C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 139, 244–256 (1996).
Hu, J. et al. Decellularization alters the unfavorable regenerative adverse microenvironment of the injured spinal cord to support neurite outgrowth. Ann. Transl. Med. 10, 934 (2022).
Chen, X., Luo, Y., Bi, H. & Yang, K. Preparation and application of acellular scaffold in tissue engineering and regenerative medicine. Chin. J. Tissue Eng. Res. 26, 591 (2022).
Xing, H. et al. Preparation of an acellular spinal cord scaffold to improve its biological properties. Mol. Med. Rep. 20, 1075–1084 (2019).
Vishwakarma, S. K., Lakkireddy, C., Bardia, A., Paspala, S. A. B. & Khan, A. A. Engineering bio-mimetic humanized neurological constructs using acellularized scaffolds of cryopreserved meningeal tissues. Mater. Sci. Eng. C., Mater. Biol. Appl. 102, 34–44 (2019).
Ham, T. R. & Leipzig, N. D. Biomaterial strategies for limiting the impact of secondary events following spinal cord injury. Biomed. Mater. 13, 024105 (2018).
Al Mamun, A. et al. Advances in immunotherapy for the treatment of spinal cord injury. Immunobiology 226, 152033 (2021).
Cox, A., Varma, A., Barry, J., Vertegel, A. & Banik, N. Nanoparticle estrogen in rat spinal cord injury elicits rapid anti-inflammatory effects in plasma, cerebrospinal fluid, and tissue. J. neurotrauma 32, 1413–1421 (2015).
Zheng, X. Q. et al. Controlled release of baricitinib from a thermos-responsive hydrogel system inhibits inflammation by suppressing JAK2/STAT3 pathway in acute spinal cord injury. Colloids Surf. B, Biointerfaces 199, 111532 (2021).
Desai, A. V., El-Bakkar, H. & Abdul-Hay, M. Novel agents in the treatment of chronic lymphocytic leukemia: a review about the future. Clin. Lymphoma Myeloma Leuk. 15, 314–322 (2015).
Ren, H. et al. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 35, 6585–6594 (2014).
Xu, C., Zhang, M., Zhang, G., Yan, S. & Yan, W. Hydrogen Sulfide Improves Functional Recovery In Rat Traumatic Spinal Cord Injury Model By Inducing Nuclear Translocation of NF-E2-related Factor 2. Biol. Pharm. Bull. 44, 1093–1100 (2021).
Jiang, Y. et al. Near-infrared light-triggered NO release for spinal cord injury repair. Sci. Adv. 6, eabc3513 (2020).
Kim, M. S. et al. Water-soluble chitosan inhibits the production of pro-inflammatory cytokine in human astrocytoma cells activated by amyloid beta peptide and interleukin-1beta. Neurosci. Lett. 321, 105–109 (2002).
Wu, W. et al. Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 35, 2355–2364 (2014).
López-Dolado, E., González-Mayorga, A., Gutiérrez, M. C. & Serrano, M. C. Immunomodulatory and angiogenic responses induced by graphene oxide scaffolds in chronic spinal hemisected rats. Biomaterials 99, 72–81 (2016).
Menezes, K., de Menezes, J. R., Nascimento, M. A., Santos Rde, S. & Coelho-Sampaio, T. Polylaminin, a polymeric form of laminin, promotes regeneration after spinal cord injury. FASEB J. 24, 4513–4522 (2010).
Austin, J. W. et al. The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials 33, 4555–4564 (2012).
Martinelli, C., Pucci, C., Battaglini, M., Marino, A. & Ciofani, G. Antioxidants and nanotechnology: promises and limits of potentially disruptive approaches in the treatment of central nervous system diseases. Adv. Health. Mater. 9, e1901589 (2020).
Luo, J., Borgens, R. & Shi, R. Polyethylene glycol immediately repairs neuronal membranes and inhibits free radical production after acute spinal cord injury. J. Neurochem. 83, 471–480 (2002).
