Abstract
Myelodysplastic syndromes (MDS) are a heterogeneous group of pre-leukemic hematopoietic disorders characterized by cytopenia in peripheral blood due to ineffective hematopoiesis and normo- or hypercellularity and morphologic dysplasia in bone marrow (BM). An inflammatory BM microenvironment and programmed cell death of hematopoietic stem/progenitor cells (HSPCs) are thought to be the major causes of ineffective hematopoiesis in MDS. Pyroptosis, apoptosis and necroptosis (collectively, PANoptosis) are observed in BM tissues of MDS patients, suggesting an important role of PANoptosis in MDS pathogenesis. Caspase 8 (Casp8) is a master regulator of PANoptosis, which is downregulated in HSPCs from most MDS patients and abnormally spliced in HSPCs from MDS patients with SRSF2 mutation. To study the role of PANoptosis in hematopoiesis, we generated inducible Casp8 knockout mice (Casp8−/−). Mx1-Cre-Casp8−/− mice died of BM failure within 10 days of polyI:C injections due to depletion of HSPCs. Rosa-ERT2Cre-Casp8−/− mice are healthy without significant changes in BM hematopoiesis within the first 1.5 months after Casp8 deletion. Such mice developed BM failure upon infection or low dose polyI:C/LPS injections due to the hypersensitivity of Casp8−/− HSPCs to infection or inflammation-induced necroptosis which can be prevented by Ripk3 deletion. However, impaired self-renewal capacity of Casp8−/− HSPCs cannot be rescued by Ripk3 deletion due to activation of Ripk1-Tbk1 signaling. Most importantly, mice transplanted with Casp8−/− BM cells developed MDS-like disease within 4 months of transplantation as demonstrated by anemia, thrombocytopenia and myelodysplasia. Our study suggests an essential role for a balance in Casp8, Ripk3-Mlkl and Ripk1-Tbk1 activities in the regulation of survival and self-renewal of HSPCs, the disruption of which induces inflammation and BM failure, resulting in MDS-like disease.

Similar content being viewed by others
Facts
-
Casp8−/− mice develop BM failure under infectious and inflammatory conditions, which can be prevented by Ripk3 deletion;
-
Casp8−/− HSCs exhibit impaired self-renewal which cannot be rescued by Ripk3 deletion;
-
Mice that received Casp8−/− BM cells developed MDS like-disease at 4 months post-transplantation.
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of pre-leukemic hematopoietic disorders characterized by persistent cytopenia in peripheral blood (PB) and normo- or hypercellularity and morphologic dysplasia in BM. Ineffective hematopoiesis has been identified as the mechanism leading to the cytopenia, while increased genetic instability and the accumulation of additional mutations are now known to be the mechanism explaining the elevated risk for leukemic transformation in MDS patients [1, 2]. An inflammatory BM micro-environment and programmed cell death (PCD) of hematopoietic stem/progenitor cells (HSPCs) have been thought to be the major causes leading to ineffective hematopoiesis [3,4,5,6]. Three types of PCD, apoptosis, necroptosis and pyroptosis were observed in BM tissues of MDS patients, suggesting PANoptosis [7,8,9]. Caspase 8 (Casp8) is a master regulator of PANoptosis, which is downregulated or abnormally spliced in HSPCs from patients with MDS [4, 10].
Casp8 was first identified as a key mediator of the extrinsic apoptotic pathway triggered by death receptors including Fas, Trail-receptors, tumor necrosis factor receptor (TNFR), and DR3 [11], as well as Toll-like receptors (TLRs) [12]. Upon ligand binding, death receptors and TLRs induce the activation of Casp8-apoptosis by triggering the formation of a death-inducing signaling complex (Fadd/pro-Casp8) for Fas and Trail-receptors [13, 14], or a complex IIa (Ripk1/Fadd/pro-Casp8) for TNFR1, DR3 and TLRs [12, 13, 15]. Under Casp8-inactive conditions, death receptors and TLRs induce necroptosis by triggering the formation of a necrosome or complex IIb (Ripk3/Ripk1/Fadd/pro-Casp8) [16]. Recent studies demonstrated that pro-Casp8 also interacts with ASC (the adaptor molecule, apoptosis-associated speck-like protein containing a CARD) to regulate Nlpr3-inflammasome activity and pyroptosis [17,18,19]. Detailed studies demonstrated that TLR signaling actually induces a much bigger complex called a PANoptosome which contains almost all the key components of the above three complexes [7, 20]. This complex of proteins mediates PANoptosis. Tak1 (TGF-β-activated kinase 1) [9, 21, 22], Zbp1 (Z-DNA binding protein 1) [9, 23], Ripk1 [24] and IRF1 [25] are the master regulators of PANoptosis, while Casp8 plays a key molecular role in regulating the switching among the three types of PCD [26, 27].
The aspartate-specific proteolytic activity of pro-Casp8 is normally inhibited by its N-terminal domains and other regulatory proteins. Activation of such activity allows Casp8 to cleave and activate both itself and its substrates. Activated Casp8 triggers mitochondria-independent apoptosis by directly activating the executioner Caspases including Casps3 and 7 or, in certain circumstances, induces mitochondrial-dependent apoptosis by cleaving Bid to produce pro-apoptotic tBid [28, 29]. Activated Casp8 also forms a noncanonical inflammasome with Card9/Bcl10/MALT1/ASC or Ripk1/ASC/Nlrp3 to cleave IL-1β, IL-18 and GSDMD for IL-1β/IL-18 production and pyroptosis [30, 31]. However, activated Casp8 restricts TNFα and TLR-induced Ripk3/Mlkl-mediated necroptosis by cleaving and degrading Ripk1 and Ripk3 and likely Mlkl as well [32,33,34]. In addition, Casp8 can also induce cell death through its catalytic-independent scaffolding activity. For example, Casp8 interacts with ASC and activates canonical Nlpr3-Casp1/11-mediated IL-1β production and pyroptosis [26, 35, 36]. Inhibition of Casp8 activity represses apoptosis but enhances necroptosis, while activation of Casp8 represses necroptosis but enhances apoptosis. The role of Casp8 in the regulation of pyroptosis is cell context-dependent [22, 26, 27, 37, 38].
In addition to regulating PCD, Casp8 is also involved in the regulation of cell proliferation/mitosis, differentiation, cytokine production and DNA damage repair. It was found that during mitosis, Ripk1/Casp8/Ripk3 form a mitotic ripoptosome complex together with Polo-like kinase 1 (Plk1). Casp8 regulates the faithful segregation of chromosomes and stability by cleaving Plk1. Inhibition of Ripk1 or Casp8 leads to chromosomal instability [39]. During radiation or chemical-induced DNA damage, Casp8 also forms a DNA damage sensor complex with Fadd/c-FLIP/Ripk1, orchestrating replication-associated DNA damage and H2AX phosphorylation [40]. DNA damage-triggered activation of nuclear Casp8 also overcomes the p53-dependent G2/M checkpoint through the cleavage of USP28, leading to drug-resistance [41]. All these studies suggested that Casp8 is essential for maintaining chromosomal stability and DNA damage repair, independent of its roles in cell death and its inflammatory function [42]. In addition, in responding to TNFα and TLR3/4 stimulation, Casp8 cleaves N4BP1 (NEDD4-binding protein 1), a negative regulator of genes encoding inflammatory cytokines, thus promoting the expression of cytokines, including IL-6, G-CSF and TNFα as well as chemokines such as CXCL1 and CCL3 [43]. Casp8 also regulates inflammatory cytokine production by activating inflammasomes in catalytic activity-dependent or independent processes [26, 27, 44].
To study the role of Casp8 in adult hematopoiesis, Kang et al. analyzed Mx1Cre-mediated Casp8 knockout (Casp8−/−) mice and reported that although there are no changes in the number of phenotypic HSPCs, functional HSPCs are significantly reduced as assessed by their ability to form myeloid or B lymphoid colonies as well as repopulating BM and lymphoid organs [45]. However, all these analyses were conducted shortly after polyI:C injections, and thus cannot distinguish Casp8-intrinsic effects from polyI:C- induced effects. In this study, we comparatively studied the hematopoietic phenotypes and HSPC functions between Mx1Cre+Casp8fx/fx and Rosa26-ERTCre+Casp8fx/fx mice as well as Mx1Cre+Casp8fx/fxRipk3−/− and Rosa26-ERTCre+Casp8fx/fxRipk3−/− mice. We found that Mx1Cre+Casp8fx/fx mice developed severe BM failure after polyI:C-induced Casp8 deletion due to the massive necroptosis of HSPCs. This could be prevented by Ripk3 deletion. Interestingly, the hematopoiesis of Rosa26-ERTCre+Casp8fx/fx mice 1.5 month after tamoxifen (TAM)-induced Casp8 deletion is comparable to that in wild-type (WT) mice. However, such mice developed MDS-like diseases 4 months after Casp8 deletion. Our study demonstrates a critical role for Casp8 in preventing infection and inflammation-induced Ripk3-mediated necroptosis in HSPCs. In addition, Casp8 also regulates HSC self-renewal by restricting Ripk1-Tbk1-mediated Type I interferon (IFN) production.
Results
Basal level activation of Casp8 limits Ripk1-Ripk3-Mlkl signaling in c-Kit+ HSPCs
Casp8 expression is higher in c-Kit+ HSPCs than that in c-Kit- BM hematopoietic cells (HCs) as shown by both RT-PCR (Fig. 1a) and Western blotting (Fig. 1b) assays. Within BM cells, granulocytes and monocytes express relatively low Casp8 (Fig. 1a). Interestingly, basal levels of Casp8 activity were detected in c-Kit+ HSPCs but not in c-Kit- HCs as demonstrated by increased number of truncated forms of Casp8 and flow cytometric analysis of active Casp8 staining (Fig. 1b, c). In addition, increased levels of truncated forms of Ripk1, Ripk3 and Mlkl were detected in c-Kit+ HSPCs (Fig. 1d). Furthermore, we found that the truncated forms of Ripk1, Ripk3 and Mlkl in c-Kit+ HSPCs can be prevented by Casp8 knockout (Fig. 1e). All these data suggest that during normal homeostatic conditions, c-Kit+ HSPCs have a basal level activation of Casp8 which restricts Ripk1-Ripk3-Mlkl signaling in c-Kit+ HSPCs by cleaving Ripk1, Ripk3 and Mlkl. However, the cleavage of Casp3, Casp7, Casp1, and Casp11 as well as the substrates PARP1 and GSDMD was not detected in c-Kit+ HSPCs, suggesting that such levels of Casp8 activation are insufficient to activate apoptosis or pyroptosis (Fig. S1).
a HSCs (LSK-CD135-CD150+CD48-), MPP1s (LSK-CD135-CD150-CD48-), MPP2/3s (LSK-CD135-CD150+/CD48+), MPP4s (LSK-CD135+CD150-CD48+/low), CMPs (LK-CD16/32-CD34+), GMPs (LK-CD16/32+CD34+), MEPs (LK-CD16/32-CD34−), CLPs (Lineage−c-KitlowSca1lowCD127+), granulocytes (CD11b+Ly6G+), monocytes (CD11b+Ly6C+), B lymphocytes (B220+) and T lymphocytes (CD3+) were purified from C57Bl6/J mice by FACS. Casp8 expression in all populations was examined by RT-PCR and normalized to the level in HSCs. Triplicate experiments were conducted. Data represent one of the two experiments performed in triplicate. **p < 0.01 compared to HSCs. b Basal levels of Casp8 activation were detected in c-Kit+ HSPCs but not in c-Kit- HCs as shown by Western blotting. c Active-Casp8 levels were examined and compared among LSK HSPCs and c-Kit- HCs from WT mice by flow cytometry. MFI (mean fluorescence intensity) ratio was counted from 3 independent experiments and normalized to gene knockout controls. **p < 0.01 compared to WT HCs. BM LSK HSPCs isolated from Casp8−/− mice were studied in parallel as controls. Levels of Ripk1, Ripk3, and Mlkl were compared between c-Kit+ HSPCs and c-Kit- HCs from WT mice (d) and between c-Kit+ HSPCs isolated from WT and Casp8−/− mice (e) by Western blotting. Data in b, d, e represent one example from the three independent experiments.
Mx1Cre+Casp8fx/fx mice developed BM failure after polyI:C-induced Casp8 deletion, which can be prevented by Ripk3 deletion
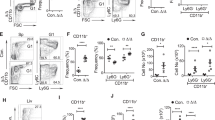
We have reported that Ripk3 signaling is not required for homeostatic hematopoiesis [46]. To study the role of Casp8 in the regulation of normal hematopoiesis, we generated Mx1Cre+Casp8fx/fx, Mx1Cre+Casp8fx/+, Casp8fx/fx (WT) and Mx1Cre+Casp8fx/fxRipk3−/− mice. At 8 weeks of age, all mice were administered polyI:C every other day for a total of three injections. Eight to ten days after these polyI:C injections, all Mx1Cre+Casp8fx/fx mice became frail as shown by hunched backs and reduced mobility, and died within 12 days, while Mx1Cre+Casp8fx/+, WT and Mx1Cre+Casp8fx/fxRipk3−/− mice were observed to be generally normal (Fig. S2). All Mx1Cre+Casp8fx/fx mice developed severe BM failure as demonstrated by a reduction of white blood cells (WBC), red blood cells (RBCs) and platelets in PB (Fig. 2a), as well as hypocellularity and a reduction in mononuclear cells (MNCs) in BM (Fig. 2b, c). Interestingly, when analyzed 6 days after the last polyI:C injection, we found that c-kit+ HSPCs in Mx1Cre+Casp8fx/fx mice had almost completely been eliminated from BM (Fig. 2d, e), while c-Kit- HC number was only modestly reduced (Fig. 2f). Compared to c-Kit- HCs, Annexin-V staining showed significantly increased cell death in c-Kit+ HSPCs in Mx1Cre+Casp8fx/fx mice, suggesting a critical role for Casp8 in the survival of HSPCs (Fig. 2g). However, all hematopoietic parameters in Mx1Cre+Casp8fx/+ mice were comparable to those in WT mice, since one allele of Casp8 is sufficient to maintain the level of Casp8 expression (Fig. S3) and hence the survival of HSPCs. In addition, all hematopoietic parameters in Mx1Cre+Casp8fx/fxRipk3−/− mice were also comparable to those in WT mice, indicating that the HSPCs in Mx1Cre+Casp8fx/fx mice died from Ripk3-mediated necroptosis (Fig. 2a–g). This notion was further supported by in vitro studies where induction of Casp8 deletion by infection of Casp8fx/fx HSPCs with Cre-expressing virus also caused massive cell death. Such cell death could be largely prevented by treatment with the Ripk1-inhibitor Necrostatin-1 or the Ripk3 inhibitor GSK872 (Fig. 2h).
a–f WT, MxCre+Casp8fx/+, MxCre+Casp8fx/fx and MxCre+Casp8fx/fxRipk3−/− mice were injected with 5 μg/g body weight polyI:C every other day for a total of three injections. PB and BM were collected from another batch of mice (6 for each genotype) 8 days after the last polyI:C injection. WBCs, RBCs, HB and platelets in PB were examined by a Hemavet 950FS (a); BM cellularity was compared by histology (b) and MNC counts (c), percentages and numbers of c-Kit+ HSPCs (d, e) and LSK HSPCs (d, f), as well as cKit- HCs, were analyzed by flow cytometry. g BM MNCs were collected two days after a single polyI:C injection (5 μg/g body weight). Death of cKit+ HSPCs and cKit- HCs was examined by Annexin-V staining. h cKit+ HSPCs were collected from Casp8fx/fx mice and infected with either Cherry-expressing virus or Cherry/Cre-expressing virus. The infected cells were then cultured in HSPC culture medium (RPMI 1640 supplied with 10% FBS, 100 ng/ml SCF, 50 ng/ml TPO, 10 ng/ml IL3 and 25 ng/ml IL6) with or without 30 μM Nec-s or 3 μM GSK’872 with medium change every other day. The percentages of Cherry+ cells were examined by FACS on the indicated days. Triplicate experiments were conducted. In (a–g). **p < 0.01 compared to other groups.
Rosa26-ERTCre+Casp8fx/fx mice exhibited normal hematopoiesis after TAM-induced Casp8 deletion
Because either polyI:C or viral infection can induce necroptosis when Casp8 activity is inhibited, there are two possible mechanisms to explain the BM failure phenotype observed in Mx1Cre+Casp8fx/fx mice: either Casp8−/− HSPCs undergo intrinsic necroptosis, or they are sensitive to polyI:C or viral infection-induced necroptosis. To distinguish between these two possible mechanisms, we generated Rosa26-ERTCre+Casp8fx/fx and WT mice. At 8 weeks of age, all mice were injected with TAM daily for 5 days to induce Casp8 deletion. Complete deletion of Casp8 in BM HSPCs and MNCs was confirmed by both PCR and Western blot assays in Rosa26-ERTCre+Casp8fx/fx mice post-TAM injection (Casp8−/− hereafter) (Fig. 3a, b). In contrast to Mx1Cre+Casp8fx/fx mice which died from BM failure after polyI:C induction, Casp8−/− mice are generally normal for two months after TAM injections. Detailed analysis of HCs, including HSPCs (including HSCs, MPP1s, MPP2/3s, MPP4s), and myeloid progenitors (including CMPs, GMPs and MEPs) in BM (Fig. S4), as well as blood cells in PB at 1.5 months post-TAM injections, demonstrated that all hematopoietic parameters in the BM of Casp8−/− mice were comparable to those of WT mice (Fig. 3c–h), suggesting that Casp8 is not essential for the survival of HSPCs during homeostasis. Functional studies demonstrated that BM cells from Casp8−/− mice displayed a reduced CFU capacity during serial replating (Fig. 3i) and reduced competitive hematopoietic repopulation capacity (CHRC) over serial transplantation when compared to BM cells from WT mice (Fig. 3j). Numbers of HSPCs were also reduced during serial transplantation (Fig. 3k), suggesting that Casp8 is required for maintaining the colony-forming capacity of HSPCs and the CHRC of HSCs under stress.
a–e WT and Rosa-CreERT+Casp8fx/fx mice were injected with 100 μg/g body weight TAM every day for 5 consecutive days to induce Casp8 deletion. BM MNCs were collected 30 days after the last TAM injection and efficient Casp8 deletion was verified by PCR (a) and Western blotting (b). PB, spleens and BM were collected from WT and Casp8−/− mice 45 days after the last TAM injection; WBCs, RBCs, HB and platelets in PB were examined by a Hemavet 950FS (c); spleens and BM cellularity were compared by MNC counts (d, e); percentages of LSK HSPCs and LK MPs (f), percentages of CMP, GMP and MEP (g), and percentages of HSCs, MPP1s, MPP2/3s and MPP4s (h) in BM of WT and Casp8−/− mice were analyzed by FACS. i–k The colony-forming ability of BM MNCs from WT and Casp8−/− mice were compared by serial replating (i); the CHRC of WT and Casp8−/− HSCs were assessed by serial transplantation (j); the percentages of donor LK MPs and LSK HSPCs from the first transplantation recipient mice were examined 10 wks. post-transplantation (k) and represent one of the three independent experiments performed in triplicate. Data in j, k are a summary of 6 mice per group. **p < 0.01 compared to WT.
HSPCs in Casp8−/− mice are more susceptible to infection and polyI:C/LPS-induced cell death
During our experiments, we transferred a batch of Casp8−/− and WT mice (five days post-TAM injections) between facilities, after having been first maintained in a quarantine room. We found that all Casp8−/− mice became sick and developed BM failure within 6-12 days of observation, characterized by pancytopenia (Fig. 4a) and reduced BM cellularity (Fig. 4b) due to significant reduction of HSPCs (Fig. 4c). All WT mice at this point were normal (Fig. 4a–c). We speculated that these mice might have bacterial infections, so we treated them with the antibiotic Baytril for 20 days. All the Casp8−/− mice in the untreated group died of BM failure, while the mice that received Baytril survived and were healthy. Hematopoietic parameters were almost completely restored in the group given Baytril (Fig. 4a–c).
a–c A batch of WT and Casp8−/− mice (5 days post last TAM injection) was transferred between facilities. Unfortunately, these mice became infected with gram-negative bacteria. All Casp8−/− mice were frail, as shown by hunched backs and reduced mobility, whereas WT mice were generally normal. Half of the mice were randomly selected and given drinking water containing the antibiotic Baytril (enrofloxacin, Bay, 0.16 mg/ml) for twelve days. The PB and BM were collected from all groups of mice. WBCs, RBCs, HB and platelets in PB were examined using a Hemavet 950FS (a); BM cellularity was compared by MNC counts (b); percentages of LSK HSPCs and LK MPs were analyzed by FACS (c). d, e. cKit+ HSPCs were isolated from WT and Casp8−/− mice and cultured in HSPC culture medium with or without LPS (100 ng/ml), polyI:C (10 μg/ml), TNFα (20 ng/ml), IFNγ (20 ng/ml) or IFNα (20 ng/ml) treatment. Cell death was examined by Annexin-V staining 24 h. after culture (d); or seeded into methylcellulose medium for colony-forming unit assay with or without LPS (100 ng/ml), polyI:C (10 μg/ml), TNFα (20 ng/ml), IFNγ (20 ng/ml) or IFNα (20 ng/ml) treatment. CFU were counted after 8 days of culturing (e). f–h WT and Casp8−/− mice were injected with vehicle, polyI:C (1 μg/g every other day), or LPS (0.2 μg/g every other day) for 16 days. PB and BM were collected five days after the last injection. WBCs, RBCs, HB and platelets in PB were analyzed using a Hemavet 950FS (f); BM cellularity was compared by MNC counts (g); percentages of c-Kit+ HSPCs in BM were examined by flow cytometry (h). Data in a–c and f–h are a summary of 5–6 mice in each group. Data in d, e represent one of the three independent experiments performed in triplicate. * and **p < 0.05 and <0.01, respectively compared to other groups.
To assess whether the BM failure that developed in Casp8−/− mice was due to the propensity of Casp8−/− HSPCs to suffer infections or inflammation-induced cell death, we compared the responses of Casp8−/− and WT HSPCs to low-dose TLR agonists or inflammatory cytokines. We found that polyI:C, LPS, IFNα, IFNγ or TNFα treatment kills significantly more Casp8−/− HSPCs than WT HSPCs (Fig. 4d). In addition, CFU assays demonstrated that polyI:C, LPS, IFNα, IFNγ or TNFα treatment significantly inhibited the colony-forming ability of Casp8−/− HSPCs but had fewer such effects on WT HSPCs (Fig. 4e). Furthermore, low dose polyI:C or LPS injections induced pancytopenia, BM failure and loss of HSPCs in Casp8−/− mice but did not do so in WT mice (Fig. 4f–h).
Ripk3 deletion prevents polyI:C-induced BM failure in Casp8−/− mice
To study whether TLR agonists or inflammatory cytokines kill Casp8−/− HSPCs through inducing Ripk3-mediated necroptosis, we generated Rosa26-ERTCre+Casp8fx/fxRipk3−/− mice. After TAM induction to induce Casp8 deletion to generate compound-knockout mice (Casp8−/−Ripk3−/−), we found that Casp8−/−Ripk3−/− mice were healthy and all hematopoietic parameters in such mice were comparable to those of WT mice (Fig. 5a-c). In addition, distinct from Casp8−/− mice, Casp8−/−Ripk3−/− mice were resistant to polyI:C-induced BM failure. All hematopoietic parameters in Casp8−/−Ripk3−/− mice were comparable to those in WT mice (Fig. 5a-c). In vitro culture studies demonstrated that, in response to polyI:C, LPS, IFNα, IFNγ or TNFα treatment, the death rate of Casp8−/−Ripk3−/− HSPCs was significantly lower than that of WT and Ripk3−/− HSPCs (Fig. 5d). In addition, polyI:C, LPS, IFNα, IFNγ or TNFα treatment significantly inhibited the colony-forming capacity of WT and Ripk3−/− HSPCs but had significantly reduced effects on Casp8−/−Ripk3−/− HSPCs (Fig. 5e). This suggests that Casp8−/− HSPCs primarily die of necroptosis. This idea is further supported by Mlkl inhibition. GW806742X is a Mlkl inhibitor [47]. We found that the polyI:C, LPS, IFNα, IFNγ or TNFα treatment-induced death of Casp8−/− HSPCs can also be prevented by GW806742X pre-treatment (Fig. S5).
a–c Batches of WT and Casp8−/−Ripk3−/− mice (10 days post last TAM injection) were injected with vehicle or polyI:C (1 ng/ml every other day) for 16 days; PB and BM were collected 5 days after the last injection. WBCs, RBCs, HB and platelets in PB were determined by a Hemavet 950FS (a), BM cellularity was compared by MNC counts (b); percentages of LK MPs and LSK HSPCs in BM were examined by flow cytometry (c). d, e cKit+ HSPCs were isolated from WT, Ripk3−/− and Casp8−/−Ripk3−/− mice and cultured in HSPC culture medium with or without LPS (300 ng/ml), polyI:C (50 μg/ml), TNFα (50 ng/ml), IFNγ (50 ng/ml) or IFNα (50 ng/ml) treatment. Cell death was examined by Annexin-V staining 24 h. after culturing (d); or seeded into methylcellulose medium for colony-forming unit forming assay with or without LPS (300 ng/ml), polyI:C (50 μg/ml), TNFα (50 ng/ml), IFNγ (50 ng/ml) or IFNα (50 ng/ml) treatment; CFU were counted on day 8 of culturing (e). Data in a–c are summary of 6 mice in each group. Data in d, e represent one of the three independent experiments performed in triplicates. * and **p < 0.05 and <0.01, respectively, compared to other groups.
Ripk3 deletion fails to fully restore the CHRC of Casp8−/− HSCs
We have reported that Ripk3 is not required for normal homeostatic hematopoiesis but does regulate HSC self-renewal during stress conditions such as serial transplantation [46]. Analysis of phenotypic HSPCs in the BM of WT, Ripk3−/− and Casp8−/−Ripk3−/− mice showed a significant increase in the LSK cell population in Casp8−/−Ripk3−/− mice compared to WT and Ripk3−/− mice (Fig. 5c). Detailed analysis suggested that the numbers of HSCs and MPP2/3 cells were increased in Casp8−/−Ripk3−/− mice compared to WT and Ripk3−/− mice, while the MPP1, MPP4, CMP, MEP and GMP populations were comparable to WT and Ripk3−/− mice (Fig. 6a, b). Consistently, we found that the colony-forming capacity of Casp8−/−Ripk3−/− BM cells was also comparable to that observed in both WT and Ripk3−/− mice (Fig. 6c). To study whether Ripk3 deletion can restore the self-renewal capacity of Casp8−/− HSCs, we compared the CHRC of WT, Casp8−/−, Ripk3−/− and Casp8−/−Ripk3−/− HSCs using serial transplantation assays. We found that, consistent with our previous reports, Rikp3−/− HSCs display improved CHRC compared to WT HSCs during serial transplantation [46]; however, the CHRC of Casp8−/−Ripk3−/− HSCs is reduced compared to WT HSCs but is significantly improved compared to that of Casp8−/− HSCs (Fig. 6d). Analyzing donor HSCs in recipient mice receiving a 3rd transplantation, we found that significantly fewer donor HSCs were maintained in the Casp8−/−Ripk3−/− transplantation compared to WT and Ripk3−/− transplantations (Fig. 6e). It was known that inflammatory cytokines impair HSC self-renewal and function. To study whether impaired HSC function in Casp8−/−Ripk3−/− mice is due to elevation of inflammatory cytokines, we compared the levels of BM inflammatory cytokines among WT, Casp8−/−, Ripk3−/− and Casp8−/−Ripk3−/−mice. We found an increased IFNβ and reduced IL1β levels in BM of Casp8−/−Ripk3−/− mice compared to other genotypes of mice (Figs. 6f and S6a), while the levels of all other cytokines were comparable among all genotypes of mice (Fig. S6b). A recent study suggested that Casp8−/− HCs produce significantly more Type-I IFN when Ripk3 is deleted due to Ripk1-mediated Tbk1 activation [48]. Type-I IFN impairs self-renewal of HSCs via the activation of Stat1 signaling and increased mitochondrial ROS [49, 50]. We speculated that the impaired CHRC of Casp8−/−Ripk3−/− HSCs might be due to the increased production of Type-I IFN. To test such a hypothesis, we examined the activities of Tbk1 and Stat1, mitochondrial Ros (mitoROS) levels as well as the expression of Type-I IFN-stimulated genes (ISG), Oas2, Irf7 and Ifnb in HSCs. We found that Tbk1 and Stat1 activities are elevated in Casp8−/−Ripk3−/− HSPCs compared to WT, Casp8−/− and Ripk3−/− HSPCs which are associated with increased mitoROS levels (Fig. 6g). We also demonstrated an increased expression of Oas2, Irf7 and Ifnb in Casp8−/−Ripk3−/− HSPCs compared to WT and Ripk3−/− HPSCs (Fig. 6h). Importantly, Ripk1 or Tbk1 inhibition could suppress Stat1 activity, mitoROS levels and the expression Oas2, Irf7 and Ifnb genes in Casp8−/−Ripk3−/− HSPCs (Fig. 6i, j). However, Mlkl inhibition failed to do so in Casp8−/−Ripk3−/− HSPCs (Fig. S7). This suggests that the elevated Tbk1-Ifnb signaling and mitochondrial ROS levels in Casp8−/−Ripk3−/− HSPCs are due to the activation of Ripk1 kinase and are independent of Mlkl.
a–g Thirty days after the last TAM injection, BM cells were collected from WT, Ripk3−/− and Casp8−/−Ripk3−/− mice and the % of CMPs, GMPs and MEPs (a) as well as HSCs, MPP1s, MPP2/3s and MPP4s (b) were assessed by flow cytometry. Colony-forming abilities of BM cells were compared by CFU assay (c); CHRC was compared by serial transplantation assay and BM cells from Casp8−/− were studied in parallel as controls (d); the % of donor HSC contributions in BM of 3rd round recipient mice were examined by flow cytometry (e). Type I IFN concentrations in the BM and sera of WT, Ripk3−/− and Casp8−/−Ripk3−/− mice were compared using LEGENDplex™ assay (f). cKit+ HSPCs were isolated from WT, Ripk3−/−, Casp8−/− and Casp8−/−Ripk3−/− mice, pStat1, pTbk1, mito-ROS levels were examined by intracellular antibody or MitoSOX staining (g). cKit+ HSPCs were collected from WT, Ripk3−/− and Casp8−/−Ripk3−/− mice and mRNA expression of the ISG genes was examined by RT-PCR and normalized to the levels in WT HSPCs (h). i, j WT and Casp8−/−Ripk3−/− mice were treated with vehicle, 2 μg/g Nes-1s (Ripk1 inhibitor), or 5 μg/g GSK8612 (Tbk1 inhibitor) for 2 days. cKit+ HSPCs were isolated from the mice 12 h. post-treatment; pStat1 and mito-ROS levels were examined by intracellular antibody or MitoSOX staining (i); the expression of ISG genes was examined by RT-PCR assay (j). Data in a–f are a summary of 6 mice in each group. Data in h–j represents one of the three independent experiments performed in triplicate. * and **p < 0.05 and <0.01, respectively, compared to other groups. MFI ratio in g and i was counted from 3 independent experiments and normalized to WT controls.
Mice transplanted with Casp8−/− BM developed MDS-like disease within 4 months
We found that some of the Casp8−/− mice developed MDS-like disease 4 months after the induction of Casp8 deletion as shown by anemia and thrombocytopenia with morphologic dysplasia of blood cells. Our Casp8−/− mice are whole body knockouts. Therefore, to study the role of Casp8 specifically in hematopoiesis, we transplanted Casp8−/− BM cells into lethally-irradiated recipient mice. Four months after transplantation, all recipient mice were terminated in order to perform hematopoietic analyses. We found that all 10 mice which had received WT BM cells were healthy with normal blood cell counts. However, 10/10 mice which had received Casp8−/− BM cells developed a certain degree of anemia, 8/10 exhibited reduced platelet numbers and 4/10 displayed splenomegaly with increased WBC counts (Fig. 7a). BM cellularity in mice which had received Casp8−/− BM cells was comparable to that in mice which had received WT BM cells (Fig. 7b). Hypersegmented neutrophils and polychromatic erythrocytes were observed in PB and BM of mice in the Casp8−/− group suggesting dysplastic myeloid cell and red blood cell morphologies (Fig. 7c). The elevated granulocyte and monocyte counts but reduced lymphocyte counts in PB suggested myeloid-biased hematopoiesis (Fig. S8a). Detailed analysis of HSPCs in BM demonstrated a myeloid-biased and accelerated aging-related hematopoietic phenotype of mice which had received Casp8−/− BM cells, as demonstrated by increased percentages of phenotypic HSC and GMP populations but reduced MPP1, MPP4, MEP and CLP populations (Fig. 7d-f). Increased death of LK and LSK HSPCs in Casp8−/− BM cells suggested an ineffective hematopoiesis that is commonly observed in MDS BM (Fig. 7g). In addition, MPP differentiation of HSCs isolated from Casp8−/− BM was significantly reduced compared to HSCs isolated from WT BM, suggesting an age associated HSC phenotype (Fig. 7h). The elevated Tnfα, IL1β, IL6 and Ifnγ in PB suggested an inflammatory reaction-associated pathogenesis (Fig. 7i). All these observations suggested an MDS-like disease in mice that had received Casp8−/− BM cells. Such hematopoietic alterations were not due to the development of autoimmune lymphoproliferative syndrome (ALPS)-like disease because none of the mice displayed autoimmune-related features at the time of analysis, as shown by negativity for autoimmune antibodies (anti-nuclear antibody and anti-dsDNA) (Fig. S8b) and normal levels of immunoglobin (Fig. S8c), as well as negativity for CD4−CD8−CD3+B220+ double-negative T-cells (Fig. S8d).
Lethally-irradiated mice were transplanted with BM cells from either WT mice or Casp8−/− mice, with each mouse receiving 2×106 BM cells. All mice were terminated for analysis 4 months after transplantation. WBCs, RBCs, HB and platelet numbers in PB were determined by a Hemavet 950FS (a), BM cellularity was compared by histology analysis of BM sections and MNC counts (b); dysplasia of blood cells was indicated by Wright’s Giemsa staining of PB smears. Hypersegmented neutrophils (arrowhead) and polychromatic erythrocytes (arrow) were present. (c); percentages of LK population (including CMP, GMP, and MEP) (d), CLP (e) and LSK HSPCs (including HSCs, MPP1, MPP2/3 and MPP4) (f) in BM were examined by flow cytometry. Cell death in LK and LSK HSPCs was examined by flow cytometry for Annexin-V staining (g). HSCs (LSK-CD150+CD135-CD48-) were purified from BM by FACS and cultured in Stemspan serum-free medium supplemented with SCF, TPO and Flt3L. Percentages of MPPs were analyzed after 48 hours of culturing (h). Levels of inflammatory cytokines including IFNβ, IFNγ, TNFα, IL1β and IL6 were measured using LEGENDplex™ assay (i).
Discussion
Infections, which are largely caused by bacteria or fungi, are major contributors to morbidity and mortality in MDS patients. Such infections exacerbate these patients’ clinical symptoms by further impairing the processes involved in hematopoiesis [51]. Inflammatory cytokines such as TNFα, IFNγ and IL1β, as well as other death receptor agonists including FasL and S100A9, are elevated in MDS. Increased incidence of all three types of PCD, apoptosis, necroptosis and pyroptosis of HSPCs, are commonly detected in BM tissues of MDS patients [7,8,9]. Casp8, the master regulator of these three types of PCD, is down-regulated in BM HCs of MDS patients, which is associated with significantly increased concentrations of Ripk1 protein and Mlkl phosphorylation [4]. Mutations of the splicing factor SRSF2 are commonly detected in MDS, which lead to mis-splicing of Casp8, resulting in truncated forms of catalytically-dead Casp8 enzymes [10]. All these clinical observations suggest that Casp8 might be involved in the pathogenesis of MDS. As is the case with MDS patients, we found that Casp8−/− mice are vulnerable to infections. The phenotype of our Casp8−/− mice resembles most of the clinical characteristics of MDS patients, including cytopenia and myelodysplasia at 4 months after induction of Casp8 deletion. By carefully comparing hematopoiesis among polyI:C and TAM-induced Casp8−/− and Casp8−/−Ripk3−/− mice, we uncovered a critical role for Casp8 in the regulation of HSPC survival and HSC self-renewal. Our study suggests that Casp8 is dispensable for homeostatic hematopoiesis in young adult mice but is essential in preventing Ripk3-mediated necroptosis during bacterial infections or inflammations. Casp8 also plays a critical role in regulating HSC function and self-renewal by restricting Ripk1-Tbk1-mediated Ifnβ production.
The role of Casp8 regulation of death-receptor signal-induced PCD has been well-documented. Casp8−/− mice are embryonically lethal due to the activation of Ripk3/Mlkl-mediated necroptosis in vascular endothelial cells [11, 52]. The significant reduction of HSPCs in the aorta–gonad–mesonephros (AGM) region and fetal livers of Casp8−/− embryos is most likely secondary to the vascular endothelial defects. The developmental defects observed in Casp8−/− mice can be completely rescued by deletion of Ripk3, suggesting a critical role for Casp8 in restricting Ripk3-mediated necroptosis during development. Casp8−/−Ripk3−/− mice develop ALPS within 4-5 months of age, resembling the phenotype of human ALPS, implicating a critical role for Casp8 in the restriction of autoimmune reactions [53, 54].
Studies of cell type-specific knockout mice suggested that Casp8 plays an essential role in regulating proliferation, mitosis/genetic stability, differentiation and cytokine production in different types of hematopoietic and immune cells. For example, in T cell-specific Casp8−/− mice, peripheral T cells are significantly reduced due to defects in proliferation stimulated by cytokines, T cell receptor mitogens, or antigen-induced activation and immune responses [55, 56]. Myeloid-specific Casp8−/− mice developed a mild systemic inflammation due to the increased numbers of Ly6Chigh and Ly6Clow splenic CD11b+F4/80+ macrophages [57]. Casp8−/− macrophages are hyperresponsive to TLR activation and exhibit aberrant M1 macrophage polarization [57]. Casp8−/− natural killer cells have defects in accumulating in response to viral infection [58]. Interestingly, all these defects can be prevented by Ripk3 deletion, suggesting a critical role for Casp8 signaling in restricting Ripk3-Mlkl-mediated necroptosis in these cell types [53, 57, 59, 60]. However, the defects in Casp8−/− B cells and dendritic cells (DCs) cannot be prevented by Ripk3 deletion. Casp8−/− B cells display defects in TLR antagonist-induced proliferation and T-independent Type I Ab responses [61,62,63]. Casp8−/− B cells also exhibit abnormal mitosis and impaired cytokinesis, which facilitate cellular transformation [64]. Approximately 50% of B cell-specific Casp8−/− mice developed lymphomas with elevated chromosomal instability within 60 weeks [42]. Casp8−/− DCs display a heightened costimulatory capacity and an elevated response to TLR signaling. DC-specific Casp8−/− mice develop a systemic autoimmune disease which can be abrogated by Ripk1 inhibition and Myd88 deletion but is exacerbated by Irf3 deletion [65].
We found that the inflammation/infection-induced HSPC necroptosis and BM failure in Casp8−/− mice can be completely prevented by Ripk3 deletion; however, the HSC self-renewal defects of Casp8−/− HSCs is mediated by Ripk1-Tbk1-Ifnβ signaling independent of Ripk3. In addition, the myelodysplastic phenotype observed in Casp8−/− mice is also independent of Ripk3 because it can also be observed in some Casp8−/−Ripk3−/− mice. We believe that such a myelodysplastic phenotype might be associated with the role of Casp8 in the regulation of mitosis, chromosomal stability and DNA damage repair [40, 42]. Future study needs to determine whether there are increased genetic mutations and chromosomal abnormalities in Casp8−/− HSPCs. In addition, Casp8 deletion or silencing has been reported in many types of solid cancers [66,67,68,69,70,71,72], and loss of Casp8 has been shown to facilitate cellular transformation in vitro [64, 73]. In hematopoietic malignancies, mutations of asp8 (within the P10 subunit) were reported in 58.21% (85/146) of AML cases, which are correlated with resistance to chemotherapy and poor prognosis for patients [74]. Thus, long-term observation is required to determine whether AML transformation occurs in Casp8−/− mice.
The roles of Casp2, Casp3 and Casp9 in hematopoietic regulation have been studied. Casp2−/− mice develop normally but show an ageing-associated phenotype with increasing oxidative stress, enhanced aneuploidy and DNA damage in their BM cells [75, 76]. Casp3 limits the sensitivity of HSPCs to Ras-Raf-Mer-Erk signaling induced by cytokines such as TPO, SCF and IL3 [77]. Mouse Casp3−/− HSCs exhibit phenotypes including premature exit from quiescence, over-proliferation and retarded differentiation due to reduced Erk signaling [77]. Casp3 also regulates B cell proliferation and differentiation, as well as the maturation of erythroid blasts and megakaryocytes [78, 79]. Casp9−/− HSCs display impaired self-renewal and CHRC due to the increased production of Ifnβ [80], which recapitulates the phenotype of our Casp8−/−Ripk3−/− HSCs despite the different mechanisms involved in the activation of Tbk1-Irf3 signaling. In Casp9−/− HSPCs, mtDNA released from mitochondria stimulates cGas/Sting-Tbk1 signaling and Ifnβ production, while in Casp8−/−Ripk3−/− HSPCs, elevated Ripk1/Tbk1 interaction stimulates Tbk1 activation and Ifnβ production. In both situations, Ifnβ impairs HSC self-renewal by triggering mitoROS generation. Elevated Ifnβ levels in serum and ISG gene expression in BM cells were reported in MDS patients [81]. Future study must determine whether Tbk1 is also activated in MDS HSPCs and contributes to impaired HSC function in MDS patients.
Materials and methods
Generation of inducible Casp8 knockout mice and Casp8/Ripk3 compound-mutant mice
Casp8fx/fx (B6.129-Casp8tm1Hed/J, Strain #:027002) [63], Mx1Cre (B6.Cg-Tg(Mx1-Cre)1Cgn/J, Strain #:003556) and RosaCreERT (B6.129-Gt(ROSA)26Sortm1(Cre/ERT2)Tyj/J, Strain #:008463) mice were purchased from JAX Laboratories. All mice were maintained in specific pathogen-free (SPF) facilities as indicated according to the standards set forth in the National Institutes of Health Guidelines for the Care and Use of Animals in the animal facility at Loyola University Medical Center and at Lanzhou University Second Hospital in a CD57B6 background. All experiments performed on animals were approved in advance by the Loyola University Institutional Animal Care and Use Committee (protocol No. 108832) or the Lanzhou University Institutional Animal Care and Use Committee. By crossing Mx1Cre mice or RosaCreERT mice with Casp8fx/fx mice and then back-crossing, we generated interferon-inducible Casp8 knockout mice (Mx1Cre+Casp8fx/fx) and tamoxifen (TAM)-inducible Casp8 knockout mice (RosaCreERT+Casp8fx/fx), respectively, and also corresponding control mice (Mx1Cre+Casp8fx/+ and Casp8fx/fx). To induce Casp8 deletion, 6–8 wks. after birth, all littermates in Mx1Cre groups were injected with polyI:C, 5 μg/g body weight, every other day for a total of three injections; mice in the RosaCreERT+ groups were given 100 mg/kg/day of TAM by intraperitoneal injection for 5 consecutive days. By back-crossing Rosa26CreERT+Casp8fx/fx or Mx1Cre+Casp8fx/+ mice with Ripk3−/− mice, we generated RosaCreERT+Casp8fx/fxRipk3−/− and Mx1Cre+Casp8fx/fxRipk3−/− mice, respectively, as well as corresponding controls. PolyI:C or TAM was injected to induce Casp8 deletion as described above. Mice genotyping was accomplished by PCR analysis of tail snip DNA using primers listed in supplementary Table 1. The efficiency of Casp8 deletion in Casp8−/− mice was determined by PCR assays using the primers listed in supplementary Table 1. To assess the responses of mice to polyI:C or LPS treatment, some of them were treated with 1 μg/g body weight of polyI:C or 0.2 μg/g body weight of LPS every other day for 16 days, as indicated.
Mouse hematopoietic phenotype analysis
Mice were humanely terminated at the indicated time points to collect peripheral blood, spleens, thymuses, and BM. PB was analyzed for WBC counts, platelet counts, RBC counts, and Hb concentration using a Hemavet 950FS (Drew Scientific Inc.). After lysis of RBCs, nucleated cells from PB, spleens, thymuses, and BM were counted and further stained with cell surface markers for phenotypic analysis by flow cytometry as described previously [46]. All the fluorescent antibodies used in flow cytometric analyses were purchased from Biolegend or eBioscience and are listed in supplementary Table 2. Stained cells were subjected to multi-color analysis using a BD LSR-FortessaTM flow cytometer. Data were analyzed using Flowjo software. All small molecular chemicals and cytokines used in this study are listed in supplementary Table 3.
Statistical analyses
The statistical analyses of all the data presented in this manuscript were performed using GraphPad Prism-9 software. Two-way ANOVA (multiple groups) and Student’s t tests (two groups) were performed to determine the statistical significance of differences among and between experimental groups. Statistical data are expressed as means ± SD. Mantel–Cox log-rank test was performed for Kaplan–Meier survival comparison between experimental groups. p < 0.05 was considered to be statistically significant.
Please find other detailed information of materials and methods in the supplementary files.
Data availability
All the original data and experimental materials related to this paper are available upon request.
References
Cazzola M, Malcovati L. Myelodysplastic syndromes-coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–8.
Raza A, Galili N. The genetic basis of phenotypic heterogeneity in myelodysplastic syndromes. Nat Rev Cancer. 2012;12:849–59.
Shetty V, Hussaini S, Alvi S, Joshi L, Shaher A, Dangerfield B, et al. Excessive apoptosis, increased phagocytosis, nuclear inclusion bodies and cylindrical confronting cisternae in bone marrow biopsies of myelodysplastic syndrome patients. Br J Haematol. 2002;116:817–25.
Zou J, Shi Q, Chen H, Juskevicius R, Zinkel SS. Programmed necroptosis is upregulated in low-grade myelodysplastic syndromes and may play a role in the pathogenesis. Exp Hematol. 2021;103:60–72.e65.
Sallman DA, List A. The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood. 2019;133:1039–48.
Basiorka AA, McGraw KL, Eksioglu EA, Chen X, Johnson J, Zhang L, et al. The NLRP3 Inflammasome functions as a driver of the myelodysplastic syndrome phenotype. Blood. 2016;128:2960–75.
Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, et al. Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. 2020;10:237.
Jiang M, Qi L, Li L, Wu Y, Song D, Li Y. Caspase-8: a key protein of cross-talk signal way in “PANoptosis” in cancer. Int J Cancer. 2021;149:1408–20.
Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis). Front Cell Infect Microbiol. 2019;9:406.
Lee SC, North K, Kim E, Jang E, Obeng E, Lu SX, et al. Synthetic lethal and convergent biological effects of cancer-associated spliceosomal gene mutations. Cancer Cell. 2018;34:225–241.e228.
Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76.
Estornes Y, Toscano F, Virard F, Jacquemin G, Pierrot A, Vanbervliet B, et al. dsRNA induces apoptosis through an atypical death complex associating TLR3 to caspase-8. Cell Death Differ. 2012;19:1482–94.
Annibaldi A, Walczak H. Death receptors and their ligands in inflammatory disease and cancer. Cold Spring Harb Perspect Biol. 2020;12:a036384.
Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19:36–41.
Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703.
Vanlangenakker N, Vanden Berghe T, Vandenabeele P. Many stimuli pull the necrotic trigger, an overview. Cell Death Differ. 2012;19:75–86.
Vajjhala PR, Lu A, Brown DL, Pang SW, Sagulenko V, Sester DP, et al. The inflammasome adaptor ASC induces procaspase-8 death effector domain filaments. J Biol Chem. 2015;290:29217–30.
Hughes SA, Lin M, Weir A, Huang B, Xiong L, Chua NK, et al. Caspase-8-driven apoptotic and pyroptotic crosstalk causes cell death and IL-1beta release in X-linked inhibitor of apoptosis (XIAP) deficiency. EMBO J. 2023;42:e110468.
Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ, et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem. 2015;290:20167–84.
Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, et al. Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. 2020;217:jem.20191644.
Malireddi RKS, Gurung P, Mavuluri J, Dasari TK, Klco JM, Chi H, et al. TAK1 restricts spontaneous NLRP3 activation and cell death to control myeloid proliferation. J Exp Med. 2018;215:1023–34.
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–9.
Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, et al. ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med. 2017;214:2217–29.
Malireddi RKS, Kesavardhana S, Karki R, Kancharana B, Burton AR, Kanneganti TD. RIPK1 distinctly regulates yersinia-induced inflammatory cell death, PANoptosis. Immunohorizons. 2020;4:789–96.
Sharma BR, Karki R, Rajesh Y, Kanneganti TD. Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis). J Biol Chem. 2023;299:105141.
Fritsch M, Gunther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP, et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature. 2019;575:683–7.
Newton K, Wickliffe KE, Maltzman A, Dugger DL, Reja R, Zhang Y, et al. Activity of caspase-8 determines plasticity between cell death pathways. Nature. 2019;575:679–82.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501.
Jost PJ, Grabow S, Gray D, McKenzie MD, Nachbur U, Huang DC, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–9.
Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, et al. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–54.
Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–73.
Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–26.
Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–67.
Chen J, Wang S, Blokhuis B, Ruijtenbeek R, Garssen J, Redegeld F. Cell death triggers induce MLKL cleavage in multiple myeloma cells, which may promote cell death. Front Oncol. 2022;12:907036.
Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515.
Henry CM, Martin SJ. Caspase-8 acts in a non-enzymatic role as a scaffold for assembly of a pro-inflammatory “FADDosome” complex upon TRAIL stimulation. Mol Cell. 2017;65:715–29.e715.
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci USA. 2018;115:E10888–E10897.
Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40.
Liccardi G, Ramos Garcia L, Tenev T, Annibaldi A, Legrand AJ, Robertson D, et al. RIPK1 and caspase-8 ensure chromosome stability independently of their role in cell death and inflammation. Mol Cell. 2019;73:413–28.e417.
Boege Y, Malehmir M, Healy ME, Bettermann K, Lorentzen A, Vucur M, et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell. 2017;32:342–59.e310.
Muller I, Strozyk E, Schindler S, Beissert S, Oo HZ, Sauter T, et al. Cancer cells employ nuclear caspase-8 to overcome the p53-dependent G2/M checkpoint through cleavage of USP28. Mol Cell. 2020;77:970–84.e977.
Hakem A, El Ghamrasni S, Maire G, Lemmers B, Karaskova J, Jurisicova A, et al. Caspase-8 is essential for maintaining chromosomal stability and suppressing B-cell lymphomagenesis. Blood. 2012;119:3495–502.
Gitlin AD, Heger K, Schubert AF, Reja R, Yan D, Pham VC, et al. Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature. 2020;587:275–80.
Newton K, Wickliffe KE, Dugger DL, Maltzman A, Roose-Girma M, Dohse M, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–31.
Kang TB, Ben-Moshe T, Varfolomeev EE, Pewzner-Jung Y, Yogev N, Jurewicz A, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–84.
Zhang L, Luo H, Ni HM, Liu S, Xing H, Zhang J, et al. Ripk3 signaling regulates HSCs during stress and represses radiation-induced leukemia in mice. Stem Cell Rep. 2022;17:1428–41.
Hildebrand JM, Tanzer MC, Lucet IS, Young SN, Spall SK, Sharma P, et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc Natl Acad Sci USA. 2014;111:15072–7.
Wang Y, Karki R, Mall R, Sharma BR, Kalathur RC, Lee S, et al. Molecular mechanism of RIPK1 and caspase-8 in homeostatic type I interferon production and regulation. Cell Rep. 2022;41:111434.
Pietras EM, Lakshminarasimhan R, Techner JM, Fong S, Flach J, Binnewies M, et al. Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. J Exp Med. 2014;211:245–62.
Walter D, Lier A, Geiselhart A, Thalheimer FB, Huntscha S, Sobotta MC, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520:549–52.
Lindquist KJ, Danese MD, Mikhael J, Knopf KB, Griffiths RI. Health care utilization and mortality among elderly patients with myelodysplastic syndromes. Ann Oncol. 2011;22:1181–8.
Yeh WC, Pompa JL, McCurrach ME, Shu HB, Elia AJ, Shahinian A, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8.
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–72.
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–7.
Zhang J, Kabra NH, Cado D, Kang C, Winoto A. FADD-deficient T cells exhibit a disaccord in regulation of the cell cycle machinery. J Biol Chem. 2001;276:29815–8.
Salmena L, Lemmers B, Hakem A, Matysiak-Zablocki E, Murakami K, Au PY, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–95.
Cuda CM, Misharin AV, Khare S, Saber R, Tsai F, Archer AM, et al. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner. Arthritis Res Ther. 2015;17:291.
Feng Y, Daley-Bauer LP, Mocarski ES. Caspase-8-dependent control of NK- and T cell responses during cytomegalovirus infection. Med Microbiol Immunol. 2019;208:555–71.
Feng Y, Livingston-Rosanoff D, Roback L, Sundararajan A, Speck SH, Mocarski ES, et al. Remarkably robust antiviral immune response despite combined deficiency in caspase-8 and RIPK3. J Immunol. 2018;201:2244–55.
Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011;208:633–41.
Zhang Y, Rosenberg S, Wang H, Imtiyaz HZ, Hou YJ, Zhang J. Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis. J Immunol. 2005;175:3033–44.
Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–61.
Beisner DR, Ch’en IL, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–73.
Krelin Y, Zhang L, Kang TB, Appel E, Kovalenko A, Wallach D. Caspase-8 deficiency facilitates cellular transformation in vitro. Cell Death Differ. 2008;15:1350–5.
Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK 3rd, Hutcheson J, et al. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. J Immunol. 2014;192:5548–60.
Mandal R, Barron JC, Kostova I, Becker S, Strebhardt K. Caspase-8: the double-edged sword. Biochim Biophys Acta Rev Cancer. 2020;1873:188357.
Soung YH, Lee JW, Kim SY, Jang J, Park YG, Park WS, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005;65:815–21.
Li C, Egloff AM, Sen M, Grandis JR, Johnson DE. Caspase-8 mutations in head and neck cancer confer resistance to death receptor-mediated apoptosis and enhance migration, invasion, and tumor growth. Mol Oncol. 2014;8:1220–30.
Cho S, Lee JH, Cho SB, Yoon KW, Park SY, Lee WS, et al. Epigenetic methylation and expression of caspase 8 and survivin in hepatocellular carcinoma. Pathol Int. 2010;60:203–11.
Soung YH, Lee JW, Kim SY, