Abstract
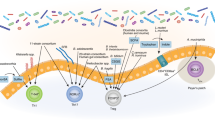
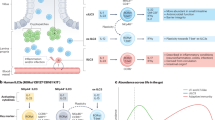
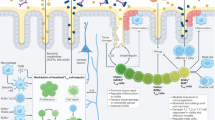
Although lymphocytes are known to circulate throughout lymphoid tissues and blood, they also establish residency in nonlymphoid organs, most prominently in barrier tissues, such as the intestines. The adaptation of T lymphocytes to intestinal environments requires constant discrimination between natural stimulation from commensal flora and food and pathogens that need to be cleared. Genetic variations that cause a defective defense or a break in tolerance along with environmental cues, such as infection or imbalances in the gut microbiota known as dysbiosis, can trigger several immune disorders via the activation of T lymphocytes in the intestines. Elucidation of the immune mechanisms that distinguish between commensal flora and pathogenic organisms may reveal therapeutic targets for the prevention or modulation of inflammatory diseases and boost the efficacy of cancer immunotherapy. In this review, we discuss the development and adaptation of T lymphocytes in the intestine, how these cells protect the host against pathogenic infections while tolerating food antigens and commensal microbiota, and the potential implications of targeting these cells for disease management and therapeutics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Darlington, D. & Rogers, A. W. Epithelial lymphocytes in the small intestine of the mouse. J. Anat. 100, 813–830 (1966).
Zeitz, M. et al. Phenotype and function of lamina propria T lymphocytes. Immunol. Res. 10, 199–206 (1991).
Hayday, A. & Gibbons, D. Brokering the peace: the origin of intestinal T cells. Mucosal Immunol. 1, 172–174 (2008).
Bai, L. & Peng, H. Generating CD8alphaalpha IELs from two sources of thymic precursors. Cell Mol. Immunol. 15, 640–641 (2018).
Starr, T. K., Jameson, S. C. & Hogquist, K. A. Positive and negative selection of T cells. Annu. Rev. Immunol. 21, 139–176 (2003).
Hogquist, K. A., Baldwin, T. A. & Jameson, S. C. Central tolerance: learning self-control in the thymus. Nat. Rev. Immunol. 5, 772–782 (2005).
Baldwin, T. A., Hogquist, K. A. & Jameson, S. C. The fourth way? Harnessing aggressive tendencies in the thymus. J. Immunol. 173, 6515–6520 (2004).
Campbell, D. J. & Butcher, E. C. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 (2002).
Agace, W. W. T-cell recruitment to the intestinal mucosa. Trends Immunol. 29, 514–522 (2008).
Masopust, D. et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 207, 553–564 (2010).
Iijima, N. & Iwasaki, A. Tissue instruction for migration and retention of TRM cells. Trends Immunol. 36, 556–564 (2015).
Svensson, M. et al. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 110, 1113–1121 (2002).
Hieshima, K. et al. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J. Immunol. 170, 1452–1461 (2003).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Shi, Y. & Mu, L. An expanding stage for commensal microbes in host immune regulation. Cell Mol. Immunol. 14, 339–348 (2017).
Wagner, N. et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Pahar, B., Lackner, A. A. & Veazey, R. S. Intestinal double-positive CD4+ CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur. J. Immunol. 36, 583–592 (2006).
Wang, X., Das, A., Lackner, A. A., Veazey, R. S. & Pahar, B. Intestinal double-positive CD4+ CD8+ T cells of neonatal rhesus macaques are proliferating, activated memory cells and primary targets for SIVMAC251 infection. Blood 112, 4981–4990 (2008).
Tiberio, L. et al. Chemokine and chemotactic signals in dendritic cell migration. Cell Mol. Immunol. 15, 346–352 (2018).
Mikulic, J., Longet, S., Favre, L., Benyacoub, J. & Corthesy, B. Secretory IgA in complex with Lactobacillus rhamnosus potentiates mucosal dendritic cell-mediated Treg cell differentiation via TLR regulatory proteins, RALDH2 and secretion of IL-10 and TGF-beta. Cell Mol. Immunol. 14, 546–556 (2017).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829 (2015).
Farache, J. et al. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 38, 581–595 (2013).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Shan, M. et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 342, 447–453 (2013).
Welty, N. E. et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. J. Exp. Med. 210, 2011–2024 (2013).
Rivollier, A., He, J., Kole, A., Valatas, V. & Kelsall, B. L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 209, 139–155 (2012).
Denning, T. L., Wang, Y. C., Patel, S. R., Williams, I. R. & Pulendran, B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat. Immunol. 8, 1086–1094 (2007).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93 (2003).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Gagliani, N. et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Cheroutre, H. & Lambolez, F. Doubting the TCR coreceptor function of CD8alphaalpha. Immunity 28, 149–159 (2008).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Huang, Y. et al. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat. Immunol. 12, 1086–1095 (2011).
Malamut, G. et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J. Clin. Invest. 120, 2131–2143 (2010).
Ma, L. J., Acero, L. F., Zal, T. & Schluns, K. S. Trans-presentation of IL-15 by intestinal epithelial cells drives development of CD8alphaalpha IELs. J. Immunol. 183, 1044–1054 (2009).
Klose, C. S. et al. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity 41, 230–243 (2014).
Reis, B. S., Hoytema van Konijnenburg, D. P., Grivennikov, S. I. & Mucida, D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity 41, 244–256 (2014).
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
Lu, X. F. et al. Preferential loss of gut-homing alpha 4 beta 7 CD4(+) T cells and their circulating functional subsets in acute HIV-1 infection. Cell. Mol. Immunol. 13, 776–784 (2016).
Klatt, N. R., Funderburg, N. T. & Brenchley, J. M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 21, 6–13 (2013).
Bhaijee, F., Subramony, C., Tang, S. J. & Pepper, D. J. Human immunodeficiency virus-associated gastrointestinal disease: common endoscopic biopsy diagnoses. Pathol. Res. Int. 2011, 247923 (2011).
Dharakul, T., Rott, L. & Greenberg, H. B. Recovery from chronic rotavirus infection in mice with severe combined immunodeficiency—virus clearance mediated by adoptive transfer of immune Cd8+ lymphocytes-T. J. Virol. 64, 4375–4382 (1990).
Adkins, B., Leclerc, C. & Marshall-Clarke, S. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4, 553–564 (2004).
Kuo, S., El Guindy, A., Panwala, C. M., Hagan, P. M. & Camerini, V. Differential appearance of T cell subsets in the large and small intestine of neonatal mice. Pediatr. Res. 49, 543–551 (2001).
Williams, A. M. et al. Effects of microflora on the neonatal development of gut mucosal T cells and myeloid cells in the mouse. Immunology 119, 470–478 (2006).
Swamy, M. et al. Intestinal intraepithelial lymphocyte activation promotes innate antiviral resistance. Nat. Commun. 6, 7090 (2015).
Tomov, V. T. et al. Persistent enteric murine norovirus infection is associated with functionally suboptimal virus-specific CD8 T cell responses. J. Virol. 87, 7015–7031 (2013).
McNeal, M. M. et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J. Virol. 76, 560–568 (2002).
Tomov, V. T. et al. Differentiation and protective capacity of virus-specific CD8(+) T cells suggest murine norovirus persistence in an immune-privileged enteric niche. Immunity 47, 723–738 e725 (2017).
Shen, H. B. & Chen, Z. W. The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell. Mol. Immunol. 15, 216–225 (2018).
Ishigame, H. et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30, 108–119 (2009).
Zenewicz, L. A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957 (2008).
Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390 (2011).
Sheridan, B. S. et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity 40, 747–757 (2014).
Romagnoli, P. A. et al. Differentiation of distinct long-lived memory CD4 T cells in intestinal tissues after oral Listeria monocytogenes infection. Mucosal Immunol. 10, 520–530 (2017).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Thiemann, S. et al. Enhancement of IFNgamma production by distinct commensals ameliorates salmonella-induced disease. Cell Host Microbe 21, 682–694 e685 (2017).
Bilate, A. M. & Lafaille, J. J. Induced CD4(+)Foxp3(+) regulatory T cells in immune tolerance. Annu. Rev. Immunol. 30, 733–758 (2012).
Boll, G. & Reimann, J. Lamina propria T-cell subsets in the small and large-intestine of euthymic and athymic mice. Scand. J. Immunol. 42, 191–201 (1995).
Kim, K. S. et al. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science 351, 858–863 (2016).
Hadis, U. et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 34, 237–246 (2011).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 107, 12204–12209 (2010).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Dolpady, J. et al. Oral probiotic VSL#3 prevents autoimmune diabetes by modulating microbiota and promoting Indoleamine 2,3-dioxygenase-enriched tolerogenic intestinal environment. J. Diabetes Res. 2016, 7569431 (2016).
Buning, J. et al. Multivesicular bodies in intestinal epithelial cells: responsible for MHC class II-restricted antigen processing and origin of exosomes. Immunology 125, 510–521 (2008).
Mucida, D. et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Coombes, J. L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Sicherer, S. H. & Sampson, H. A. Food allergy: epidemiology, pathogenesis, diagnosis, and treatment. J. Allergy Clin. Immunol. 133, 291–307 (2014).
Meresse, B., Ripoche, J., Heyman, M. & Cerf-Bensussan, N. Celiac disease: from oral tolerance to intestinal inflammation, autoimmunity and lymphomagenesis. Mucosal Immunol. 2, 8–23 (2009).
Podolsky, D. K. Inflammatory bowel disease (2). N. Engl. J. Med. 325, 1008–1016 (1991).
Neurath, M. F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 14, 269–278 (2017).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Liu, T. C. & Stappenbeck, T. S. Genetics and pathogenesis of inflammatory bowel disease. Annu. Rev. Pathol.: Mech. Dis., Vol. 11 11, 127–148 (2016).
Seo, S. U. et al. Distinct commensals induce interleukin-1beta via NLRP3 inflammasome in inflammatory monocytes to promote intestinal inflammation in response to injury. Immunity 42, 744–755 (2015).
Ramanan, D. et al. Helminth infection promotes colonization resistance via type 2 immunity. Science 352, 608–612 (2016).
Powrie, F. et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1, 553–562 (1994).
Leppkes, M. et al. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology 136, 257–267 (2009).
Wedebye Schmidt, E. G. et al. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 19, 1567–1576 (2013).
Berg, D. J. et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J. Clin. Invest. 98, 1010–1020 (1996).
Ostanin, D. V. et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146 (2009).
Fuss, I. J. et al. Both IL-12p70 and IL-23 are synthesized during active Crohn’s disease and are down-regulated by treatment with anti-IL-12 p40 monoclonal antibody. Inflamm. Bowel Dis. 12, 9–15 (2006).
Monteleone, G. et al. Interleukin 12 is expressed and actively released by Crohn’s disease intestinal lamina propria mononuclear cells. Gastroenterology 112, 1169–1178 (1997).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Monteleone, I., Pallone, F. & Monteleone, G. Th17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Med. 9, 122 (2011).
Neurath, M. F. IL-23: a master regulator in Crohn disease. Nat. Med. 13, 26–28 (2007).
De Nitto, D., Sarra, M., Cupi, M. L., Pallone, F. & Monteleone, G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr. Pharm. Des. 16, 3656–3660 (2010).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Reinisch, W. et al. A dose escalating, placebo controlled, double blind, single dose and multidose, safety and tolerability study of fontolizumab, a humanised anti-interferon gamma antibody, in patients with moderate to severe Crohn’s disease. Gut 55, 1138–1144 (2006).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
Maxwell, J. R. et al. Differential roles for interleukin-23 and interleukin-17 in intestinal immunoregulation. Immunity 43, 739–750 (2015).
Awasthi, A. & Kuchroo, V. K. IL-17A directly inhibits TH1 cells and thereby suppresses development of intestinal inflammation. Nat. Immunol. 10, 568–570 (2009).
El-Behi, M. et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12, 568–575 (2011).
Codarri, L. et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12, 560–567 (2011).
Tjon, J. M., van Bergen, J. & Koning, F. Celiac disease: how complicated can it get? Immunogenetics 62, 641–651 (2010).
Keuning, J. J., Pena, A. S., van Leeuwen, A., van Hooff, J. P. & va Rood, J. J. HLA-DW3 associated with coeliac disease. Lancet 1, 506–508 (1976).
Stepniak, D. & Koning, F. Celiac disease—sandwiched between innate and adaptive immunity. Hum. Immunol. 67, 460–468 (2006).
Ju, J. M., Marietta, E. V. & Murray, J. A. Generating transgenic mouse models for studying Celiac disease. Methods Mol. Biol. 1326, 23–33 (2015).
Yokoyama, S., Takada, K., Hirasawa, M., Perera, L. P. & Hiroi, T. Transgenic mice that overexpress human IL-15 in enterocytes recapitulate both B and T cell-mediated pathologic manifestations of celiac disease. J. Clin. Immunol. 31, 1038–1044 (2011).
Bouziat, R. et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 356, 44–50 (2017).
Cellier, C. et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet 356, 203–208 (2000).
Mention, J. J. et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 125, 730–745 (2003).
Di Sabatino, A. et al. Epithelium derived interleukin 15 regulates intraepithelial lymphocyte Th1 cytokine production, cytotoxicity, and survival in coeliac disease. Gut 55, 469–477 (2006).
Maiuri, L. et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology 119, 996–1006 (2000).
Eksteen, B. et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9 + gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 200, 1511–1517 (2004).
Seidel, D. et al. CD8 T cells primed in the gut-associated lymphoid tissue induce immune-mediated cholangitis in mice. Hepatology 59, 601–611 (2014).
Ali, A. H., Carey, E. J. & Lindor, K. D. Current research on the treatment of primary sclerosing cholangitis. Intractable Rare Dis. Res. 4, 1–6 (2015).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Petrovic, A. et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood 103, 1542–1547 (2004).
Murai, M. et al. Peyer’s patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat. Immunol. 4, 154–160 (2003).
Lee, Y. K., Menezes, J. S., Umesaki, Y. & Mazmanian, S. K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108(Suppl 1), 4615–4622 (2011).
Yang, X. D. et al. A predominant role of integrin alpha 4 in the spontaneous development of autoimmune diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 91, 12604–12608 (1994).
Hanninen, A., Salmi, M., Simell, O. & Jalkanen, S. Mucosa-associated (beta 7-integrinhigh) lymphocytes accumulate early in the pancreas of NOD mice and show aberrant recirculation behavior. Diabetes 45, 1173–1180 (1996).
Yang, X. D., Sytwu, H. K., McDevitt, H. O. & Michie, S. A. Involvement of beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the development of diabetes in obese diabetic mice. Diabetes 46, 1542–1547 (1997).
Yu, H. et al. Intestinal type 1 regulatory T cells migrate to periphery to suppress diabetogenic T cells and prevent diabetes development. Proc. Natl. Acad. Sci. USA 114, 10443–10448 (2017).
Marino, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Hebbandi Nanjundappa, R. et al. A gut microbial mimic that hijacks diabetogenic autoreactivity to suppress colitis. Cell 171, 655–667 e617 (2017).
Wu, S. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016–1022 (2009).
Erdman, S. E. & Poutahidis, T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol. Pathol. 38, 76–87 (2010).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Zitvogel, L., Ma, Y., Raoult, D., Kroemer, G. & Gajewski, T. F. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 359, 1366–1370 (2018).
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2018YFA0508000) (S.Z.); the National Natural Science Foundation of China (81822021, 91842105, 31770990, 81788101 and 81821001) (S.Z.), and (81871284) (H.M.); and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29030101) (S.Z.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Ma, H., Tao, W. & Zhu, S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol 16, 216–224 (2019). https://doi.org/10.1038/s41423-019-0208-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0208-2
Keywords
This article is cited by
-
Immunity to Cryptosporidium: insights into principles of enteric responses to infection
Nature Reviews Immunology (2024)
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
Single-cell map of dynamic cellular microenvironment of radiation-induced intestinal injury
Communications Biology (2023)
-
Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment
Chinese Medicine (2022)
-
Tissue engineering of the gastrointestinal tract: the historic path to translation
Journal of Biological Engineering (2022)