Luo, J., Borgens, R. & Shi, R. Polyethylene glycol improves function and reduces oxidative stress in synaptosomal preparations following spinal cord injury. J. Neurotrauma 21, 994–1007 (2004).
Cho, Y., Shi, R., Ivanisevic, A. & Borgens, R. B. Functional silica nanoparticle-mediated neuronal membrane sealing following traumatic spinal cord injury. J. Neurosci. Res. 88, 1433–1444 (2010).
Yao, Y. et al. Reactive oxygen species (ROS)-responsive biomaterials mediate tissue microenvironments and tissue regeneration. J. Mater. Chem. B. 7, 5019–5037 (2019).
Zhang, T. et al. Reactive oxide species-scavenging lipid-polymer nanoparticles for neuroprotection after spinal cord injury. Appl. Mater. Today 24, 101109 (2021).
Rao, S. et al. Traditional Chinese medicine active ingredients-based selenium nanoparticles regulate antioxidant selenoproteins for spinal cord injury treatment. J. Nanobiotechnol. 20, 278 (2022).
Jiang, X. et al. Functional resveratrol-biodegradable manganese doped silica nanoparticles for the spinal cord injury treatment. Mater. today Bio. 13, 100177 (2022).
Wei, H. & Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem. Soc. Rev. 42, 6060–6093 (2013).
Pirmohamed, T. et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 46, 2736–2738 (2010).
Kim, J. W. et al. Functional recovery of contused spinal cord in rat with the injection of optimal-dosed cerium oxide nanoparticles. Adv. Sci. 4, 1700034 (2017).
Pal, A. et al. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int J. Nanomed. 8, 2259–2272 (2013).
Bauer, H. C., Krizbai, I. A., Bauer, H. & Traweger, A. “You Shall Not Pass”-tight junctions of the blood brain barrier. Front. Neurosci. 8, 392 (2014).
Gao, J., Khang, M., Liao, Z., Detloff, M. & Lee, J. S. Therapeutic targets and nanomaterial-based therapies for mitigation of secondary injury after spinal cord injury. Nanomedicine 16, 2013–2028 (2021).
Cohen, D. M. et al. Blood-spinal cord barrier permeability in experimental spinal cord injury: dynamic contrast-enhanced MRI. NMR Biomed. 22, 332–341 (2009).
Yu, D. S. et al. Curcumin improves the integrity of blood-spinal cord barrier after compressive spinal cord injury in rats. J. Neurol. Sci. 346, 51–59 (2014).
Requejo-Aguilar, R. et al. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials 113, 18–30 (2017).
Joshi, H. P. et al. CORM-2-solid lipid nanoparticles maintain integrity of blood-spinal cord barrier after spinal cord injury in rats. Mol. Neurobiol. 57, 2671–2689 (2020).
Rauch, M. F. et al. Engineering angiogenesis following spinal cord injury: a coculture of neural progenitor and endothelial cells in a degradable polymer implant leads to an increase in vessel density and formation of the blood-spinal cord barrier. Eur. J. Neurosci. 29, 132–145 (2009).
Tran, K. A. et al. Vascularization of self-assembled peptide scaffolds for spinal cord injury repair. Acta Biomater. 104, 76–84 (2020).
Matsushita, T. et al. Diffuse and persistent blood-spinal cord barrier disruption after contusive spinal cord injury rapidly recovers following intravenous infusion of bone marrow mesenchymal stem cells. Exp. Neurol. 267, 152–164 (2015).
Badner, A. et al. Early intravenous delivery of human brain stromal cells modulates systemic inflammation and leads to vasoprotection in traumatic spinal cord injury. Stem Cells Transl. Med. 5, 991–1003 (2016).
Ropper, A. E. et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc. Natl Acad. Sci. USA 114, E820–e829 (2017).
Pang, Q. M. et al. Neuroinflammation and scarring after spinal cord injury: therapeutic roles of MSCs on inflammation and Glial scar. Front. Immunol. 12, 751021 (2021).
Dyck, S. M. & Karimi-Abdolrezaee, S. Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system. Exp. Neurol. 269, 169–187 (2015).
Mukherjee, N. et al. Targeting Chondroitin sulfate proteoglycans: an emerging therapeutic strategy to treat CNS injury. ACS Chem. Neurosci. 11, 231–232 (2020).
Tran, A. P., Warren, P. M. & Silver, J. New insights into glial scar formation after spinal cord injury. Cell Tissue Res. 387, 319–336 (2022).
Bradbury, E. J. et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640 (2002).
McKay, C. A. et al. An injectable, calcium-responsive composite hydrogel for the treatment of acute spinal cord injury. ACS Appl Mater. Interfaces 6, 1424–1438 (2014).
Chen, C. et al. Bioinspired hydrogel electrospun fibers for spinal cord regeneration. Adv. Funct. Mater. 29, 1806899 (2019).
Mann, A. P. et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat. Commun. 7, 11980 (2016).
Wang, Q. et al. Novel multi-drug delivery hydrogel using scar-homing liposomes improves spinal cord injury repair. Theranostics 8, 4429–4446 (2018).
Sun, G. et al. Synthesis and characterization of a silica-based drug delivery system for spinal cord injury therapy. Nano-micro Lett. 11, 23 (2019).
Papa, S. et al. Functionalized nanogel for treating activated astrocytes in spinal cord injury. J. Control. Rel. 330, 218–228 (2021).
Li, Z., Wang, Q., Hu, H., Zheng, W. & Gao, C. Research advances of biomaterials-based microenvironment-regulation therapies for repair and regeneration of spinal cord injury. Biomed Mater. 16, 052002 (2021).
Azizi, M. et al. ChABC-loaded PLGA nanoparticles: A comprehensive study on biocompatibility, functional recovery, and axonal regeneration in animal model of spinal cord injury. Int. J. Pharm. 577, 119037 (2020).
Lee, H., McKeon, R. J. & Bellamkonda, R. V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl Acad. Sci. USA 107, 3340–3345 (2010).
Faulkner, J. R. et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci. 24, 2143–2155 (2004).
Fu, Q. et al. Engrafted peripheral blood-derived mesenchymal stem cells promote locomotive recovery in adult rats after spinal cord injury. Am. J. Transl. Res. 9, 3950–3966 (2017).
Hejrati, N. & Fehlings, M. G. A review of emerging neuroprotective and neuroregenerative therapies in traumatic spinal cord injury. Curr. Opin. Pharmacol. 60, 331–340 (2021).
Kaplan, B. & Levenberg, S. The role of biomaterials in peripheral nerve and spinal cord injury: a review. Int J. Mol. Sci. 23, 1244 (2022).
Stokols, S. et al. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 12, 2777–2787 (2006).
Shahriari, D., Koffler, J. Y., Tuszynski, M. H., Campana, W. M. & Sakamoto, J. S. Hierarchically ordered porous and high-volume polycaprolactone microchannel scaffolds enhanced axon growth in transected spinal cords. Tissue Eng. Part A 23, 415–425 (2017).
Siebert, J. R., Eade, A. M. & Osterhout, D. J. Biomaterial approaches to enhancing neurorestoration after spinal cord injury: strategies for overcoming inherent biological obstacles. Biomed. Res Int 2015, 752572 (2015).
Álvarez, Z. et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021).
Cao, D. D., Li, L. & Chan, W. Y. MicroRNAs: Key regulators in the central nervous system and their implication in neurological diseases. Int J. Mol. Sci. 17, 842 (2016).
Zhang, N. et al. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv. Sci. 8, e2100805 (2021).
Anderson, M. A. et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400 (2018).
Loh, N. K., Woerly, S., Bunt, S. M., Wilton, S. D. & Harvey, A. R. The regrowth of axons within tissue defects in the CNS is promoted by implanted hydrogel matrices that contain BDNF and CNTF producing fibroblasts. Exp. Neurol. 170, 72–84 (2001).
Tobias, C. A. et al. Grafting of encapsulated BDNF-producing fibroblasts into the injured spinal cord without immune suppression in adult rats. J. Neurotrauma 18, 287–301 (2001).
Tobias, C. A. et al. Alginate encapsulated BDNF-producing fibroblast grafts permit recovery of function after spinal cord injury in the absence of immune suppression. J. Neurotrauma 22, 138–156 (2005).
Lai, B. Q. et al. Stem cell-derived neuronal relay strategies and functional electrical stimulation for treatment of spinal cord injury. Biomaterials 279, 121211 (2021).
Li, X. et al. Scaffold-facilitated locomotor improvement post complete spinal cord injury: Motor axon regeneration versus endogenous neuronal relay formation. Biomaterials 197, 20–31 (2019).
Dulin, J. N. & Lu, P. Bridging the injured spinal cord with neural stem cells. Neural Regen. Res. 9, 229–231 (2014).
Assinck, P., Duncan, G. J., Hilton, B. J., Plemel, J. R. & Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 20, 637–647 (2017).
Lu, P., Kadoya, K. & Tuszynski, M. H. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr. Opin. Neurobiol. 27, 103–109 (2014).
Lai, B. Q., Zeng, X., Ding, Y., Li, G. & Zeng, Y. S. Research progress of neuronal relay strategies in repairing spinal cord injuries (in Chinese). Sci. Sin. Vitae 50, 1013–1024 (2020).
Ma, D. et al. A novel hydrogel-based treatment for complete transection spinal cord injury repair is driven by microglia/macrophages repopulation. Biomaterials 237, 119830 (2020).
Zheng, C. et al. Bio-C (Modified hyaluronic acid-coated-collagen tube) implants enable functional recovery after complete spinal cord injury. Pharmaceutics 14, 596 (2022).
Li, X. et al. A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/β-catenin signaling for spinal cord injury repair. Biomaterials 183, 114–127 (2018).
Yuan, T. et al. Highly permeable DNA supramolecular hydrogel promotes neurogenesis and functional recovery after completely transected spinal cord injury. Adv. Mater. 33, e2102428 (2021).
Lai, B. Q. et al. The integration of NSC-derived and host neural networks after rat spinal cord transection. Biomaterials 34, 2888–2901 (2013).
Zeng, X. et al. Integration of donor mesenchymal stem cell-derived neuron-like cells into host neural network after rat spinal cord transection. Biomaterials 53, 184–201 (2015).
Lai, B. Q. et al. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials 109, 40–54 (2016).
Wu, G. H. et al. Recovery of paralyzed limb motor function in canine with complete spinal cord injury following implantation of MSC-derived neural network tissue. Biomaterials 181, 15–34 (2018).
Guo, Y. S. & Cao, H. Q. Thoughts on spinal cord injury and regenerative repair (in Chinese). Bull. Natl Nat. Sci. Found. China 32, 354–357 (2018).
Lai, B. Q. et al. A modular assembly of spinal cord-like tissue allows targeted tissue repair in the transected spinal cord. Adv. Sci. 5, 1800261 (2018).
Siebert, J. R. & Osterhout, D. J. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 119, 176–188 (2011).
Pendleton, J. C. et al. Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ. Exp. Neurol. 247, 113–121 (2013).
Unal, D. B., Caliari, S. R. & Lampe, K. J. Engineering biomaterial microenvironments to promote myelination in the central nervous system. Brain Res. Bull. 152, 159–174 (2019).
Li, X. et al. Engineering an in situ crosslinkable hydrogel for enhanced remyelination. FASEB J. 27, 1127–1136 (2013).
Führmann, T. et al. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials 83, 23–36 (2016).
Li, Y. et al. Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules 15, 319–326 (2014).
Lee, S. et al. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922 (2012).
Leipzig, N. D. & Shoichet, M. S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30, 6867–6878 (2009).
Shah, S. et al. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 26, 3673–3680 (2014).
Chen, B. K. et al. GDNF Schwann cells in hydrogel scaffolds promote regional axon regeneration, remyelination and functional improvement after spinal cord transection in rats. J. Tissue Eng. Regen. Med. 12, e398–e407 (2018).
Yang, F. L., Li, P., Yang, L., Zhang, X. Y. & Wu, H. Y. Research progress of Schwann cells and repair of spinal cord injury (in Chinese). J. Chongqing Med. Univ. 47, 39–43 (2022).
Duncan, G. J. et al. Locomotor recovery following contusive spinal cord injury does not require oligodendrocyte remyelination. Nat. Commun. 9, 3066 (2018).
Liu, S., Schackel, T., Weidner, N. & Puttagunta, R. Biomaterial-supported cell transplantation treatments for spinal cord injury: challenges and perspectives. Front Cell Neurosci. 11, 430 (2017).
Anderson, K. D. et al. Safety of autologous human schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma 34, 2950–2963 (2017).
Mackay-Sim, A. et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain 131, 2376–2386 (2008).
Tabakow, P. et al. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 22, 1591–1612 (2013).
Mendonça, M. V. et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 5, 126 (2014).
Oraee-Yazdani, S. et al. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal cord. 54, 102–109 (2016).
Shin, J. C. et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. 2015, 630932 (2015).
Bozkurt, G. et al. Chitosan channels containing spinal cord-derived stem/progenitor cells for repair of subacute spinal cord injury in the rat. Neurosurgery 67, 1733–1744 (2010).
Park, S. S. et al. Functional recovery after spinal cord injury in dogs treated with a combination of Matrigel and neural-induced adipose-derived mesenchymal Stem cells. Cytotherapy 14, 584–597 (2012).
Ma, T., Wu, J., Mu, J. & Gao, J. Biomaterials reinforced MSCs transplantation for spinal cord injury repair. Asian J. Pharm. Sci. 17, 4–19 (2022).
Khaing, Z. Z. et al. High molecular weight hyaluronic acid limits astrocyte activation and scar formation after spinal cord injury. J. Neural Eng. 8, 046033 (2011).
Seyedhassantehrani, N., Li, Y. & Yao, L. Dynamic behaviors of astrocytes in chemically modified fibrin and collagen hydrogels. Integr. Biol. : Quant. Biosci. Nano Macro 8, 624–634 (2016).
Miller, C., Shanks, H., Witt, A., Rutkowski, G. & Mallapragada, S. Oriented Schwann cell growth on micropatterned biodegradable polymer substrates. Biomaterials 22, 1263–1269 (2001).
Chen, B. K. et al. Axon regeneration through scaffold into distal spinal cord after transection. J. Neurotrauma 26, 1759–1771 (2009).
Liu, T. et al. Recent advances in cell and functional biomaterial treatment for spinal cord injury. Biomed. Res Int 2022, 5079153 (2022).
Teng, Y. D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl Acad. Sci. USA 99, 3024–3029 (2002).
Slotkin, J. R. et al. Biodegradable scaffolds promote tissue remodeling and functional improvement in non-human primates with acute spinal cord injury. Biomaterials 123, 63–76 (2017).
Theodore, N. et al. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: a clinical pilot study for safety and feasibility. Neurosurgery 79, E305–E312 (2016).
Kim, K. D. et al. A study of probable benefit of a bioresorbable polymer scaffold for safety and neurological recovery in patients with complete thoracic spinal cord injury: 6-month results from the INSPIRE study. J. Neurosurg. Spine. 1–10 (2021).
Han, Q. et al. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng. Part A 15, 2927–2935 (2009).
Han, Q. et al. The promotion of neural regeneration in an extreme rat spinal cord injury model using a collagen scaffold containing a collagen binding neuroprotective protein and an EGFR neutralizing antibody. Biomaterials 31, 9212–9220 (2010).
Han, S. et al. The linear-ordered collagen scaffold-BDNF complex significantly promotes functional recovery after completely transected spinal cord injury in canine. Biomaterials 41, 89–96 (2015).
Li, X. et al. Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl Mater. Interfaces 7, 13960–13971 (2015).
Xiao, Z. et al. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Science China. Life Sci. 59, 647–655 (2016).
Zhao, Y. et al. Clinical study of neuroregen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 26, 891–900 (2017).
Tang, F. et al. Long-term clinical observation of patients with acute and chronic complete spinal cord injury after transplantation of NeuroRegen scaffold. Science China. Life Sci. 65, 909–926 (2022).
Xiao, Z. et al. Significant improvement of acute complete spinal cord injury patients diagnosed by a combined criteria implanted with neuroregen scaffolds and mesenchymal stem cells. Cell Transplant. 27, 907–915 (2018).
Chen, W. et al. NeuroRegen scaffolds combined with autologous bone marrow mononuclear cells for the repair of acute complete spinal cord injury: a 3-year clinical study. Cell Transplant. 29, 963689720950637 (2020).
Delaere, P. R. & Van Raemdonck, D. Commentary: The sobering truth about tracheal regeneration. J. Thorac. Cardiovasc. Surg. 159, 2537–2539 (2020).
Li, N. & Leung, G. K. Oligodendrocyte precursor cells in spinal cord injury: a review and update. Biomed. Res Int. 2015, 235195 (2015).
Yiu, G. & He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 7, 617–627 (2006).
Abbas, W. A. et al. Recent advances in the regenerative approaches for traumatic spinal cord injury: materials perspective. ACS Biomater. Sci. Eng. 6, 6490–6509 (2020).
Guan, T., Li, J., Chen, C. & Liu, Y. Self-assembling peptide-based hydrogels for wound tissue repair. Adv. Sci. 9, e2104165 (2022).
Neishabouri, A., Soltani Khaboushan, A., Daghigh, F., Kajbafzadeh, A. M. & Majidi Zolbin, M. Decellularization in tissue engineering and regenerative medicine: evaluation, modification, and application methods. Front. Bioeng. Biotechnol. 10, 805299 (2022).
Li, Z. & Tan, B. H. Towards the development of polycaprolactone based amphiphilic block copolymers: molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 45, 620–634 (2014).
Amani, H., Kazerooni, H., Hassanpoor, H., Akbarzadeh, A. & Pazoki-Toroudi, H. Tailoring synthetic polymeric biomaterials towards nerve tissue engineering: a review. Artif. Cells, Nanomed. Biotechnol. 47, 3524–3539 (2019).
BaoLin, G. & Ma, P. X. Synthetic biodegradable functional polymers for tissue engineering: a brief review. Science China. Chemistry 57, 490–500 (2014).
Jeong, H. J. et al. Fabrication of three-dimensional composite scaffold for simultaneous alveolar bone regeneration in dental implant installation. Int. J. Mol. Sci. 21, 1863 (2020).
Schnell, E. et al. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials 28, 3012–3025 (2007).
Zhou, X. et al. Polycaprolactone electrospun fiber scaffold loaded with iPSCs-NSCs and ASCs as a novel tissue engineering scaffold for the treatment of spinal cord injury. Int J. Nanomed. 13, 6265–6277 (2018).
Singh, N. K. et al. Uric acid released from poly(ε-caprolactone) fibers as a treatment platform for spinal cord injury. J. Tissue Eng. Regen. Med. 15, 14–23 (2021).
Kong, X. B. et al. Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen. Res. 12, 1003–1008 (2017).
Rao, S. S., Han, N. & Winter, J. O. Polylysine-modified PEG-based hydrogels to enhance the neuro-electrode interface. J. Biomater. Sci. Polym. Ed. 22, 611–625 (2011).
Caldwell, A. S., Rao, V. V., Golden, A. C. & Anseth, K. S. Porous bio-click microgel scaffolds control hMSC interactions and promote their secretory properties. Biomaterials 232, 119725 (2020).
Ciciriello, A. J. et al. Acute implantation of aligned hydrogel tubes supports delayed spinal progenitor implantation. ACS Biomater. Sci. Eng. 6, 5771–5784 (2020).
Tyler, B., Gullotti, D., Mangraviti, A., Utsuki, T. & Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 107, 163–175 (2016).
Santoro, M., Shah, S. R., Walker, J. L. & Mikos, A. G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 107, 206–212 (2016).
Saini, P., Arora, M. & Kumar, M. Poly(lactic acid) blends in biomedical applications. Adv. Drug Deliv. Rev. 107, 47–59 (2016).
Liu, Z. H. et al. Co-delivery of Docosahexaenoic Acid and brain-derived neurotropic factor from electrospun aligned core-shell fibrous membranes in treatment of spinal cord injury. Pharmaceutics 14, 321 (2022).
de Ruiter, G. C. et al. Methods for in vitro characterization of multichannel nerve tubes. J. Biomed. Mater. Res. A 84, 643–651 (2008).
Lee, J. Y., Bashur, C. A., Goldstein, A. S. & Schmidt, C. E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 30, 4325–4335 (2009).
Mehrasa, M., Asadollahi, M. A., Ghaedi, K., Salehi, H. & Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int J. Biol. Macromol. 79, 687–695 (2015).
Pan, S. et al. Graphene oxide-PLGA hybrid nanofibres for the local delivery of IGF-1 and BDNF in spinal cord repair. Artificial cells. Nanomed. Biotechnol. 47, 651–664 (2019).
Shen, K. et al. Anti-Inflammatory nanotherapeutics by targeting matrix metalloproteinases for immunotherapy of spinal cord injury. Small 17, e2102102 (2021).
Ismail, M. et al. Genistein loaded nanofibers protect spinal cord tissue following experimental injury in rats. Biomedicines 6, 96 (2018).
Bakshi, A. et al. Mechanically engineered hydrogel scaffolds for axonal growth and angiogenesis after transplantation in spinal cord injury. J. Neurosurg. Spine 1, 322–329 (2004).
Hejcl, A. et al. Macroporous hydrogels based on 2-hydroxyethyl methacrylate. Part 6: 3D hydrogels with positive and negative surface charges and polyelectrolyte complexes in spinal cord injury repair. J. Mater. Sci. Mater. Med. 20, 1571–1577 (2009).
Ruzicka, J. et al. The effect of iPS-derived neural progenitors seeded on laminin-coated pHEMA-MOETACl hydrogel with dual porosity in a rat model of chronic spinal cord injury. Cell Transplant. 28, 400–412 (2019).
Pertici, V. et al. The use of poly(N-[2-hydroxypropyl]-methacrylamide) hydrogel to repair a T10 spinal cord hemisection in rat: a behavioural, electrophysiological and anatomical examination. ASN Neuro 5, 149–166 (2013).
Woerly, S., Pinet, E., de Robertis, L., Van Diep, D. & Bousmina, M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel). Biomaterials 22, 1095–1111 (2001).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82272531, 81902234, 82102627, 81972088, 82072469, 82172470, 82072471, 82002335), the Chenguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (21CGA45), Shanghai Science & Technology Commission Excellent Academic Leader Project (21XD1404800), Shanghai Shenkang Hospital Development Center Clinical Innovation Project (SHDC2020CR6018), and Shanghai Changzheng Hospital Clinical Innovation Project (2020YLCYJ-Z04).
Author information
Authors and Affiliations
Contributions
K.C, and W.Y. wrote the main manuscript text. G.Z. and Z.X. prepared figures. C.Y., Y.W. and Z.Y. helped search and collect relevant articles. W.Y. * and B.H. * made instructive revisions to the article. H.C. *General design for the article and financial support. All authors reviewed the manuscript. K.C. and W.Y. contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, K., Yu, W., Zheng, G. et al. Biomaterial-based regenerative therapeutic strategies for spinal cord injury. NPG Asia Mater 16, 5 (2024). https://doi.org/10.1038/s41427-023-00526-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-023-00526-4