Abstract
The combined use of peptides, nanomaterials, and hydrogels is a promising strategy for chronic skin wound healing, which remains a huge clinical challenge. Here, we optimized the RL-QN15 peptide, which was shown to be a pro-healing drug candidate in our previous research, to obtain the cyclic heptapeptide (CyRL-QN15) with considerable therapeutic potency against skin wounds. Furthermore, a Zn2+-crosslinked sodium alginate (ZA) hydrogel containing hollow polydopamine (HPDA) nanoparticles loaded with CyRL-QN15 (HPDAlCyRL-QN15/ZA hydrogel) was prepared and characterized, which significantly enhanced the pro-healing potency of CyRL-QN15. At the cellular level, this nontoxic hydrogel accelerated the proliferation, migration, tube formation, and scratch healing of skin cells, regulated the secretion of cytokines from macrophages, directly scavenged free radicals, and decreased reactive oxygen species. Moreover, the HPDAlCyRL-QN15/ZA hydrogel significantly accelerated the healing of full-thickness skin wounds in type 2 diabetic mice by promoting the transition of macrophages to the M2 phenotype to reduce inflammation and cause re-epithelialization, formation of granulation tissue, deposition of collagen, and angiogenesis. Of note, the hydrogel also facilitated wound healing of diabetic patient skin cultured ex vivo. Overall, the HPDAlCyRL-QN15/ZA hydrogel presents a novel therapeutic strategy for clinical chronic skin wound (diabetic ulcer) healing.
Similar content being viewed by others
Introduction
Wound healing is a complex physiological process that maintains the structural integrity of the body and consists of hemostasis, inflammation, proliferation, and remodeling stages1,2. Wounds that fail to heal within a normal time frame are considered chronic wounds. Chronic wounds affect 0.2% to 1% of the population in developed countries, posing an increasing health and economic burden on society3. At present, chronic wound treatment lacks effective targeted therapies, focusing instead on optimizing controllable healing factors4,5. Therefore, exploring innovative intervention strategies to promote chronic skin wound healing remains essential.
Various novel interventions have been developed for chronic skin wounds, including the use of bioactive peptides, hydrogels, nanomaterials, and tissue engineering. In particular, bioactive peptides, hydrogel dressings, and nanomaterials have received considerable attention6,7. Several bioactive peptides derived from amphibian skin, such as OA-GL12, cathelicidin-OA1, cathelicidin-NV, and RL-QN15, have shown significant potential as novel pro-healing agents in the treatment of skin wounds8,9,10,11,12. A variety of biomaterials (e.g., hydrogels, nanofibers, and films) have also been used in the treatment of chronic wounds13. Hydrogels are three-dimensional (3D) networks formed by cross-linking hydrophilic polymer chains, with properties similar to those of the extracellular matrix (ECM). They are considered ideal scaffolds for wound healing due to their ability to absorb wound exudates, maintain a moist environment, and promote fibroblast proliferation and keratinocyte migration14,15. Sodium alginate (SA) consists of different ratios of β-1,4-linked repeating units of D-mannuronic acid (M) and L-glutamine (G)16. The high L-glutamine (G) block content of alginate enables the formation of an insoluble gel network by building bridges in the polymer network with divalent cations such as Zn2+17. In addition, Zn2+ is an essential element for cell proliferation and angiogenesis and has shown excellent results in chronic skin wound healing18. Nanomaterials have been widely used in wound repair due to their unique surface properties, physiological activities, adjustable porous structure, outstanding biocompatibility, and drug loading ability19. Hollow polydopamine (HPDA) nanoparticles exhibit excellent surface permeability, load-carrying capacity, antioxidant activity, and controllable morphology, thus representing an ideal drug delivery system for chronic skin wound healing20. Therefore, incorporating peptides, nanomaterials, and hydrogels to create combination agents will lead to novel strategies for the treatment of chronic skin wounds. At present, however, relevant reports on skin wound healing remain scarce.
Optimization of existing bioactive peptides is an effective approach for developing novel agents, such as ziconotide, exenatide, bivalirudin, and captopril21,22. We previously identified a novel pro-healing peptide RL-QN15 from frog skin secretions, which contains intramolecular disulfide bonds without posttranslational modifications23. At low concentrations, RL-QN15 showed remarkable therapeutic potential in the healing of acute wounds, chronic wounds, skin fibrosis, oral ulcers, and full-thickness skin wounds in pigs10. We also developed a novel strategy to promote dermal wound healing by loading RL-QN15 into HPDA nanoparticles, which increased the pro-healing ability of the peptide19. However, further refinement of the RL-QN15 structure is important to reduce costs and increase activity and thus facilitate the development of novel pro-healing drugs.
In the current study, we optimized the structure of RL-QN15 and obtained a shorter cyclic heptapeptide (CyRL-QN15) with excellent skin wound healing activity. Furthermore, we successfully prepared and characterized a Zn2+ cross-linked SA hydrogel containing HPDA nanoparticles loaded with CyRL-QN15 (HPDAlCyRL-QN15/ZA hydrogel) for chronic skin wound healing. At the cellular level, this nontoxic hydrogel accelerated the proliferation, migration, tube formation, and scratch healing of skin cells, regulated the secretion of cytokines from macrophages, directly scavenged free radicals, and decreased reactive oxygen species (ROS). The HPDAlCyRL-QN15/ZA hydrogel also showed excellent therapeutic effects on full-thickness diabetic skin wounds in mice and full-thickness ex vivo foot skin wounds from diabetic patients. This study presents a prospective HPDAlCyRL-QN15/ZA hydrogel for chronic skin wound healing and emphasizes the potential of combined therapy based on peptides, nanomaterials, and hydrogels for the clinical treatment of chronic skin trauma.
Experimental section
Animal ethics statement and informed consent
Male Kunming and C57BL/6 mice (20–24 g, 6–8 weeks old) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China). All animal care and handling procedures were approved by and followed the requirements of the Ethics Committee of Kunming Medical University (kmmu20220069).
All human skin samples were obtained with informed consent from diabetic patients undergoing amputation surgeries at the Department of Endocrinology, Affiliated Hospital of Yunnan University (Kunming, Yunnan, China). Skin collection was approved by the Ethics Committee of the Affiliated Hospital of Yunnan University (2021103). Informed consent confirmed that the patients voluntarily donated their skin for wound healing research with no financial payment. This research abides by the Declaration of Helsinki principles.
Synthesis and stability of peptides
The RL-QN15 peptide, reduced linear peptide of RL-QN15 without disulfide bonds (ReRL-QN15), linear octapeptide in front of RL-QN15 disulfide bonds (LiRL-QN15), and cyclic heptapeptide composed of an RL-QN15 disulfide-bonded circular structure (CyRL-QN15) (purity > 95%) were commercially synthesized by Bioyeargene Biotechnology Co., Ltd. (Wuhan, China). The structure of CyRL-QN15 was predicted using PEP-FOLD3 online service9. The stability of RL-QN15 and CyRL-QN15 was evaluated according to previous research24.
Cell culture
Human keratinocytes (HaCaT cells), human skin fibroblasts (HSFs), human umbilical vein endothelial cells (HUVECs), and mouse macrophages (RAW 264.7 cells) were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/high glucose medium (BI, Israel) supplemented with 1% double antibodies (penicillin and streptomycin) and 10% fetal bovine serum (FBS, Gibco) at 37 °C in a humidified atmosphere of 5% CO2.
Keratinocyte scratch healing assay
The pro-healing effects of CyRL-QN15 on HaCaT cells were evaluated according to a previous study25. Specific experimental methods are detailed in section S2.2 of the Supplementary Information.
Effects of RL-QN15-modified peptides on full-thickness skin wounds in mice
The healing effects of CyRL-QN15 on full-thickness skin wounds in mice were examined according to a previous study10. Specific experimental methods are detailed in section S2.3 of the Supplementary Information.
Preparation and characterization of HPDAlCyRL-QN15/ZA hydrogel
Specific experimental methods for the preparation and characterization of the HPDAlCyRL-QN15/ZA hydrogel are detailed in section S2.4 of the Supplementary Information.
Loading and release of HPDAlCyRL-QN15/ZA hydrogel against CyRL-QN15
The loading efficiency of HPDA and hydrogel against CyRL-QN15 and the release efficiency of the HPDAlCyRL-QN15/ZA hydrogel against CyRL-QN15 were determined according a previous study19.
Biocompatibility and degradation of the HPDAlCyRL-QN15/ZA hydrogel
The toxicity of the HPDAlCyRL-QN15/ZA hydrogel was evaluated in C57BL/6 mice with full-thickness skin wounds and HaCaT cells using the live/dead cell viability assay according to a previous study19. The degradation of ZA, HPDA/ZA, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA in vitro and in vivo was determined according to previous studies26,27. Specific experimental methods are detailed in section S2.5 of the Supplementary Information.
Assessment of cell proliferation
Cell proliferation was determined using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay according to previous methods19. Specific experimental methods are detailed in section S2.6 of the Supplementary Information.
In vitro HUVEC migration and tube formation assays
Angiogenesis experiments were performed in vitro according to a previous study28. Specific experimental methods are detailed in section S2.7 of the Supplementary Information.
Influence of samples on cytokine levels involved in healing
RAW 264.7 cells (2 × 104 cells/well) were cultured in 6-well plates and incubated with lipopolysaccharide (LPS), vehicle (phosphate-buffered saline, PBS), CyRL-QN15, HPDAlCyRL-QN15, or HPDAlCyRL-QN15/ZA for 24 h based on previous research19. The supernatants were collected to detect the effects on the release of cytokines (transforming growth factor-β1, TGF-β1; tumor necrosis factor-α, TNF-α) using enzyme-linked immunosorbent assay (ELISA) kits (NeoBioscience, Shanghai, China).
Antioxidant activity of the HPDAlCyRL-QN15/ZA hydrogel
The antioxidant activity of the HPDAlCyRL-QN15/ZA hydrogel was evaluated based on free radical scavenging ability and reduction of intracellular reactive oxygen species (ROS). Specific experimental methods are detailed in Section S2.8 of the Supplementary Information.
Effects of samples on chronic diabetic skin wounds in mice
To evaluate the wound healing effects of the HPDAlCyRL-QN15/ZA hydrogel, chronic full-thickness skin wounds in diabetic mice were established according to an earlier study10. Specific experimental methods are detailed in Section S2.9 of the Supplementary Information.
Effects of the HPDAlCyRL-QN15/ZA hydrogel on cytokine secretion in skin wounds
Specimens were acquired from the central area of full-thickness skin wounds in diabetic mice on Days 3, 7, and 14 postoperation, homogenized in ice-cold saline (weight/volume = 1:9) at 4 °C and centrifuged at 12000 × g for 20 min at 4 °C to collect the supernatant. The levels of TNF-α and TGF-β1 were detected using ELISA kits (NeoBioscience, Shanghai, China).
Histological analysis and immunohistochemical and immunofluorescence staining
To investigate tissue regeneration, including macrophage polarization (F4/80; inducible nitric oxide synthase, INOS; arginase, ARG), keratinocyte proliferation (Ki67), deposition of collagen (collagen type I, COL I; collagen type III, COL III), angiogenesis (vascular endothelial growth factor, VEGF; α-smooth muscle actin, α-SMA; platelet endothelial cell adhesion molecule, CD31) and the expression of inflammatory factors (interleukin-1β, IL-1β; interleukin-10, IL-10) in skin wounds after different treatments, wound tissues underwent hematoxylin and eosin (H&E), Masson trichrome, periodic acid-Schiff (PAS), and immunohistochemical staining and immunofluorescence analysis according to a previous study29. Specific experimental methods are detailed in section S2.10 of the Supplementary Information.
Human ex vivo diabetic skin wound model
The pro-tissue regenerative activity of the HPDAlCyRL-QN15/ZA hydrogel was examined using a modified human skin wound healing assay30,31,32. Specific experimental methods are detailed in section S2.11 of the Supplementary Information.
Results and discussion
The RL-QN15-optimized cyclic heptapeptide CyRL-QN15 promoted keratinocyte scratch repair and full-thickness skin wound healing in mice
Structural optimization of existing bioactive peptides is important for the development of novel drugs. We previously revealed that the bioactive peptide RL-QN15 exhibits considerable therapeutic effects on skin wounds10. As shown in Fig. 1A, RL-QN15 consists of 15 amino acid residues and contains a pair of intramolecular disulfide bonds. Disulfide bonds are critical to the function of amphibian-derived bioactive peptides10,33,34. To optimize RL-QN15 and obtain peptides with shorter amino acid sequences and stronger activities, we synthesized ReRL-QN15, LiRL-QN15, and CyRL-QN15 (Fig. 1A). As shown in Fig. 1B, CyRL-QN15 contains seven amino acids and links two-terminal cysteine residues to form a closed-loop structure. The predicted structure of CyRL-QN15 also demonstrated a closed-loop structure (Fig. 1C).
A Amino acid sequences of RL-QN15, ReRL-QN15, LiRL-QN15, and CyRL-QN15. B Chemical structure of CyRL-QN15. C Predicted structure of CyRL-QN15. D, E Representative and quantitative plots of the effects of RL-QN15, ReRL-QN15, LiRL-QN15, and CyRL-QN15 on scratch repair of HaCaT cells. Data represent the mean ± standard deviation (SD), generated from three independent experiments performed in triplicate. ** indicates P < 0.01. F, G Representative and quantitative plots of the effects of RL-QN15, ReRL-QN15, LiRL-QN15, and CyRL-QN15 on wound healing in Kunming mice with full-thickness skin wounds in vivo. Data represent the mean ± SD, n = 15. * and ** indicate P < 0.05 and P < 0.01, respectively.
At a concentration of 1 nM, LiRL-QN15 and ReRL-QN15 did not promote HaCaT cell scratch repair, whereas CyRL-QN15 exhibited similar HaCaT cell scratch pro-healing effects as RL-QN15 (Fig. 1D). The cell scratch repair rates of ReRL-QN15, LiRL-QN15, RL-QN15, and CyRL-QN15 were 55.1%, 60.8%, 88.1%, and 90.5%, respectively (Fig. 1E).
The therapeutic effects of CyRL-QN15 on full-thickness skin wounds in mice were explored (Fig. 1F). At a concentration of 1 nM, the wound healing rates of RL-QN15 and CyRL-QN15 conditions were 91.8% and 94.0%, respectively, higher than those of the positive control rh-bFGF (87.7%) and the ReRL-QN15 (60.0%) and LiRL-QN15 (63.1%) conditions (Fig. 1G). Peptide stability is critical in skin wound treatment, and therefore, we examined the stability of CyRL-QN15 in plasma. The half-life of CyRL-QN15 in plasma was 8.07 h, longer than that of RL-QN15 (7.79 h), indicating greater stability (Fig. S1A, B). Thus, CyRL-QN15 exhibited pro-trauma repair activity comparable to that of RL-QN15 but had better stability and lower synthesis costs than RL-QN15, suggesting that it may be a better pro-healing drug candidate.
Characterization and properties of the HPDAlCyRL-QN15/ZA hydrogel
In the current study, we prepared HPDAlCyRL-QN15/ZA hydrogel containing HPDA nanoparticles loaded with CyRL-QN15 for skin wound healing. As shown in Fig. S2, Zn2+ promoted the formation of ZA and HPDAlCyRL-QN15/ZA hydrogels with greater cross-linking than the SA hydrogel. Transmission electron microscopy (TEM) revealed that the HPDA nanoparticles loaded with CyRL-QN15 had an average grain size of approximately 50 nm, with a spherical morphology and hollow structure, indicating excellent loading capacity (Fig. S3A, B). The morphology and structure of ZA and HPDAlCyRL-QN15/ZA hydrogels were characterized by scanning electron microscopy (SEM), showing a 3D network of microporous structures, which form the basis of the moisture retention and swelling of gels (Fig. 2A, B). Due to the ability of HPDAlCyRL-QN15 to interact with the internal molecules of the ZA hydrogel, the HPDAlCyRL-QN15/ZA hydrogel showed an increased cross-linking density and a denser surface, with a much lower porosity (48.52 ± 0.40%) compared to the ZA hydrogel (56.78 ± 0.10%) (Fig. 2C).
A, B SEM images of the ZA and HPDAlCyRL-QN15/ZA hydrogels, respectively. The scale (100 μm and 20 μm) is represented by the line in the lower left corner of the figure. C Porosity of the ZA and HPDAlCyRL-QN15/ZA hydrogels. Data represent the mean ± SD, generated from three independent experiments. * indicates P < 0.05. D, E FTIR and XPS analyses of the SA, ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA, respectively. F Swelling ratio of the ZA and HPDAlCyRL-QN15/ZA hydrogels. G, H Loading efficiency of CyRL-QN15 and the efficiency of its slow-release from the HPDAlCyRL-QN15, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA. Data represent the mean ± SD, generated from three independent experiments performed in triplicate.
The four Fourier transform infrared (FTIR) spectral curves of the crystal structures of SA, ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA were shown in Fig. 2D. The peak at 3384 cm−1 in the SA curve corresponded to the stretching vibration of O-H, which shifted to 3380 cm−1 and 3344 cm−1 with the formation of ZA and HPDAlCyRL-QN15/ZA hydrogels, respectively. The shift in the HPDAlCyRL-QN15/ZA hydrogel toward lower stretching energy may be related to the presence of imine, amine, catechol, and other groups in HPDA that cause stretching vibrations of the -OH group (Fig. 2D). In addition, the characteristic peak of the -COO stretching vibration at 1610 cm−1 in SA shifted to 1607 and 1599 cm−1 in the ZA and HPDAlCyRL-QN15/ZA hydrogels, respectively (Fig. 2D). After HPDAlCyRL-QN15 was embedded in the HPDAlCyRL-QN15/ZA hydrogel, the characteristic peak of 1220 cm−1 for HPDAlCyRL-QN15 shifted to 1226 cm−1 due to the intermolecular interaction between CyRL-QN15 and alginate, while the SA and ZA hydrogels remained at 1215 cm−1, indicating successful introduction of CyRL-QN15 into the HPDAlCyRL-QN15/ZA hydrogel (Fig. 2D). The X-ray photoelectron spectroscopy (XPS) spectra of SA, ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA were shown in Fig. 2E. Notably, Na1s, Zn2p, O1s, N1s, C1s, and S2p signal peaks were detected in the HPPDAlCyRL-QN15/ZA hydrogel, indicating the presence of SA, Zn2+, and cyclic peptides with disulfide bonds in the composite. Thus, based on these results, the multifunctional HPDAlCyRL-QN15/ZA hydrogel containing HPDA, CyRL-QN15, and Zn2+ was successfully prepared.
A decreased cross-linking density of the hydrogel results in higher swelling properties35. As seen in Fig. 2F, the ZA hydrogel showed excellent swelling properties, while the HPDAlCyRL-QN15/ZA hydrogel showed a lower swelling ratio. The lower swelling ratio of the HPDAlCyRL-QN15/ZA hydrogel may be related to HPDAlCyRL-QN15 interacting with the internal molecules of the ZA hydrogel and increasing its cross-linking density, consistent with the SEM and porosity results. The mechanical properties of hydrogels applied to wounds or tissue engineering are crucial36; thus, we determined the rheological and compression properties of the hydrogels. The rheological properties of storage modulus (G’) and loss modulus (G”) of ZA, HPDA/ZA, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA hydrogels were measured in the frequency range of 0–10 Hz at 37 °C. The results showed that G’ was greater than G” for all groups, with the HPDAlCyRL-QN15/ZA hydrogel showing the most significant difference, indicating greater flexibility (Fig. S4A). The G’ and G” values of the HPDAlCyRL-QN15/ZA hydrogel did not change with increasing oscillation frequency, indicating that the HPDAlCyRL-QN15/ZA hydrogel has excellent stability (Fig. S4A). The compression properties of the HPDAlCyRL-QN15/ZA hydrogel were enhanced by the cross-linking of Zn2+ and the internal molecular interactions between HPDAlCyRL-QN15 and ZA, demonstrating higher compressive strength (more than 250 kPa to 80% strain) than the ZA, HPDA/ZA, and CyRL-QN15/ZA hydrogels (Fig. S4B).
The loading of CyRL-QN15 and its slow-release from CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA were evaluated. As shown in Fig. 2G, the loading efficiencies of HPDAlCyRL-QN15/ZA, HPDAlCyRL-QN15, and CyRL-QN15/ZA against CyRL-QN15 were 44.61, 50.62, and 87.86%, respectively. When dispersed in PBS, HPDAlCyRL-QN15, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA released CyRL-QN15 into the solvent in a sustained manner (Fig. 2H). As shown in Fig. 2H, the efficiency of CyRL-QN15 release from CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA sequentially decreased, with release rates of >50% at 24 h, reaching 93.51, 79.89, and 73.48% at 48 h, respectively. The sustained-release of CyRL-QN15 by the HPDAlCyRL-QN15/ZA hydrogel prolonged the effects of the peptide, and the release rates of CyRL-QN15 peaked at 48 h, thus exerting effects on the inflammatory and proliferative phases of wound repair. In summary, we successfully prepared the HPDAlCyRL-QN15/ZA hydrogel with excellent mechanical properties and CyRL-QN15 loading and slow-release properties.
Biocompatibility and degradation of the HPDAlCyRL-QN15/ZA hydrogel
Biocompatibility is an important property regarding the potential safety of novel biomaterials37. As shown in Fig. S5A, nearly all HaCaT cells were stained with calcein-AM ester (green fluorescence), with very few dead cells stained with PI (red fluorescence), indicating that ZA, HPDA, CyRL-QN15, HPDA/ZA, CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA exhibited no toxicity toward HaCaT cells. The C57BL/6 mouse dorsal skin wound toxicity assay also revealed that ZA, HPDA, CyRL-QN15, HPDA/ZA, CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA caused no mortality in mice, and histological sections of major organs (heart, liver, spleen, lung, and kidney) from treated mice showed no abnormalities (Fig. S5B). The degradation of hydrogels in vivo is also crucial for their application in skin wound healing38. Thus, we evaluated the degradation of hydrogels in vitro and in vivo (Fig. S6). The results showed that the hydrogels gradually decreased in size with increasing incubation time under hyaluronidase and collagenase treatment; after 120 h of treatment, the remaining amounts of ZA, HPDA/ZA, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA hydrogels were 19.8, 23.2, 23.4, and 29.8%, respectively (Fig. S6A). The ZA, HPDA/ZA, CyRL-QN15/ZA, and HPDAlCyRL-QN15/ZA hydrogels also showed great degradation efficacy when injected subcutaneously into the abdomens of mice, with all hydrogels fully degrading two weeks after injection (Fig. S6B). In conclusion, the HPDAlCyRL-QN15/ZA hydrogel showed negligible cytotoxicity, excellent biocompatibility, and excellent degradation properties, providing a foundation for its pro-healing therapeutic potential in skin wounds.
HPDAlCyRL-QN15/ZA hydrogel promoted cell proliferation and scratching healing
The MTS results showed that ZA, HPDA/ZA, CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA exhibited significantly proliferation-promoting activity toward HaCaT cells, HSFs, and HUVECs (Fig. 3A–C) but did not affect the proliferation of macrophages (Fig. 3D). The HPDAlCyRL-QN15/ZA hydrogel showed the most significant cell pro-proliferation effect, indicating that the multifunctional HPDAlCyRL-QN15/ZA hydrogel, incorporating Zn2+, HPDA, and ZA, greatly enhanced the cell proliferation-promoting activity of CyRL-QN15.
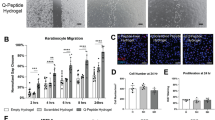
A–D Effects of vehicle (PBS), ZA, HPDA nanoparticles, HPDA/ZA, CyRL-QN15, CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA conditions on the proliferation of HaCaT cells, HSFs, HUVECs, and RAW 264.7 cells. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. *, **, ***, and **** indicate P < 0.05, P < 0.01, P < 0.001, and P < 0.0001, respectively. E Representative images showing the pro-healing effects of the samples against HaCaT cell scratches. F Quantification of the pro-healing effects of the samples against HaCaT cell scratches. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. * and ** indicate P < 0.05 and P < 0.01, respectively.
The cell wound pro-healing potential of the HPDAlCyRL-QN15/ZA hydrogel was demonstrated using a keratinocyte scratch assay (Fig. 3E). Compared to the vehicle (52.02 ± 4.02%), ZA and HPDA/ZA showed no obvious pro-healing effects, while CyRL-QN15, CyRL-QN15/ZA, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA showed great pro-healing activity, with scratch repair rates of 89.51 ± 3.88, 90.84 ± 2.75, 92.83 ± 2.75, and 95.23 ± 4.49%, respectively (Fig. 3F). CyRL-QN15 exhibited excellent scratch healing activity, and its scratch healing activity was significantly enhanced by HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA.
The HPDAlCyRL-QN15/ZA hydrogel regulated the release of cytokines from macrophages, promoted angiogenesis, and exerted antioxidant activities
Macrophages play a pivotal role in wound healing, particularly in the secretion of cytokines and inflammatory factors that regulate the wound repair process, thereby facilitating skin wound healing39. CyRL-QN15 significantly decreased the expression of TNF-α induced by LPS but promoted the expression of TGF-β1. More importantly, the HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA enhanced the regulatory activity of CyRL-QN15 on cytokine release from macrophages (Fig. 4A, B). The HPDAlCyRL-QN15/ZA hydrogel exerted the best effects, reducing the expression of TNF-α from 850.89 ± 75.25 pg/mL (LPS) to 674.83 ± 34.48 pg/mL while promoting the expression of TGF-β1 from 99.45 ± 20.96 pg/mL (vehicle) to 315.50 ± 63.50 pg/mL (Fig. 4A, B).
A Effects of vehicle (PBS), CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA conditions on TNF-α release in RAW 264.7 cells stimulated with LPS. B Effects of vehicle (PBS), CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA conditions on TGF-β1 release in RAW 264.7 cells. C, E Representative images showing HUVEC migration and quantitative analysis, respectively. Scale bar: 200 μm. D, F Representative images of HUVEC tube formation and quantitative analysis, respectively. Scale bar: 200 μm. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. *, **, and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
The effects of the HPDAlCyRL-QN15/ZA hydrogel on HUVEC migration and tube formation were explored (Fig. 4C, D). Compared to the vehicle, CyRL-QN15 significantly promoted HUVEC migration (by 1.23-fold), while the HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA significantly enhanced the cell pro-migration activity of CyRL-QN15 (by 1.42- and 1.58-fold, respectively) (Fig. 4E). After treatment with CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA, HUVEC tube formation reached 231 ± 26.94, 286.33 ± 6.01, and 347.33 ± 14.19, respectively (Fig. 4F). These results indicate that the HPDAlCyRL-QN15/ZA hydrogel significantly enhanced the activity of CyRL-QN15, especially by its loading and slow release from HPDA, as well as the pro-proliferative and angiogenic activities of Zn2+.
Excessive production of ROS in the wound area can cause oxidative stress, leading to cellular DNA damage and impaired angiogenesis; therefore, removal of excessive ROS effectively promotes wound healing40,41. CyRL-QN15 showed no free radical scavenging capacity, whereas HPDA exhibited free radical scavenging and ROS reducing activities; therefore, both HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA exhibited free radical scavenging and ROS reducing activities (Fig. S7). As seen in Fig. S7A, B, the HPDAlCyRL-QN15/ZA hydrogel showed direct scavenging activity against 2, 2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+) (53.01 ± 2.84%) and 2,2-diphenyl-1-picrylhydrazyl (DPPH free radicals) (42.06 ± 2.89%), with similar activity as the positive control vitamin C (VC). The intracellular scavenging ability of the HPDAlCyRL-QN15/ZA hydrogel against ROS in HUVECs stimulated with hydrogen peroxide (H2O2) was also assessed by flow cytometry. ROS intensity increased significantly (4123.66 ± 415.54) following H2O2 stimulation but decreased significantly under HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA treatment (2795 ± 214.41 and 2285.66 ± 134.50, respectively) (Fig. S7C, D). In conclusion, the multifunctional HPDAlCyRL-QN15/ZA hydrogel not only exhibited regulatory effects on cytokine release in macrophages but also promoted HUVEC migration and free radical scavenging and reduced excessive ROS in HUVECs. Thus, this hydrogel shows excellent therapeutic potential for the treatment of chronic skin injuries, such as diabetic wounds.
The HPDAlCyRL-QN15/ZA hydrogel promoted full-thickness wound healing, epidermal regeneration, and collagen deposition in diabetic mice
The application of multifunctional hydrogel dressings with anti-inflammatory, antioxidant, and angiogenic activities provides a new strategy for skin wound treatment, especially chronic wound healing42. With its superior biocompatibility, the multifunctional HPDAlCyRL-QN15/ZA hydrogel showed excellent potential for the treatment of chronic skin wounds (Figs. 3, 4, S5, and S7). Thus, we further investigated its pro-healing activity on diabetic skin wounds in mice. Wounds in type 2 diabetic mice were treated with the different compounds and then monitored at different times to determine the change in wound area (Fig. 5A). The results showed that the repairing effect of CyRL-QN15 on wounds was significantly better than that of the vehicle or commercial dressing (Tegaderm), and the HPDAlCyRL-QN15/ZA hydrogel significantly enhanced the skin regenerative effects of CyRL-QN15. Notably, after 14 days of treatment, the HPDAlCyRL-QN15/ZA hydrogel-treated wounds were almost completely healed without obvious scarring, while the CyRL-QN15- and HPDAlCyRL-QN15-treated wounds were mostly healed with a small amount of scabbing. In contrast, the Tegaderm- and vehicle-treated wounds were not healed and showed considerable scarring. On Day 14, compared with the vehicle (66.38 ± 3.41%), the skin tissue repair rates of the CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA were 88.74 ± 2.14, 92.64 ± 2.72, and 95.23 ± 4.49%, respectively, demonstrating that the HPDAlCyRL-QN15/ZA hydrogel exhibited the highest pro-regeneration effects on diabetic skin wounds in mice (Fig. 5B).
A Representative images of wounds in each group during the healing process and schematic of the wound healing process in the five groups. B Quantitative data of wound repair rates at different time points in the five groups. C Representative H&E staining images of wound specimens from different groups on Days 3, 7, and 14. Masson trichrome and PAS staining on Day 14. Es eschar, NE newborn epithelium, GT granulation tissue. Scale bar: 500 µm. D–F Quantification of regenerated epidermis thickness, granulation tissue thickness, and collagen deposition in wounds. All data represent the mean ± SD generated from three independent experiments performed in triplicate. *, **, and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively.
H&E staining was performed to evaluate skin wound healing and regeneration on Days 3, 7, and 14 (Fig. 5C–F). From Days 3 to 14, wounds treated with the HPDAlCyRL-QN15/ZA hydrogel exhibited the best regeneration of epidermal integrity and thickness. On Day 14, CyRL-QN15 treatment showed superior neo-epidermal (173 ± 6.23 μm) and granulation tissue thickness (576.33 ± 9.80 μm) in the wound compared to the vehicle and Tegaderm groups. Notably, compared to treatment with CyRL-QN15, wounds treated with the HPDAlCyRL-QN15/ZA hydrogel exhibited better therapeutic efficacy, in which neo-epidermal and granulation tissue thicknesses reached 198 ± 5.09 μm and 669 ± 8.64 μm, respectively (Fig. 5D, E). As shown in Fig. S8A, C, compared with the vehicle group (99.95 ± 26.97), immunofluorescence staining of Ki67 in injured tissue on postoperative Day 14 also indicated that the HPDAlCyRL-QN15/ZA hydrogel (279.52 ± 25.60) significantly enhanced the expression of the epidermal cell proliferation marker Ki67.
Masson trichrome and PAS staining showed significantly greater collagen deposition and basement membrane completion in the regenerated tissue with HPDAlCyRL-QN15/ZA hydrogel treatment (Fig. 5C). As seen in Fig. S8B, D, E, the HPDAlCyRL-QN15/ZA hydrogel-treated wounds showed significantly higher positive staining intensity for collagen types I and III (COL I and COL III, respectively) compared with the other treatment groups, consistent with its promotion of fibroblast proliferation and collagen deposition. These findings indicated that the HPDAlCyRL-QN15/ZA hydrogel enhanced the pro-healing effects of CyRL-QN15 in chronic skin wounds, resulting in shortened healing time, thicker neo-epidermis, superior granulation tissue formation, and greater collagen deposition.
The HPDAlCyRL-QN15/ZA hydrogel promoted macrophage polarization (M1 to M2) to reduce inflammation and angiogenesis
Markers of the macrophage M1 (F4/80/INOS) and M2 phenotypes (F4/80/ARG) were stained with immunofluorescence to evaluate the effects of the HPDAlCyRL-QN15/ZA hydrogel on macrophage polarization (Fig. 6A, B). Immunofluorescence showed that the positive rate of F4/80/INOS (INOS+/F4/80+) in the vehicle, Tegaderm, CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA groups increased sequentially on Day 3 (95.99 ± 14.42, 138.09 ± 29.11, 152.08 ± 22.16, 174.30 ± 22.43, and 185.29 ± 8.08, respectively) (Fig. 6C). On Day 7, the INOS+/F4/80+ ratio in the different groups decreased sequentially, from 98.96 ± 10.05 in the vehicle group to 57.34 ± 11.89 in the CyRL-QN15 group and 27.81 ± 6.22 in the HPDAlCyRL-QN15/ZA hydrogel group (Fig. 6D). On Days 3 and 7, the positive rate of ARG/F4/80 (ARG+/F4/80+) showed the opposite trend to INOS+/F4/80+ in CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA, with the greatest increase being seen in the HPDAlCyRL-QN15/ZA hydrogel (from 24.97 ± 8.02 to 308.26 ± 40.72) (Fig. 6E), which indicated the greatest increase in M2 phenotype macrophages in the wound tissue (Fig. 6E, F). These results suggested that CyRL-QN15 regulated macrophage polarization and the HPDAlCyRL-QN15/ZA hydrogel significantly enhanced CyRL-QN15 activity.
A Representative images of F4/80, INOS, and ARG staining on Day 3. Scale bar: 50 µm. B Representative images of F4/80, INOS, and ARG staining on Day 7. Scale bar: 50 µm. C, D Ratio of INOS-positive to F4/80-positive macrophages (INOS+/F4/80+) on Days 3 and 7. E, F Ratio of ARG-positive to F4/80-positive macrophages (ARG+/F4/80+) on Days 3 and 7. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. * and ** indicate P < 0.05 and P < 0.01, respectively.
As shown in Fig. S9A, B, the expression level of TNF-α decreased after CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA treatment, whereas the expression level of TGF-β1 showed the opposite pattern, consistent with the in vitro results. After 7 days of treatment with CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA, the expression levels of IL-1β decreased, while the expression levels of IL-10 increased (Fig. S9C–E). Thus, CyRL-QN15 promoted macrophage polarization to the M2 phenotype to reduce inflammation, while the HPDAlCyRL-QN15/ZA hydrogel enhanced the regulatory activity of CyRL-QN15.
As M2 macrophages and Zn2+ can promote angiogenesis18,43 and both CyRL-QN15 and HPDAlCyRL-QN15/ZA promoted the transformation of macrophages to M2, their effects on angiogenesis were explored by immunofluorescence analysis of VEGF, CD31, and α-SMA in skin wounds. VEGF can promote endothelial cell migration and angiogenesis, CD31 and α-SMA are common markers of angiogenesis, and α-SMA also promotes wound contraction44. Compared with the vehicle, the positive staining intensities of VEGF, α-SMA, and CD31 were enhanced after treatment with CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA, indicating promotion of angiogenic activity (Figs. 7A–D, S10A, B). CyRL-QN15 enhanced the expression of CD31, and HPDAlCyRL-QN15 markedly enhanced the expression levels of VEGF, α-SMA, and CD31 (Figs. 7C, D, S10B). Notably, the HPDAlCyRL-QN15/ZA hydrogel showed the greatest promotion of angiogenesis, increasing the expression levels of VEGF, α-SMA, and CD31 by 3.1-, 2.6-, and 3.3-fold, respectively, compared to the vehicle (Figs. 7C, D, S10B). CyRL-QN15 significantly promoted angiogenesis in diabetic mouse skin wounds. The HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA enhanced CyRL-QN15 activity, with the HPDAlCyRL-QN15/ZA hydrogel exhibiting the strongest angiogenic activity due to the combination of Zn2+ and CyRL-QN15. In summary, the HPDAlCyRL-QN15/ZA hydrogel markedly enhanced CyRL-QN15 peptide activity, suppressed the inflammatory response by stimulating macrophage polarization from the M1 to M2 phenotype, and promoted Ki67, VEGF, CD31, α-SMA, COL I, and COL III expression to accelerate epithelialization and facilitate blood vessel regeneration, collagen deposition, and granulation tissue regeneration. Thus, this hydrogel exhibits excellent therapeutic potential in the healing of chronic diabetic skin wounds.
A, B Representative images of immunofluorescence staining of VEGF and α-SMA in diabetic mice on Day 14 after treatment. Scale bar: 50 µm. C, D Quantitative data of VEGF and α-SMA in wounds, respectively. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. * and ** indicate P < 0.05 and P < 0.01, respectively.
The HPDAlCyRL-QN15/ZA hydrogel boosted skin wound repair in cultured ex vivo diabetic patient skin
The HPDAlCyRL-QN15/ZA hydrogel exhibited similar anti-inflammatory and angiogenesis-promoting properties as the multifunctional GelMA/AA/Cu and PC/GO/Met hydrogels, showing excellent therapeutic effects on chronic diabetic wounds at the animal level45,46. In addition, the HPDAlCyRL-QN15/ZA hydrogel also exhibited free radical scavenging and oxidative stress reducing activities, which are essential for the recovery of cellular function in diabetic wound areas (Fig. S7). To better explore the therapeutic effects of the HPDAlCyRL-QN15/ZA hydrogel on skin wounds in diabetic patients, an ex vivo diabetic skin wound healing model was established (Fig. 8A, B). PAS staining showed that the skin in all treatment groups maintained a normal structure, with no epidermal detachment and no significant changes at the dermal-epidermal junction, indicating successful construction of the model (Fig. 8C). Compared with the vehicle (0.56 ± 0.07 mm and 0.29 ± 0.05 mm), the HPDAlCyRL-QN15/ZA hydrogel significantly stimulated re-epithelialization, with a markedly thicker epidermis (0.77 ± 0.07 mm) and epidermal migration (0.5 ± 0.05 mm) from the edge to the center of the wound (Fig. 8D, E). The level of collagen deposition in granulation tissue is a principal marker of wound healing47. As shown in Fig. 8F, collagen deposition in the HPDAlCyRL-QN15/ZA hydrogel group (81.62 ± 7.90%) was significantly higher than that in the HPDAlCyRL-QN15 (70.21 ± 5.71%), CyRL-QN15 (65.86 ± 4.38%), and vehicle groups (58.15 ± 5.00%).
A, B The HPDAlCyRL-QN15/ZA hydrogel promoted re-epithelialization in an ex vivo diabetic skin wound healing model. C Representative images of H&E-, Masson trichrome-, and PAS-stained wound cross-sections of isolated wound healing models treated with PBS (vehicle), CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA after 7 days of in vitro incubation. Scale bar: 200 µm. D–F Comparison of length, height, and collagen content of epidermal migration in wounds treated with PBS (posttreatment), CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA after 7 days. G, H TNF-α and TGF-β1 expression levels in diabetic patient skin after 7 days of in vitro simulated wound tissue treatment. Data represent the mean ± SD, generated from three independent experiments performed in triplicate. * and ** indicate P < 0.05 and P < 0.01, respectively.
The ELISA results showed that the expression level of TNF-α in the in vitro diabetic skin wounds was significantly lower in the CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA groups than in the vehicle group, while the expression level of TGF-β1 was significantly higher after HPDAlCyRL-QN15 and especially HPDAlCyRL-QN15/ZA treatment (Fig. 8G, H). Immunofluorescence staining of the vascular regeneration-related markers CD31 and α-SMA was performed to detect angiogenesis in ex vivo foot skin wounds of diabetic patients (Fig. S11). As shown in Fig. S11B, D, α-SMA expression in the CyRL-QN15, HPDAlCyRL-QN15, and HPDAlCyRL-QN15/ZA groups differed insignificantly from that in the vehicle (PBS) group. In contrast, the expression levels of CD31 in the HPDAlCyRL-QN15 and HPDAlCyRL-QN15/ZA groups were 169.60 ± 9.63 and 170.10 ± 10.92%, respectively, significantly higher than that of CyRL-QN15 (150.32 ± 12.80%) (Fig. S11A, C). These results demonstrated that the HPDAlCyRL-QN15/ZA hydrogel inhibited inflammation, promoted re-epithelialization, and accelerated angiogenesis, thus exhibiting excellent therapeutic effects on ex vivo diabetic patient skin wounds and providing a new strategy and candidate for the treatment of chronic wounds.
Conclusions
The HPDAlCyRL-QN15/ZA hydrogel accelerated the proliferation, migration, and tube formation of skin cells, regulated the secretion of cytokines, and directly scavenged free radicals and ROS. Interestingly, the HPDAlCyRL-QN15/ZA hydrogel markedly accelerated the healing of diabetic skin wounds by promoting re-epithelialization, granulation tissue formation, collagen deposition, and angiogenesis and by reducing inflammation. Thus, the HPDAlCyRL-QN15/ZA hydrogel can be used as a novel therapeutic strategy for the clinical treatment of chronic skin wounds.
References
Li, D. et al. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J. Clin. Invest. 125, 3008–3026 (2015).
Larouche, J., Sheoran, S., Maruyama, K., & Martino, MIM. Immune regulation of skin wound healing: Mechanisms and novel therapeutic targets. Adv. Wound Care 7, 209–31 (2018).
Gianino, E., Miller, C. & Gilmore, J. Smart wound dressings for diabetic chronic wounds. Bioengineering 5, 51 (2018).
Liu, Y. et al. Application of nanomaterial in hydrogels related to wound healing. J. Nanomater. 2022, 1–11 (2022).
Ezhilarasu, H., Vishalli, D., Dheen, S. T., Bay, B.-H. & Srinivasan, D. K. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials 10, 1234 (2020).
Wang, M. et al. Nanomaterials applied in wound healing: Mechanisms, limitations, and perspectives. J. Control Release 337, 236–47 (2021).
Weng, T. et al. Nanomaterials for the delivery of bioactive factors to enhance angiogenesis of dermal substitutes during wound healing. Burns Trauma 10, tkab049 (2022).
Zhang, Y., Wang, Q.-Q., Zhao, Z. & Deng, C.-J. Animal secretory endolysosome channel discovery. Zool. Res. 42, 141–152 (2021).
Cao, X. et al. Cathelicidin-OA1, a novel antioxidant peptide identified from an amphibian, accelerates skin wound healing. Sci. Rep. 8, 943 (2018).
Wang, Y. et al. Discovery of a novel short peptide with efficacy in accelerating the healing of skin wounds. Pharm. Res. 163, 105296 (2021).
Song, Y. et al. A short peptide potentially promotes the healing of skin wound. Biosci. Rep. 39, BSR20181734 (2019).
Wu, J. et al. A frog cathelicidin peptide effectively promotes cutaneous wound healing in mice. Biochem. J. 475, 2785–2799 (2018).
Murray, R. Z., West, Z. E., Cowin, A. J. & Farrugia, B. L. Development and use of biomaterials as wound healing therapies. Burns Trauma 7, s41038–018-0139-7 (2019).
Wang, C. et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 9, 65–76 (2019).
Huang, R., Hu, J., Qian, W., Chen, L. & Zhang, D. Recent advances in nanotherapeutics for the treatment of burn wounds. Burns Trauma 9, tkab026 (2021).
Zheng, Z. et al. A cannabidiol-containing alginate based hydrogel as novel multifunctional wound dressing for promoting wound healing. Biomater. Adv. 134, 112560 (2021).
Zhang, M. et al. Zn(2+)-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 143, 235–242 (2020).
Lin, P. H. et al. Zinc in wound healing modulation. Nutrients 10, 16 (2017).
Sun, H. et al. Hollow polydopamine nanoparticles loading with peptide RL-QN15: A new pro-regenerative therapeutic agent for skin wounds. J. Nanobiotechnol. 19, 304 (2021).
Ou, Q. et al. More natural more better: Triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J. Nanobiotechnol. 19, 237 (2021).
Bordon, K. C. F. et al. From animal poisons and venoms to medicines: Achievements, challenges and perspectives in drug discovery. Front. Pharm. 11, 1132 (2020).
Yang, X., Wang, Y., Wu, C. & Ling, E. A. Animal venom peptides as a treasure trove for new therapeutics against neurodegenerative disorders. Curr. Med. Chem. 26, 4749–4774 (2019).
Li, C., Wang, J., Zhao, M., Zhang, S. & Zhang, Y. Toll-like receptor 4 antagonist FP7 alleviates lipopolysaccharide-induced septic shock via NF-kB signaling pathway. Chem. Biol. Drug Des. 97, 1151–1157 (2021).
Nguyen, L. T. et al. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PloS One. 5, e12684 (2010).
Fu, Y. et al. Amphibian-derived peptide homodimer promotes regeneration of skin wounds. Biomed. Pharmacother. 146, 112539 (2022).
Chen, X. et al. Preparation and application of quaternized chitosan- and AgNPs-base synergistic antibacterial hydrogel for burn wound healing. Molecules 26, 4037 (2021).
Wang, X. et al. Preparation of antimicrobial hyaluronic acid/quaternized chitosan hydrogels for the promotion of seawater-immersion wound healing. Front. Bioeng. Biotechnol. 7, 360 (2019).
Wu, D. et al. Exosomes derived from bone mesenchymal stem cells with the stimulation of Fe3O4 nanoparticles and static magnetic field enhance wound healing Through Upregulated miR-21-5p. Int. J. Nanomed. 15, 7979–7993 (2020).
Shalin, S. C., Ferringer, T. & Cassarino, D. S. PAS and GMS utility in dermatopathology: Review of the current medical literature. J. Cutan. Pathol. 47, 1096–1102 (2020).
Ueck, C. et al. Comparison of in-vitro and ex-vivo wound healing assays for the investigation of diabetic wound healing and demonstration of a beneficial effect of a triterpene extract. PloS One 12, e0169028 (2017).
Gherardini, J., van Lessen, M., Piccini, I., Edelkamp, J. & Bertolini, M. Human wound healing ex vivo model with focus on molecular markers. Methods Mol. Biol. 2154, 249–254 (2020).
Stone, R. II, Wall, J. T., Natesan, S. & Christy, R. J. PEG-plasma hydrogels increase epithelialization using a human ex vivo skin model. Int. J. Mol. Sci. 19, 3156 (2018).
Yang, X., Lee, W. H. & Zhang, Y. Extremely abundant antimicrobial peptides existed in the skins of nine kinds of Chinese odorous frogs. J. Proteome Res. 11, 306–319 (2012).
Li, X. et al. OM-LV20, a novel peptide from odorous frog skin, accelerates wound healing in vitro and in vivo. Chem. Biol. Drug Des. 91, 126–136 (2018).
Hoti, G. et al. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 14, 478 (2021).
Qu, J. et al. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 183, 185–199 (2018).
Su, J., Li, J., Liang, J., Zhang, K. & Li, J. Hydrogel preparation methods and biomaterials for wound dressing. Life 11, 1016 (2021).
Zhang, M. et al. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 266, 118100 (2021).
Aitcheson, S. M., Frentiu, F. D., Hurn, S. E., Edwards, K. & Murray, R. Z. Skin wound healing: Normal macrophage function and macrophage dysfunction in diabetic wounds. Molecules 26, 4917 (2021).
Wu, Y. et al. A spatiotemporal release platform based on pH/ROS stimuli-responsive hydrogel in wound repairing. J. Control Release 341, 147–165 (2022).
Qi, Y. et al. A thermoreversible antibacterial zeolite-based nanoparticles loaded hydrogel promotes diabetic wound healing via detrimental factor neutralization and ROS scavenging. J. Nanobiotechnol. 19, 414 (2021).
Liang, Y., He, J. & Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 15, 12687–12722 (2021).
Wang, K. et al. Exosomes laden self-healing injectable hydrogel enhances diabetic wound healing via regulating macrophage polarization to accelerate angiogenesis. Chem. Eng. J. 430, 132664 (2022).
Jia, J. et al. AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvasc. Res. 105, 103–108 (2016).
Liang, Y. et al. pH/Glucose dual responsive metformin release hydrogel dressings with adhesion and self-healing via dual-dynamic bonding for athletic diabetic foot wound healing. ACS Nano 16, 3194–3207 (2022).
Chen, J. et al. Antibacterial adhesive self-healing hydrogels to promote diabetic wound healing. Acta Biomater. 146, 119–130 (2022).
Chen, K. et al. Injectable melatonin-loaded carboxymethyl chitosan (CMCS)-based hydrogel accelerates wound healing by reducing inflammation and promoting angiogenesis and collagen deposition. J. Mater. Sci. Technol. 63, 236–245 (2021).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (32060212, 81760648, and 82160159); Program for Innovative Research Team in the Ministry of Education of China (IRT17-R49); Major Science and Technology Project of Yunnan Province (202202AA100004); Key project from Yunnan Key Laboratory of Pharmacology for Natural Products (YKLPNP-K2301); Science and Technology Leadership Talent Project in Yunnan, China (2017HA010); Key Project of Yunnan Applied Basic Research Project-Kunming Medical University Union Foundation (202101AY070001-006); Yunnan Applied Basic Research Project Foundation (2019FB128); Project of Yunnan Applied Basic Research Project-Kunming Medical University Union Foundation (202101AY070001-036); and Innovative Team of Precise Prevention and Treatment against Metabolic Diseases of Yunnan University.
Author information
Authors and Affiliations
Contributions
X.Y. and Y.W. designed the project. X.Y., Y.W., Y.Y., and L.H. received financial support for the project. X.Y. and Y.W. designed and supervised the project and commented on the project. Z.F., H.S., Y.W., and C.L. structured and characterized the nanospheres. Y.W., J.N., Y.L., K.G., and Y.L. performed the in vivo experiments and analyzed the data. Y.W., Z.F., H.S., Y.W., C.L., D.S., Q.J., S.Y., and N.L. performed the SEM, FTIR, and XPS in vitro experiments and analyzed the data. Y.Y., L.H., J.N., and Y.Z. provided skin tissue samples from diabetic patients after amputation. Z.F., H.S., Y.W., and C.L. wrote the paper. All authors contributed to the discussion during the whole project. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, Z., Sun, H., Wu, Y. et al. A cyclic heptapeptide-based hydrogel boosts the healing of chronic skin wounds in diabetic mice and patients. NPG Asia Mater 14, 99 (2022). https://doi.org/10.1038/s41427-022-00444-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-022-00444-x