Abstract
Autophagy enables the maintenance of nutrient recycling and metabolic homeostasis through a multistep lysosomal degradation pathway, and it has been demonstrated that autophagy can act as a tumor suppressor or tumor promoter, depending on the tumor microenvironment (TME). The dual role of autophagy in tumorigenesis results in two opposing therapeutic strategies, namely, inhibition versus promotion. However, due to the protective mechanisms of tumor cells and the absence of specific strategies for autophagy regulation, the modulation of autophagy has become a major consideration in cancer treatment. Owing to their unique properties, nanoparticles (NPs) have demonstrated excellent potential for overcoming these limitations. Here, we provide a summary of the latest progress in autophagy-targeting NPs for effective cancer treatment, and we conclude with recent advances in relevant clinical and preclinical studies. This summary of typical autophagy-targeted nano-drug delivery systems aims to provide references and expand ideas for researchers intending to explore this field. Finally, we provide an outlook on the potential of autophagy modulation in cancer treatment, and several key objective problems are carefully highlighted.
Similar content being viewed by others
Introduction
Cancer is one of the main diseases adversely impacting the survival and health of human beings. According to the latest statistics, ~19 million people were affected by cancer in 2020, and there were ~10 million cancer deaths1. There is, therefore, an urgent need for effective cancer treatments. Autophagy has undergone continuous and in-depth research since its discovery by Klionsky2, and its role in cancer has attracted extensive attention. Autophagy is the main cellular pathway for the degradation of long-lived proteins and cytoplasmic organelles, thereby providing protective mechanisms for maintaining cell homeostasis and resisting adverse external environments3,4. Depending on the tumor microenvironment (TME) context, autophagy exhibits different characteristics in cancer. In well-balanced cells, autophagy is a powerful barrier to tumor development. Autophagy regulates many important physiological processes to ensure the survival of normal cells5. However, autophagy plays a dual role in established tumors6,7. A moderate level of autophagy (i.e., a stage involving the accumulation of autophagosomes and the degradation of harmful foreign substances in the autophagolysosome before the critical threshold) protects the tumor from an unfavorable external environment and promotes its growth8. Once the level of autophagy exceeds the critical threshold (i.e., once extensive cytoplasmic vacuolization occurs, culminating in phagocytic uptake and consequent lysosomal degradation9), this overactivation can trigger autophagic death of tumor cells10. The dual role of autophagy in tumors has led to the emergence of two opposite anticancer strategies, namely, inhibition versus promotion. The most appropriate strategy needs to be based on the existing situation.
However, because of the protective mechanisms of tumors, such as multidrug resistance (MDR) (the resistance of cancer cells to multiple chemotherapeutic drugs with different structures and mechanisms of action11), tumor immune escape12 and even the protection conferred by autophagy8, proper application of autophagy modulation has become a major consideration in cancer treatment. Currently, nonspecificity and off-target effects of autophagy-related drugs limit their use. With the continuous advances in nanotechnology, the excellent performance of nanoparticles (NPs, referring to particulate materials with 50% or more of the constituent particles having one or more external dimensions ranging from 1–100 nanometers13) in the fields of tumor diagnosis and treatment have been extensively studied14,15. Novel and efficient nano-drug delivery systems have demonstrated excellent potential for overcoming the limitations due to nonspecificity and off-target effects to regulate the TME and synergize multiple therapeutic modalities16,17,18. It has been shown that properly modified NPs possess superior targeting performance and good biological safety profiles, both of which are beneficial in regard to the modulation of autophagy. This review summarizes the findings of recent research, including clinical and preclinical studies, on autophagy-targeting NPs and the inhibition or promotion of autophagy in cancer treatment (Scheme 1) to facilitate the exploration of autophagy modulation in cancer treatment. By enumerating the various autophagy-targeting nano-drug delivery systems, this paper provides reference cases for researchers with an interest in autophagy and cancer treatment, and it provides valuable insights regarding the emergence of novel and more advanced autophagy-targeting nano-drug delivery systems.
The potential of autophagy modulation in tumor treatment is well recognized, but this does not mean that the modulation of autophagy is limited to this domain. It is also of considerable relevance to other diseases that have long plagued human beings, such as influenza19, neurodegenerative diseases20,21, and inflammation22.
Autophagy, cancer, and nanoparticles
Autophagy is a highly conserved catabolic process that maintains cellular homeostasis by transporting waste or hazardous substances to the lysosome. The level of autophagy is significantly enhanced under harsh conditions, such as starvation, in which engulfment of the cytoplasm and other organelles occurs to ensure that the cells have sufficient nutrients for survival3,23. Based on how autophagy substrates are delivered to the lysosome, autophagy can be divided into three types: microautophagy, chaperone-mediated autophagy (CMA), and macroautophagy3. Microautophagy relies on the direct uptake of cytoplasmic materials by invagination of the lysosomal membrane24. CMA involves the translocation of lysosomal-associated membrane protein 2-dependent autophagy substrates that bind to the cytoplasmic chaperone of the heat shock protein family across the lysosomal membrane25. Macroautophagy involves special double-membrane vesicles, known as autophagosomes, which gradually isolate autophagic cargo and deliver the cargo to lysosomes by membrane fusion. An organelle formed by the fusion of an autophagosome and a lysosome is often called an autophagolysosome26. Macroautophagy is by far the most common form of autophagy. Therefore, unless specified otherwise, the term autophagy in this article refers to macroautophagy. Several molecular signaling pathways are involved in autophagy, including the mechanistic target of rapamycin kinase (mTOR), which is the primary negative regulator of autophagy, as well as 5ʹ AMP-activated protein kinase and class III phosphatidylinositol-3-kinase (PI3K), which are the two types of autophagy-promoting kinases. As shown in Fig. 1, the process of autophagy involves the initiation, formation, and expansion of autophagosomes, fusion between autophagosomes and lysosomes, and the degradation of capsule contents. Autophagy begins with the activation of the ULK1 (also known as autophagy-related gene ATG1) complex, which includes several components, such as ULK1, ULK2, ATG13, ATG101, and FIP200, and activates a class III PI3K complex composed of VPS34, VPS15, ATG14, Beclin1, UVRAG (also known as p63), and AMBRA127. Expansion of the autophagosome membrane depends on the incorporation of ATG5-ATG12 complexes and ATG16 with the help of ATG7 and ATG10. ATG4B, along with ATG7, conjugates LC3-I and lipid phosphatidylethanolamine to form LC3-II. Ultimately, the autophagosome fuses with the lysosome with the assistance of syntaxin 17 (STX17), the contents are degraded, and the macromolecular precursors are recycled or used to fuel metabolic pathways28.
Many studies have shown that reduced expression of autophagy-related genes (such as Beclin1) can increase cancer in mice. Moreover, enhanced expression of these genes (such as Beclin1 and Atg5) can inhibit the occurrence of breast cancer in tumor-bearing mice. Autophagy deficiency may lead to tumor formation29, while autophagy may prevent cancer. Autophagy is intimately involved in cancer, and its function becomes more complicated as cancer develops and depends on the availability of nutrients, the stress level of the microenvironment, and the presence of immune surveillance30,31,32. The loss of the ability of a cell to stop proliferating when it comes into contact with neighboring cells is a hallmark of the malignant transformation, growth, invasion, and metastasis of tumors. It has been reported that contact with inhibitory cells (as occurs at high cell density) impairs the formation of autophagosomes33. Moreover, a study has shown that the inhibition of autophagy enhances chemotherapeutic drug-induced apoptosis34. The rapid proliferation of tumor cells causes a high demand for nutrients. In the limited nutrient environment of the TME, autophagy can promote interaction between the tumor and the matrix, thereby promoting tumor growth. However, when the level of autophagy exceeds the critical threshold, overactivated autophagy no longer exerts a protective effect and instead kills tumor cells by triggering autophagic cell death35. When the normal autophagic catabolism of cells is altered (whether interrupted or enhanced), the normal physiological functioning of cells will be affected, which can lead to cell death. Hence, both the inhibition and promotion of autophagy are considered feasible strategies in cancer treatment, and the specific choice should be based on the actual situation.
There is, however, currently no intervention to regulate autophagy. Although rapamycin, chloroquine (CQ), hydroxychloroquine (HCQ), and several other drugs licensed for human use can activate or inhibit autophagy, they have not been developed for this purpose. Some challenges hinder the development of regulators of clinical autophagy. Many chemical reagents that can be used to activate or inhibit autophagy have inherently low pharmacological specificity for their targets. For example, acute rapamycin administration leads to the relatively specific inhibition of mTORC1 through FK506 binding protein 1A, and prolonged rapamycin exposure can promote the decomposition of mTORC236. Another problem related to specificity stems from the complex structure of tissues, which generally contain several different cell types and participate in a wide range of homologous and heterologous interactions. Most of the currently available autophagy regulators have poor specificity because they do not preferentially target a single cell type. Moreover, several components of the autophagy mechanism operate at the interface of multiple cellular processes; that is, they also mediate autophagy-independent functions. For instance, rapamycin results in strong immunosuppression because it blocks T-cell proliferation37. These issues limit the application of autophagy modulation in cancer treatment. Due to their unique properties, NPs offer significant advantages in overcoming these challenges: they can improve the therapeutic index of drugs by increasing efficacy or by reducing toxicity; by allowing more effective targeting of tissues, cells, or organelles; and by enhancing the pharmaceutical properties of therapeutic molecules (such as stability, solubility, plasma half-life, and tumor accumulation)14. Researchers have designed and generated nano-drug delivery systems with different properties according to their respective purposes. For example, the solubility and stability of NPs are increased by the modification of special groups; the timely and accurate release of drugs is achieved by the addition of various stimulation-sensitive groups (such as disulfide, hydrazine, hydrazone, and thioketal bonds), and different types of NPs (such as polymer micelles, liposomes, and metal-organic frameworks) are generated to meet different treatment needs. The emergence of nano-drug delivery systems with different functions has led to very significant advances in cancer treatment, solving many of the problems that have stymied conventional pharmacological agents. There is also a unique connection between NPs and autophagy. Because of their size, NPs are readily taken up by cells, thereby leading to autophagy38,39. The mechanism of autophagy induction by nanomaterials is thought to be mediated mainly by intracellular oxidative stress. Under external stress, the phagocytosis of foreign bodies increases, while under some pathological conditions, mitochondrial respiration is enhanced, and a large number of incompletely reduced oxygen atoms accumulate, resulting in the generation of a large number of reactive oxygen species (ROS) and apoptosis40,41. Autophagy induced by NPs may be a cellular defense mechanism against NP toxicity42. A new study of CQ has found that it can reduce the immunological clearance of NPs by resident macrophages in the liver, increase the tumor accumulation of nanodrugs, and improve drug delivery and efficacy by suppressing autophagy43. This means that the combination of autophagy and NPs has great potential for tumor therapy. Some researchers have already devised different forms of autophagy-targeting nano-drug delivery systems to treat tumors, leading to breakthroughs in cancer treatment. The therapeutic strategy of autophagy-targeting nano-drug delivery systems has several advantages. First, these entities can be accurately directed to tumor cells, thereby reducing the nonspecific function of autophagy regulators, enhancing the accumulation of drugs at tumor sites, and consequently enhancing the antitumour efficacy. At the same time, this strategy allows strong interference with the normal autophagy process, such as direct interruption of a certain link or promotion of the catabolism process of autophagy, resulting in disruption of the normal physiology of cells, which can eventually lead to tumor cell death. The different effects of interference with autophagy to different treatment strategies: induction of autophagy versus inhibition of autophagy.
In practice, the choice between inhibition and promotion of autophagy is controversial, as it may depend on the role of autophagy in tumor development. As long as autophagy exerts a positive effect on the treatment of certain cancers, strategies that promote autophagy remain desirable. However, when autophagy adversely affects cancer treatment, inhibition of autophagy is the appropriate strategy. Depending on the type of cancer, therapy should involve an appropriate treatment in combination with autophagy. For example, superficial tumors, such as skin cancer, are more amenable to treatment with phototherapy. Recurrent tumors can be treated with immunotherapy to reduce recurrence and tumor metastasis and to improve prognosis. Chemotherapy is suitable for the treatment of most tumors. As tumors have a variety of survival regulatory mechanisms, comprehensive therapies are becoming increasingly necessary. The choice of therapy should ensure that the treatment is as effective, safe, and convenient as possible.
The following are a number of specific treatment strategies relevant to autophagy. To make the information clearer, key aspects of the strategies below are also listed in Table 1. Thus, Table 1 provides basic information, and more detailed descriptions of the strategies are provided in the text below.
Strategies for autophagy inhibition
Autophagy is a protective mechanism when tumor cells have not reached the critical autophagy threshold; in this situation, the inhibition of autophagy greatly promotes tumor cell apoptosis. Thus, the inhibition of autophagy can enhance antitumour response when combined with other therapies (such as chemotherapy, phototherapy or immunotherapy).
Chemotherapeutic nanoparticles for autophagy inhibition
Chemotherapy is the most commonly used cancer treatment. However, the side effects of chemotherapeutics against normal cells are significant due to nonspecific cytotoxicity. Moreover, most antitumour drugs are small hydrophobic molecules, and their solubility, biological metabolism, and other properties are often unsatisfactory, which severely restricts their clinical application. As mentioned above, there have been significant recent advances in nanotechnology44,45,46. Nano-drug delivery systems can overcome the challenges related to solubility, biological metabolism, and nonspecific cytotoxicity. However, other issues cannot readily be addressed, including MDR and the immunological clearance of NPs by resident macrophages. Recent studies have shown that there is a close relationship between autophagy and chemotherapy. It has been shown that antitumour drugs can induce mild autophagy and thus protect tumor cells, which is also one of the reasons underlying MDR47 and immunological clearance by macrophages43. Traditional chemotherapy still suffers from this problem, and researchers have generated nanocomposites to increase drug accumulation in tumor cells to offset the effect of MDR48,49,50,51. However, this approach can only alleviate but not completely overcome MDR. Due to the negative effects of mild autophagy on chemotherapy, inhibitory strategies have become a promising approach, and the use of autophagy inhibitors is proving to be the most convenient and effective method52,53,54.
As a new material, stimulus-responsive amphiphilic polymers have attracted increasing attention for cancer treatment. Previous studies have shown that these stimulus-responsive nanocarriers can minimize side effects and greatly improve therapeutic effectiveness by responding to changes in the TME over time55. Some researchers have already used amphiphilic polymers with autophagy inhibitors and chemotherapeutics to overcome MDR in cancer treatment. For example, as illustrated in Fig. 2A, Wuliji et al.56 generated polymeric micelles based on an amphiphilic polymer hyperbranched polyacylhydrazone (HPAH) conjugated with doxorubicin (DOX), which encapsulated the autophagy inhibitor LY294002 (LY) for the treatment of oral squamous cell carcinoma. HPAH has a large number of acylhydrazine groups for further conjugation and has good water solubility and low cytotoxicity, making it an excellent drug delivery vector. LY is an autophagy inhibitor that inhibits PI3K signaling pathways57. The chemotherapeutic drug DOX can bind to HPAH through the hydrazone bond and form hydrophobic terminals of amphiphilic micelles. The autophagy inhibitor LY can then be encapsulated in the core of the self-assembled HPAH-DOX micelles. Then, when HPAH-DOX is endocytosed by tumor cells, the pH-sensitive hydrazone bond is cleaved, releasing LY and DOX. LY helps to reduce the immunological clearance of NPs by macrophages, and it makes tumor cells more sensitive to DOX as MDR is reduced. Based on the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the HPAH-DOX/LY group had lower cell viability than the HPAH-DOX group, demonstrating that the polymer codelivery system combining chemotherapeutic drugs with autophagy inhibitors resulted in an enhanced anticancer effect. According to the fluorescence images, the HPAH-DOX group exhibited stronger fluorescence intensity than the free Rh123 group (hydrophobic fluorescent probe Rh123 was loaded into micelles to replace LY, as LY had no discernible fluorescence). This means that the encapsulation of Rh123 in HPAH-DOX micelles increases their solubility and enhances their delivery to cells. However, conjugation of the prodrug by covalent bonds suffers from a limitation: a low graft rate leads to a weak loading capacity, and overcoming this limitation is the key to further development.
A Schematic diagram of HPAH-DOX/LY, which was designed to overcome multidrug resistance through the combination of autophagy inhibition and chemotherapy. B Schematic diagram of 3-MA@ZIF-8 NPs, a controllable drug delivery system consisting of MOF nanoparticles encapsulating autophagy inhibitors. C Schematic diagram of lysosome-retained supramolecular nanogel SiPT to overcome multidrug resistance. SiPT is composed of ultrabright organosilica nanodots (OSiNDs), the photosensitizer tetraphenylporphinesulfonate (TPPs), and methoxy-poly(ethylene glycol)-poly(L-glutamic acid sodium salt) (PEG-PLE). D Schematic diagram of photothermal therapeutic iron oxide nanoparticle (IONP) used to block autophagic flux. E Schematic diagram of D&H-A-A&C nanoparticles combined with a PD-L1 inhibitor to inhibit autophagy for enhanced cancer immunotherapy.
Metal-organic frameworks (MOFs) are excellent drug delivery systems due to their high porosity, large surface area, and adjustable functionalities. MOFs are made of metal ions and organic ligands and have been frequently used in oncology therapy58. Autophagy has also been explored in relation to the use of MOFs as delivery vectors in tumor treatment. Chen et al.59 used a type of MOF called a zeolitic imidazole framework (ZIF-8) crystal as the drug vehicle for the autophagy inhibitor 3-methyladenine (3-MA), which is pH-sensitive and has a high drug-loading capacity (Fig. 2B). 3-MA@ZIF-8 dissociates the bonding between zinc and 2-methylimidazole (MeIM), resulting in a loss of its characteristic crystalline nature and release of 3-MA in the acidic environment of the tumor. The release of MeIM from ZIF-8 can lead to an alkaline environment and disrupt the pH balance in the target cells, thereby causing cell toxicity. 3-MA, as an autophagy inhibitor, can inhibit the class III PI3K (Vps34)/Beclin-1 complex and interfere with the formation of autophagosomes, thus enhancing the sensitivity of tumor cells and consequently enhancing apoptosis. The MTT assay showed that 3-MA@ZIF-8 exhibited the highest cytotoxicity, indicating the superiority of the combination of autophagy inhibitors with MOFs. Unfortunately, despite passive targeting due to the low particle size, the absence of active targeting may still lead to nonspecific toxicity. The biodistribution results confirmed that 3-MA@ZIF-8 accumulated in the organs of the reticuloendothelial system, including the lung, liver, and spleen.
Phototherapeutic nanoparticles for autophagy inhibition
Studies have shown that the inhibition of autophagy can enhance the sensitivity of tumor cells to radiotherapy60. Despite the enhanced toxicity of radiotherapy toward cancer cells, side effects such as the killing of normal cells that proliferate rapidly and increase the incidence of cancer limit the use of radiotherapy. Phototherapy is a new method for tumor ablation and a safe model for cancer treatment61. It is commonly used due to its noninvasive nature and the ease of temporospatial control. Phototherapy can be categorized into photodynamic therapy (PDT) and photothermal therapy (PTT), both of which can induce autophagy, causing repression of tumor apoptosis62,63. Hence, the inhibition of autophagy can sensitize tumor cells to phototherapy and enhance antitumour efficiency.
PDT photosensitizers can kill tumor cells by generating cytotoxic ROS with near-infrared radiation, and the residual tumor cell debris can be released as antigens to induce the maturation of dendritic cells (DCs) and promote infiltration of cytotoxic T lymphocytes into the tumor site, which is beneficial for tumor treatment64,65. PTT takes advantage of thermal-sensitive materials that can convert light energy into heat to ablate tumors66,67,68.
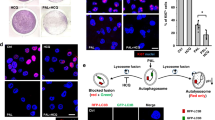
There are several connections between autophagy and phototherapy. As mentioned above, phototherapy can induce autophagy and reduce tumor cell apoptosis. Therefore, many researchers have inhibited autophagy to sensitize tumor cells to phototherapy. Autophagy inhibitors are the most commonly used tools for this, although there are other ways to attenuate autophagy. For instance, Zhang et al.69 devised a new strategy to inhibit autophagy that involves the destruction of lysosomes (Fig. 2C). Specifically, they generated a supramolecular nanogel SiPT composed of ultrabright organosilica nanodots (OSiNDs), the photosensitizer tetraphenylporphinesulfonate (TPPS), and methoxy-poly(ethylene glycol)-poly(L-glutamic acid sodium salt) (PEG-PLE). OSiNDs allow long-term lysosomal imaging by aggregating in acidic lysosomes. The photosensitizer TPPS can generate ROS and enhance apoptosis in tumor cells. The copolymer PEG-PLE has the advantages of biocompatibility and water solubility. When SiPT reached the tumor cells, they aggregated in the lysosome to form larger aggregates and downregulated the exocytosis of lysosomes from the cells. TPPS could then induce apoptosis by irradiation of the cells with a 532 nm laser, and the combination of SiPT and laser led to inhibition of autophagy by the destruction of lysosomes. Transmission electron microscopy (TEM) images showed that SiPT formed small aggregates comprising two or three NPs in a weakly acidic environment (pH = 6.0) and further clustered in a more acidic medium (pH = 4.5), thus demonstrating their ability to accumulate in acidic environments. Confocal fluorescence images obtained with LysoBlue indicated that the lysosomes were damaged, and autophagy was probably involved. Western blotting was performed to verify the regulation of autophagy in this process. Lower p62 expression levels and higher LC3-II/LC3-I ratios were observed in free TPPS in the laser group, which indicated the enhancement of autophagy. The combined application of SiPT and laser irradiation increased the expression of p62 and LC3-II, suggesting that fusion between autophagosomes and lysosomes was blocked and that autophagy was inhibited due to lysosome damage after PDT treatment. In conclusion, SiPT exhibited potential for resistance to MDR. However, the in vivo fluorescence images showed that although SiPT was better than free TPPS at targeting tumors, its distribution in other sites could still be observed, indicating the disadvantages of nonactive targeting.
PTT has also been associated with autophagy inhibition. As shown in Fig. 2D, Ren et al.70 generated iron oxide NPs (IONPs) that were used in combination with the autophagy inhibitor CQ for cancer treatment. IONPs have good biocompatibility, low cytotoxicity, MRI imaging features, and, most importantly, thermal sensitivity, thus allowing tumor cell ablation by laser irradiation71. It has also been reported that IONPs induce autophagy and resistance to apoptosis72. The study used IONPs and CQ with irradiation with an 808 nm laser, and the results of cytotoxicity and cell apoptosis assays showed that the IONP + CQ group exhibited the strongest effect. Compared with the IONP group without CQ, the IONP + CQ group had lower cell viability, demonstrating that autophagy inhibition boosted the efficacy of phototherapy. With the ability to be actively targeted, this type of NP may exhibit improved performance.
Although phototherapy has some advantages, as mentioned above, its poor penetration restricts its clinical application to superficial cancer. Finding new methods to achieve deeper penetrability is an ideal research direction.
Immunotherapeutic nanoparticles for autophagy inhibition
Autophagy is also involved in supporting the survival of dormant tumors, and it may be crucial for the regeneration of these tumor cells. A recent study has shown that dormant tumors from autophagy-deficient animals are reactivated when transplanted into an animal with uncompromised autophagy. This shows that autophagy in the TME is crucial for the regeneration of dormant tumors, which suggests that the inhibition of autophagy could be combined with immunotherapy to prevent tumor recurrence and metastasis73.
Immune-deficient mice are more likely to develop cancer than mice with a normal immune system, indicating that cancer is not only a genetic disease but also an immune disease74. The immune system is a natural barrier against tumors. However, tumors have developed effective measures for immune evasion. Tumors evade immune surveillance in two ways: (1) immunoselection, the growth of poorly immunogenic tumor cells, and (2) immunosubversion, the destruction of the immune system12. Therefore, enhancing targeted recognition of tumors by the immune system is a key objective in tumor immunotherapy.
With the rapid development of immunotherapy, a variety of tumor suppression methods have been developed, including tumor vaccines, immune checkpoint blockade, and chimeric antigen receptor T cells75,76. The relationship between autophagy and the immune system is complicated, and some studies have shown that autophagy probably facilitates tumor escape from immune surveillance, leading to resistance to antitumour immunotherapy77. Therefore, autophagy inhibition has a salutary effect on tumor immunotherapy.
Glioma, the most common primary cancer of the human central nervous system, can ensure its survival by upregulating the expression of PD-L1 and increasing autophagy. Ruan et al. demonstrated that agminated gold nanoparticles (AuNPs) activated by legumain increased DOX accumulation in glioma sites78. Despite the enhancement of the efficacy of chemotherapy, glioma cells could still devise several mechanisms to survive. Therefore, they further devised a drug vehicle, referred to as D&H-A-A&C, accompanied by immunotherapy and autophagy inhibition, to treat glioma79 (Fig. 2E). D&H-A-A&C is a combination of two NPs: D&H-A-AK and D&H-A-CABT. D&H-A-AK is composed of Ala-Ala-Asn-Cys-Lys-polyethylene glycol-thiol (AK-PEG-SH)-modified AuNPs with pH-sensitive DOX and HCQ as prodrugs. D&H-A-CABT is composed of 2-cyano-6-amino-benzothiazole-polyethylene glycol-thiol (CABT-PEG-SH)-modified AuNPs coloaded with DOX and HCQ prodrugs. When D&H-A-A&C reaches the tumor site by passive targeting and enters the cells, in the presence of legumain, D&H-A-AK can be hydrolyzed to expose the 1,2-thiolamino groups and form AuNP aggregates, which occurs by a click cycloaddition with the contiguous cyano group on D&H-A-CABT. The AuNP aggregates can block the exocytosis of NPs, and more DOX and HCQ are then released in tumor cells through the stimulation of the acidic tumor environment. While DOX exerts its cytotoxic effect, it induces an increase in the expression of PD-L1 and the level of autophagy by inhibiting the mTOR pathway. At this point, HCQ inhibits the formation of autolysosomes by destroying lysosomes, thus inhibiting autophagy. The involvement of PD-L1 inhibitors can inhibit the DOX-induced immune escape mechanism of tumor cells, thus enhancing the immune response. Finally, the three agents synergistically enhanced the antitumour effect, and the in vivo TUNEL results showed that D&H-A-A&C had the greatest apoptosis-inducing ability.
Other therapeutic nanoparticles for autophagy inhibition
In addition to the more familiar therapies, there are also a number of novel antitumour therapies. Ferroptosis is another form of regulated cell death characterized by the accumulation of lethal lipid hydroperoxides and could conceivably be used as a new strategy for cancer treatment80. Ferroptosis can be induced by the excess ROS produced through the Fenton reaction between Fe2+ and H2O2, leading to tumor cell death81. This mechanism of cell death was explored recently. Zhang et al.82 generated CA4-FeAlg/HCQ nanogels, and they combined ferroptosis with autophagy inhibition (Fig. 3A). When the CA4-FeAlg/HCQ nanogels reached the tumor vascular sites, the vascular blocker combretastatin A4 (CA4) was released, which disrupted tumor vessels. The results of the immunofluorescence assay of PE-CD31 showed that the CA4-FeAlg/HCQ group had the lowest tumor vascular density. However, it caused a lack of nutrition in tumors and an increase in the level of autophagy. The latter provided nutrients by breaking down cell contents, thereby resisting the therapeutic effects of CA4. The FeAlg/HCQ was subsequently broken down into small nanogel particles, which facilitated deep penetration into the tumor. When entering tumor cells, because Fe3+ was reduced to Fe2+ by excess glutathione (GSH) in tumor cells, the connection between sodium alginate (Alg) and the Fe3+ of FeAlg/HCQ was cleaved, and HCQ was rapidly released to inhibit CA4-induced autophagy by alkalizing lysosomes. Lysosomal damage by CA4-FeAlg/HCQ demonstrated a powerful inhibitory effect. At the same time, Fe2+ catalyzed the conversion of hydrogen peroxide in the tumor cells into cytotoxic hydroxyl radicals and enhanced the antitumour effect. However, its distribution in the liver, lung, and kidney in vivo suggests that there is still a degree of nonspecific distribution, which may cause adverse side effects.
Sonodynamic therapy (SDT), a novel emerging treatment, can achieve deeper tissue penetration than phototherapy and has been used for cancer treatment. However, SDT can induce autophagy and render tumor cells resistant to SDT-mediated apoptosis. Feng et al.83 devised a biomimetic CCM-HMTNPs/HCQ nanoplatform, which uses a cancer cell membrane as the outer membrane and hollow porous titanium dioxide nanoparticles (HMTNPs) as the basic framework (Fig. 3B). The cancer cell membrane enabled the nanoplatform to escape phagocytosis by macrophages and actively target tumors through homologous targeting ability. Western blotting and cellular uptake assays indicated that CCM-HMTNPs retained CD44 and CD47 to prevent macrophage phagocytosis, actively targeted cancer cells, and promoted cell uptake. HMTNPs generated ROS by ultrasound activation and induced apoptosis. HCQ, an internally loaded autophagy inhibitor, blocked autophagic flux and cut off the nutrient supply from damaged organelles to eliminate resistance to SDT. Cell viability assays demonstrated that the cytotoxicity of CCM-HMTNPs/HCQ was twofold higher than that of CCM-HMTNPs, indicating that autophagy inhibition enhances the therapeutic effect of SDT. However, the involvement of cancer cell membranes may cause unknown side effects, and this approach should hence be used with caution, even though it may enhance the active targeting of tumors.
In addition to these innovative therapies, there are also other methods for improving the antitumour effects associated with autophagy inhibition, such as homeostatic perturbation therapy84, pharmacophore hybridization85, and calcium interference86. These methods are not discussed further in this review.
Strategies for autophagy induction
Although autophagy inhibition is effective for tumor treatment, it is difficult to ensure the complete suppression of autophagy using autophagy inhibitors. Previous studies have shown that once the level of autophagy exceeds the critical threshold, overactivation leads to autophagic cell death, enhanced antigen presentation, and increased immune cell recruitment87. Based on this, researchers have already used autophagy overactivation to induce tumor cell death, which has been found to yield impressive results.
Chemotherapeutic nanoparticles for autophagy induction
Similar to the inhibition of autophagy, inducers of autophagy are also used to promote this cellular process. Shang et al.88 generated PLT@BPQDs-HED NPs that combine chemotherapeutic drugs with the activation of autophagy (Fig. 4A). Hederagenin (HED) is a free drug used for cancer treatment that has poor targeting ability and weak antitumour activity in vivo. Therefore, it has been modified with a platelet membrane to improve its shortcomings. The platelet membrane (PLT) targets tumor cells by binding to the CD44 receptor and P-selectin. Moreover, PLTs are biological membranes that exist naturally in organisms, and their antigenicity is weak, which can reduce uptake by macrophages and increase the retention time of drugs in vivo. Black phosphorus quantum dots (BPQDs) are a type of quantum dot with excellent tissue penetration, biocompatibility, and passive targeting ability. The acidic conditions in tumor cells increase the degradation of BPQDs, accelerating the release of chemotherapeutic drugs. Most importantly, BPQD can promote autophagy by upregulating Beclin1 expression and by promoting the conversion of LC3-I to LC3-II to induce autophagic cell death. The safety, targeting ability, and efficacy of the drug delivery platforms were confirmed by hemolysis, TEM, and CCK-8 assays. At a BPQD concentration of 2.0 mg/ml, the hemolysis ratio was only ~0.5%, which is considered safe, and PLT considerably enhanced the biocompatibility of BPQDs. TEM images showed a significant accumulation of PLT@BPQDs-HED NPs, confirming their targeting ability. At the same concentration, the cell viability was lowest with PLT@BPQDs-HED, indicating that it had the strongest antitumour effect. As a biomolecule, the blood cell membrane is more prone to denaturation and is more difficult to preserve than polymer materials and MOFs, which limits the clinical application.
A Schematic diagram of PLT@BPQDs-HED. B Schematic diagram of CD-Ce6-3BP NPs synergistically inducing autophagic cell death. C Schematic diagram of the on-demand autophagy cascade amplification nanoparticle (ASN) for enhanced immunotherapy. D Schematic diagram of the self-assembled nanoactivator NP-B-OVA directly boosting autophagy of dendritic cells in situ.
Phototherapeutic nanoparticles for autophagy induction
Autophagy induction can facilitate PDT. Hypoxia is a prominent feature in many solid tumors, and the hypoxic TME is the main obstacle in PDT. However, PDT can be used if the hypoxia in tumor cells is improved. The strategy usually involves decreasing the consumption of oxygen or increasing the supply of oxygen. ROS-triggered autophagy simultaneously exhibits an anti-apoptotic effect in PDT, thus enabling tumor cells to survive. Researchers usually use autophagy inhibitors to overcome this problem, although it is very difficult to completely inhibit autophagy. Deng et al.89 devised a supramolecular nanoplatform, CD-Ce6-3BP NPs (Fig. 4B), comprising 3-bromopyruvate (3-BP) and chlorin e6 (Ce6) conjugated with α-cyclodextrin (CD) to form prodrugs through pH-sensitive hydrazone bonds. CD-based prodrugs and poly(ethylene glycol)-b-poly(2-methacryloyloxyethyl phosphorylcholine) (PEG-b-PMPC) constitute the final CD-Ce6-3BP NPs through host–guest interactions. The modification of CD and PEG-b-PMPC increased the solubility, biocompatibility, and plasma half-life in vivo. The pH-sensitive hydrazone bond is cleaved in acidic lysosomes at pH 5.5, and 3-BP and Ce6 are released. 3-BP, which is an inhibitor of hexokinase-II (HK-II) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), can reduce the energy supply, significantly reducing intracellular oxygen consumption and prompting famine-induced autophagy by inhibiting glycolysis and mitochondrial respiration. The oxygen consumption of CD-Ce6-3BP NPs was 50% lower than that of CD-Ce6 NPs without 3-BP. The photosensitizer Ce6 promoted the generation of ROS and triggered antiapoptotic autophagy under irradiation with a 660 nm laser. Finally, ROS-induced autophagy and starvation-induced autophagy synergistically led to overactivated autophagy in tumor cells and promoted antitumour effects. Moreover, alleviating tumor hypoxia may be an effective way to inhibit tumor metastasis. The bioluminescence imaging photographs of the 4T1 lung metastases in mice showed that CD-Ce6-3BP NPs had the greatest ability to inhibit tumor metastasis.
Immunotherapeutic nanoparticles for autophagy induction
According to several studies, particularly by Kroemer and colleagues, the inhibition of autophagy is not necessarily a good strategy in cancer treatment because it reduces the immune response of antitumour T cells90. The rationale is that autophagy in dead tumor cells is necessary for the occurrence of immunogenic cell death (ICD), which leads to effective recognition by the immune system and activation of durable antitumour immune responses.
Due to the positive role of autophagy in immunotherapy, taking advantage of augmented autophagy is common. He et al.91 designed on-demand autophagy cascade amplification nanoparticles (ASNs), which were very effective at taking advantage of different TME to achieve a hierarchical release function (Fig. 4C). ASNs are prepared by self-assembling C-TFG monomers, which are sensitive to the autophagy enzyme ATG4 and coated with oxaliplatin (OXA)-grafted hyaluronic acid (HA) prodrug (HA-OXA) by electrostatic binding. When ASNs enter the blood circulation, they are able to actively target tumor sites. After entering tumor cells, OXA is first dissociated from HA-OXA by hyaluronidase and recovers its active form as a result of the reduction effect of GSH. Free OXA induces mild autophagy, which can contribute to the secretion of ATG4 in tumor cells and enhance cancer immunotherapy by the secretion of ATP, which recruits DCs and triggers ICD. C-TFG micelles are cleaved, and the autophagy inducer STF-62247 is released to induce autophagic cell death. The MTT assay showed that the IC50 of ASN was 6.477 ± 0.811 × 10−6 M, which is 3.81-fold lower than that of OXA alone, which indicated the strongest antitumour effect. When the autophagy inhibitor 3-MA was added, the cell viability of the OXA group decreased because the protective autophagy induced by OXA was suppressed and the antitumour efficacy was enhanced. There was no significant difference between the AIN (the ASN analog unable to respond to only ATG4) and the AIN + 3-MA groups. In contrast, the cell viability of the ASN group incubated with 3-MA increased, indicating a higher level of autophagy, which induced autophagic cell death instead of continually protecting the cells. These findings highlight the role of autophagy cascade amplification, and this study is an outstanding example of immediate autophagy-responsive NPs, which is a useful conceptual framework for the further construction of autophagy-targeting NPs. However, the immune response is not long-lasting, which is a limitation common to immunotherapy.
Wang et al.92 adopted another strategy to enhance immunotherapy by activating DCs in situ. As depicted in Fig. 4D, they generated NP-B-OVA nanoactivators based on polymers made of a hydrophobic monomer (HDDA), a pH-responsive monomer (DBPA), and hydrophilic amino-terminated polyethylene glycol (PEG-NH2). The autophagy promoter Beclin1 and antigen peptide OVA257–264 were conjugated with the polymers. With immune stimulation induced by OVA257–264, Beclin1 enhanced DC autophagy, increased the immune response, and improved the antitumour efficacy. DCs stimulated in vitro were injected into C57BL/6 mice, and the flow cytometry results indicated that NP-B-OVA showed an approximately twofold increase in CD8+ T cells compared with the non-Beclin1 group as a result of autophagy promotion. However, exogenous antigens triggered unexpected immune rejection.
Other therapeutic nanoparticles for autophagy induction
There are additional ways to promote autophagy in cancer treatment. It should be kept in mind that conventional PTT is limited by the use of high temperature, which causes severe pain. However, a new therapy, ultrafast low-temperature photothermal therapy (LTPTT), induces PTT at 38–43 °C to overcome this drawback. Osteosarcoma is a common malignant tumor in adolescents and is prone to relapse and metastasis. Deng et al.93 generated GFS particles composed of the tumor-targeting ligand folic acid, the photothermal material graphene oxide (GO), and the heat shock protein 90 (HSP90) inhibitor SNX-2112 with LTPTT (Fig. 5A). When GFS particles enter tumor cells, GO breaks down and releases SNX-2112 under 808 nm laser light. It has been shown that autophagy can protect tumor cells in the presence of HSP90 but kills tumor cells in its absence. Therefore, the released SNX-2112 can inhibit HSP90, which leads to downregulation of the AKT pathway and consequently to autophagic cell death at low temperatures and thermal pain relief. The main cause of death was studied using flow cytometry. The cell viability of free SNX-2112 under 808 nm laser light was ~80%, and that of the non-SNX-2112 GF group was ~60%. This means that LTPTT without autophagy modulation and SNX-2112 alone are incapable of killing tumor cells. The combination of the two strategies provides the required efficacy, and it may be necessary to assess the relationship between safety and clinical efficacy.
The regulation of tumor metabolism is potentially an effective way to kill tumors. The rapid proliferation of tumor cells requires that they optimize the utilization of nutrients to adapt to nutrient-deficient conditions. Researchers have inhibited tumor growth and metastasis by disrupting the metabolic reprogramming of tumor cells. However, this nutritional restriction may lead to metabolic flexibility in tumors, which involves filling the metabolic pool of one nutrient with another nutrient to maintain growth and survival. Autophagy also plays a role in nutrient metabolism in tumors. Under nutrient-deficient conditions, autophagy degrades cellular components and provides nutrients for tumor cells to ensure their survival. Although excessive autophagy can lead to tumor cell death, the effect of conventional autophagy inducers is not sufficient. Therefore, Guo et al.94 devised a codelivery system, ARPNP, which is composed of the autophagy inducer rapamycin, anti-PFKFB4 siRNA, and a nucleoprotein targeting aptamer AS1411 (Fig. 5B). PFKFB4 is a metabolic enzyme of fructose-2,6-bisphosphatase-4, which can promote aggressive metastatic tumors and synthesize glycolytic stimulating factors. Downregulation of PFKFB4 can inhibit the SRC3/Akt/mTOR pathway, which impedes tumor cell killing and promotes autophagy to induce tumor cell apoptosis. ARPNP can synchronously regulate glycolysis and autophagy, ensuring that autophagy plays an antitumour role while inhibiting tumors from reprogramming metabolism and disrupting cancer cell homeostasis. 4T1 cell uptake experiments showed that the fluorescence intensity gradually increased with an increase in aptamer modification. However, there did not appear to be a significant difference between 20 and 40% modification, which may be due to the saturation of nucleolin. Either way, modification of the aptamer AS1411 increased the accumulation of ARPNP in tumor cells. As expected, ARPNP showed the strongest ability to inhibit PFKFB4 expression and initiate autophagy, according to western blot and acridine orange staining. However, the 40-day mouse survival rate showed that only 50% of the mice in the ARPNP group survived, which suggests that more attention should be given to its biological safety.
Clinical and preclinical studies
Preclinical and clinical studies of autophagy-related tumor therapies are ongoing. Many preclinical studies have demonstrated that suppressing autophagy during tumor therapy appears to be a good approach. Since the initial finding of Amaravadi et al.95, numerous in vitro models, genetically engineered mouse models, and patient-derived xenograft mouse models have shown that the combination of different anticancer drugs and autophagy inhibition can produce better therapeutic results30,96. HCQ and CQ are currently the only drugs used in clinical practice to inhibit autophagy. They are also thought to improve the prognosis. For example, in an institutional study, routine chemotherapy for glioblastoma was found to extend median survival and reduce mortality in the CQ treatment group, thus demonstrating the safety of CQ and the potential for extended survival97. Moreover, an experiment involving HCQ in combination with the chemotherapy drug doxorubicin to treat canine lymphoma proved the safety of this combination strategy and provided useful evidence for clinical research on autophagy targeting98. In clinical trials, the results obtained by the use of autophagy inhibitors vary significantly. Although it has been shown that treatment with CQ can prolong the median survival time of glioblastoma, the results of HCQ combined with chemotherapy and radiotherapy in phase I/II trials showed that the survival rate of patients with glioblastoma was not significantly improved99. The different results may be ascribed to the dose-limiting toxicities, which prevent the full efficacy of HCQ from being reached.
Kroemer and colleagues have shown that it may not be a good idea to inhibit autophagy in tumor treatment, especially as it can reduce the antitumour T-cell response100,101. They have also shown that enhanced autophagy can promote tumor immunotherapy102. However, there is a caveat, as they focused on tumor models with high immunogenicity, such as the CT26 colon cancer mouse model, which may have affected the results. For example, in one experiment, poorly immunogenic mouse B16 melanoma and 4T1 breast cancer cells were used, and it was found that the immune response of tumor-bearing mice with high autophagy levels was equivalent to that of mice with normal autophagy levels103.
In cancer treatment, the inhibition or promotion of autophagy is not specifically aimed at tumor cells, which makes it necessary to consider the systemic toxicity of inhibition or promotion of autophagy. In a preclinical study, researchers knocked out the necessary genes for autophagy in adult mice, which led to all of the mice dying104. This indicates that it is more meaningful to specifically target tumor autophagy, and the advantages of nano-drug delivery systems should be kept in mind in this regard. Table 2 summarizes a number of autophagy-targeting NPs in clinical and preclinical studies.
Few NP constructs that target autophagy are suitable for preclinical and clinical trials. As mentioned above, the autophagy modulators in preclinical and clinical trials are generally not specific to autophagy. There is, therefore, a need for further concerted research efforts to create state-of-the-art nano-drug delivery systems to improve the application of autophagy modulation in tumor therapy.
Conclusions and outlook
Owing to their unique characteristics, nano-drug delivery systems have shown potential for tumor treatment. As tumor cells grow more quickly than normal cells and the TME differs from the normal physiological environment, it is possible to devise NPs that selectively target tumors. With increased understanding of the mechanisms of autophagy from physiological and medical studies, it is now recognized that autophagy plays a remarkable role in tumor therapy as a physiological phenomenon that occurs broadly in eukaryotic cells. Autophagy-targeting NPs have become a new research field, and they exhibit opposing effects depending on the tumor status (i.e., inhibition versus promotion). This review summarizes the various strategies devised to date to inhibit or promote autophagy. Researchers have utilized different types of nanomaterials, such as polymeric micelles and MOFs, to regulate autophagy in a timely and accurate manner. Compared with traditional therapies (such as chemotherapy, phototherapy and immunotherapy), the combination of NPs with autophagy modulators exhibits stronger antitumour effects. The results of clinical and preclinical studies suggest that the development of autophagy-targeting NPs needs to advance toward tumor targeting, biosafety and combination strategies.
From this summary of autophagy-targeting NPs, it can be seen that the three main ways to influence autophagy in cancer treatment are the direct use of autophagy-related proteins or their coding sequences; the use of autophagy inhibitors or activators confirmed by clinical or preclinical studies; and the synthesis of materials whose physical or chemical characteristics have been proven to regulate the level of autophagy. Although this approach has already achieved surprising results, there are still some challenges associated with the development of autophagy-targeting NPs. First, many scientific questions remain unanswered because research on the regulation of autophagy by nanomaterials is still in its infancy. Due to the complexity of the TME, there is still a long way to go before NPs are suitable for clinical applications, such as the precise targeting of tumor cells. Assessment of the critical threshold between mild autophagy and acute autophagy requires more precise and quantitative criteria rather than a simple qualitative and malleable distinction. This may require new and more advanced intracellular markers, which can only be expected from further advances in this area of research. It is thought that overcoming these limitations will contribute to the improvement of strategies for the use of autophagy-targeting NPs in the field of tumor therapy.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Klionsky, D. J. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat. Rev. Mol. Cell Biol. 8, 931–937 (2007).
Levine, B. & Klionsky, D. J. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell 6, 463–477 (2004).
Hernandez-Caceres, M. P. et al. Mechanobiology of autophagy: the unexplored side of cancer. Front. Oncol. 11, 632956 (2021).
Galluzzi, L. et al. Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880 (2015).
Bhutia, S. K. et al. Autophagy: cancer’s friend or foe? Adv. Cancer Res. 118, 61–95 (2013).
White, E. & DiPaola, R. S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 15, 5308–5316 (2009).
White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 12, 401–410 (2012).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Galluzzi, L. et al. Molecular definitions of autophagy and related processes. EMBO J. 36, 1811–1836 (2017).
Holohan, C., Van Schaeybroeck, S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6, 715–727 (2006).
Rauscher, H., Sokull-Klüttgen, B. & Stamm, H. The European Commission’s recommendation on the definition of nanomaterial makes an impact. Nanotoxicology 7, 1195–1197 (2013).
Shi, J., Kantoff, P. W., Wooster, R. & Farokhzad, O. C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017).
Xia, H. et al. Reduction-sensitive polymeric micelles as amplifying oxidative stress vehicles for enhanced antitumor therapy. Colloids Surf. B Biointerfaces 203, 111733 (2021).
Yan, J. et al. Multifunctional nanoparticles self-assembled from polyethylenimine-based graft polymers as efficient anticancer drug delivery. Colloids Surf. B Biointerfaces 155, 118–127 (2017).
Yan, J. et al. In situ injection of dual-delivery PEG based MMP-2 sensitive hydrogels for enhanced tumor penetration and chemo-immune combination therapy. Nanoscale 13, 9577–9589 (2021).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Disco. 20, 101–124 (2021).
Villeret, B. et al. Silver nanoparticles impair retinoic acid-inducible gene I-mediated mitochondrial antiviral immunity by blocking the autophagic flux in lung epithelial cells. ACS Nano 12, 1188–1202 (2018).
Liu, J. et al. A self-assembled alpha-synuclein nanoscavenger for Parkinson’s disease. ACS Nano 14, 1533–1549 (2020).
Sun, H. et al. A tauopathy-homing and autophagy-activating nanoassembly for specific clearance of pathogenic tau in Alzheimer’s disease. ACS Nano 15, 5263–5275 (2021).
Liu, Y. et al. Polypeptide nano-Se targeting inflammation and theranostic rheumatoid arthritis by anti-angiogenic and NO activating AMPKalpha signaling pathway. J. Mater. Chem. B 6, 3497–3514 (2018).
Hara, T. et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Li, W. W., Li, J. & Bao, J. K. Microautophagy: lesser-known self-eating. Cell. Mol. Life Sci. 69, 1125–1136 (2012).
Cuervo, A. M. & Wong, E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 24, 92–104 (2014).
Galluzzi, L., Bravo-San Pedro, J. M., Levine, B., Green, D. R. & Kroemer, G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Disco. 16, 487–511 (2017).
Liang, X. H. et al. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 402, 672–676 (1999).
Levy, J. M. M., Towers, C. G. & Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 17, 528–542 (2017).
Levine, B. Cell biology: autophagy and cancer. Nature 446, 745–747 (2007).
Amaravadi, R., Kimmelman, A. C. & White, E. Recent insights into the function of autophagy in cancer. Genes Dev. 30, 1913–1930 (2016).
Degenhardt, K. et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 10, 51–64 (2006).
Amaravadi, R. K. et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin. Cancer Res. 17, 654–666 (2011).
Pavel, M. et al. Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. Nat. Commun. 9, 2961 (2018).
Machado-Neto, J. A. et al. Autophagy inhibition potentiates ruxolitinib-induced apoptosis in JAK2(V617F) cells. Invest. New Drugs 38, 733–745 (2020).
Arakawa, S. et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 24, 1598–1608 (2017).
Schreiber, K. H. et al. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell 14, 265–273 (2015).
Calne, R. Y. et al. Rapamycin for immunosuppression in organ allografting. Lancet 2, 227 (1989).
Afeseh Ngwa, H. et al. Manganese nanoparticle activates mitochondrial dependent apoptotic signaling and autophagy in dopaminergic neuronal cells. Toxicol. Appl. Pharmacol. 256, 227–240 (2011).
Nowak, J. S. et al. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol. Lett. 224, 84–92 (2014).
Dewaele, M., Maes, H. & Agostinis, P. ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 6, 838–854 (2010).
Wong, C. H. et al. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS ONE 5, e9996 (2010).
Mao, B. H., Tsai, J. C., Chen, C. W., Yan, S. J. & Wang, Y. J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 10, 1021–1040 (2016).
Pelt, J. et al. Chloroquine and nanoparticle drug delivery: a promising combination. Pharm. Ther. 191, 43–49 (2018).
Yan, J. et al. Mitochondria-targeted tetrahedral DNA nanostructures for doxorubicin delivery and enhancement of apoptosis. J. Mater. Chem. B 8, 492–503 (2020).
Chen, H., Zhang, W., Zhu, G., Xie, J. & Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2, 17024 (2017).
Mahvi, D. A., Liu, R., Grinstaff, M. W., Colson, Y. L. & Raut, C. P. Local cancer recurrence: the realities, challenges, and opportunities for new therapies. CA Cancer J. Clin. 68, 488–505 (2018).
Zhong, Z. & Virshup, D. M. Wnt signaling and drug resistance in cancer. Mol. Pharm. 97, 72–89 (2020).
Yan, J. et al. Redox-responsive polyethyleneimine/tetrahedron DNA/doxorubicin nanocomplexes for deep cell/tissue penetration to overcome multidrug resistance. J. Control Release 329, 36–49 (2021).
Lepeltier, E. et al. Nanomedicine to target multidrug resistant tumors. Drug Resist. Updat. 52, 100704 (2020).
Livney, Y. D. & Assaraf, Y. G. Rationally designed nanovehicles to overcome cancer chemoresistance. Adv. Drug Deliv. Rev. 65, 1716–1730 (2013).
Shapira, A., Livney, Y. D., Broxterman, H. J. & Assaraf, Y. G. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist. Updat. 14, 150–163 (2011).
Xin, L. et al. METase/lncRNA HULC/FoxM1 reduced cisplatin resistance in gastric cancer by suppressing autophagy. J. Cancer Res. Clin. Oncol. 145, 2507–2517 (2019).
Liang, B. et al. Inhibition of autophagy sensitizes MDR-phenotype ovarian cancer SKVCR cells to chemotherapy. Biomed. Pharmacother. 82, 98–105 (2016).
Wu, J. et al. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J. Exp. Clin. Cancer Res. 37, 272 (2018).
Ge, Z. & Liu, S. Functional block copolymer assemblies responsive to tumor and intracellular microenvironments for site-specific drug delivery and enhanced imaging performance. Chem. Soc. Rev. 42, 7289–7325 (2013).
Saiyin, W. et al. Sequential release of autophagy inhibitor and chemotherapeutic drug with polymeric delivery system for oral squamous cell carcinoma therapy. Mol. Pharm. 11, 1662–1675 (2014).
Ebrahimi, S. et al. Targeting the Akt/PI3K signaling pathway as a potential therapeutic strategy for the treatment of pancreatic cancer. Curr. Med. Chem. 24, 1321–1331 (2017).
Wu, M. X. & Yang, Y. W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 29, 1606134 (2017).
Chen, X. et al. MOF nanoparticles with encapsulated autophagy inhibitor in controlled drug delivery system for antitumor. ACS Appl. Mater. Interfaces 10, 2328–2337 (2018).
Apel, A., Herr, I., Schwarz, H., Rodemann, H. P. & Mayer, A. Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer Res. 68, 1485–1494 (2008).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Zhang, Y. et al. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 9, 4236 (2018).
Domagala, A. et al. Inhibition of autophagy sensitizes cancer cells to Photofrin-based photodynamic therapy. BMC Cancer 18, 210 (2018).
Yu, X. et al. Inhibiting metastasis and preventing tumor relapse by triggering host immunity with tumor-targeted photodynamic therapy using photosensitizer-loaded functional nanographenes. ACS Nano 11, 10147–10158 (2017).
Chen, J. et al. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials 237, 119827 (2020).
Wang, C. et al. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 26, 8154–8162 (2014).
Liu, Y., Bhattarai, P., Dai, Z. & Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 48, 2053–2108 (2019).
Pan, Q. et al. In-situ drug generation and controllable loading: rational design of copper-based nanosystems for chemo-photothermal cancer therapy. Chem. Eng. J. 409, 128222 (2021).
Zhang, X. et al. Endosome/lysosome-detained supramolecular nanogels as an efflux retarder and autophagy inhibitor for repeated photodynamic therapy of multidrug-resistant cancer. Nanoscale Horiz. 5, 481–487 (2020).
Ren, X. et al. Blocking autophagic flux enhances iron oxide nanoparticle photothermal therapeutic efficiency in cancer treatment. ACS Appl Mater. Interfaces 10, 27701–27711 (2018).
Colombo, M. et al. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 41, 4306–4334 (2012).
Khan, M. I. et al. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials 33, 1477–1488 (2012).
Katheder, N. S. et al. Microenvironmental autophagy promotes tumour growth. Nature 541, 417–420 (2017).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Huang, J. et al. Nanomedicine‐boosting tumor immunogenicity for enhanced immunotherapy. Adv. Funct. Mater. 31, 2011171 (2021).
Ward, E. M., Flowers, C. R., Gansler, T., Omer, S. B. & Bednarczyk, R. A. The importance of immunization in cancer prevention, treatment, and survivorship. CA Cancer J. Clin. 67, 398–410 (2017).
Jiang, G. M. et al. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer 18, 17 (2019).
Ruan, S. et al. Increased gold nanoparticle retention in brain tumors by in situ enzyme-induced aggregation. ACS Nano 10, 10086–10098 (2016).
Ruan, S. et al. Aggregable nanoparticles-enabled chemotherapy and autophagy inhibition combined with Anti-PD-L1 antibody for improved glioma treatment. Nano Lett. 19, 8318–8332 (2019).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Zhang, H. et al. Positioning remodeling nanogels mediated codelivery of antivascular drug and autophagy inhibitor for cooperative tumor therapy. ACS Appl Mater. Interfaces 12, 6978–6990 (2020).
Feng, Q. et al. Cancer cell membrane-biomimetic nanoplatform for enhanced sonodynamic therapy on breast cancer via autophagy regulation strategy. ACS Appl Mater. Interfaces 11, 32729–32738 (2019).
Wan, S. S., Zhang, L. & Zhang, X. Z. An ATP-regulated ion transport nanosystem for homeostatic perturbation therapy and sensitizing photodynamic therapy by autophagy inhibition of tumors. ACS Cent. Sci. 5, 327–340 (2019).
Ma, Z. et al. Pharmacophore hybridisation and nanoscale assembly to discover self-delivering lysosomotropic new-chemical entities for cancer therapy. Nat. Commun. 11, 4615 (2020).
An, J. et al. Nanoenabled disruption of multiple barriers in antigen cross-presentation of dendritic cells via calcium interference for enhanced chemo-immunotherapy. ACS Nano 14, 7639–7650 (2020).
Martins, I. et al. Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy 8, 413–415 (2012).
Shang, Y. et al. Platelet-membrane-camouflaged black phosphorus quantum dots enhance anticancer effect mediated by apoptosis and autophagy. ACS Appl Mater. Interfaces 11, 28254–28266 (2019).
Deng, Y. et al. 3-Bromopyruvate-conjugated nanoplatform-induced pro-death autophagy for enhanced photodynamic therapy against hypoxic tumor. ACS Nano 14, 9711–9727 (2020).
Rao, S., Yang, H., Penninger, J. M. & Kroemer, G. Autophagy in non-small cell lung carcinogenesis: a positive regulator of antitumor immunosurveillance. Autophagy 10, 529–531 (2014).
Wang, X. et al. On-demand autophagy cascade amplification nanoparticles precisely enhanced oxaliplatin-induced cancer immunotherapy. Adv. Mater. 32, e2002160 (2020).
Wang, Y. et al. In situ manipulation of dendritic cells by an autophagy-regulative nanoactivator enables effective cancer immunotherapy. ACS Nano 13, 7568–7577 (2019).
Deng, X. et al. Ultrafast low-temperature photothermal therapy activates autophagy and recovers immunity for efficient antitumor treatment. ACS Appl Mater. Interfaces 12, 4265–4275 (2020).
Guo, Q. et al. Click-nucleic-acid-containing codelivery system inducing collapse of cellular homeostasis for tumor therapy through bidirectional regulation of autophagy and glycolysis. ACS Appl Mater. Interfaces 12, 57757–57767 (2020).
Amaravadi, R. K. et al. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J. Clin. Investig. 117, 326–336 (2007).
Thorburn, A., Thamm, D. H. & Gustafson, D. L. Autophagy and cancer therapy. Mol. Pharm. 85, 830–838 (2014).
Sotelo, J., Briceño, E. & López-González, M. A. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 144, 337–343 (2006).
Barnard, R. A. et al. Phase I clinical trial and pharmacodynamic evaluation of combination hydroxychloroquine and doxorubicin treatment in pet dogs treated for spontaneously occurring lymphoma. Autophagy 10, 1415–1425 (2014).
Rosenfeld, M. R. et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 10, 1359–1368 (2014).
Townsend, K. N. et al. Autophagy inhibition in cancer therapy: metabolic considerations for antitumor immunity. Immunol. Rev. 249, 176–194 (2012).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Starobinets, H. et al. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J. Clin. Investig. 126, 4417–4429 (2016).
Karsli-Uzunbas, G. et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Disco. 4, 914–927 (2014).
Fang, J. H. et al. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv. Health. Mater. 3, 1250–1260 (2014).
Wang, L. et al. Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single-walled carbon nanotubes. Biomaterials 34, 262–274 (2013).
Shim, G. et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J. Control Release 155, 60–66 (2011).
Tang, S. et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (β-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials 48, 1–15 (2015).
Acknowledgements
The authors thank the National Science Foundation of China (Nos. 51803098 and 51773188), Key Project of Natural Science Foundation of Shandong Province (No. ZR2020KE016) and China Postdoctoral Natural Science Foundation (No. 2021TQ0160) for financial support.
Author information
Authors and Affiliations
Contributions
Y.L. conceived and designed the topic of this review. X.L. and J.Y. collected the literature and wrote the article. Z.Z. and J.C. collected the literature and summarized the insert figures and tables. B.H. and Y.S. reviewed and revised this review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Long, X., Yan, J., Zhang, Z. et al. Autophagy-targeted nanoparticles for effective cancer treatment: advances and outlook. NPG Asia Mater 14, 71 (2022). https://doi.org/10.1038/s41427-022-00422-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41427-022-00422-3
This article is cited by
-
Biosynthesis of palladium, platinum, and their bimetallic nanoparticles using rosemary and ginseng herbal plants: evaluation of anticancer activity
Scientific Reports (2024)
-
Persuasive phytoestrogenic imidazole-based selenium N-heterocyclic carbenes: electronic, structural, and in silico anticancer potential investigations
Structural Chemistry (2024)
-
Modulation of Autophagy in Gastric Cancer Cells and Sensitization to 5-Fluorouracil by Combination Therapy with Se–FA Nanoparticles
Journal of Cluster Science (2024)
-
The impact of nanomaterials on autophagy across health and disease conditions
Cellular and Molecular Life Sciences (2024)
-
In situ self-assembly for cancer therapy and imaging
Nature Reviews Materials (2023)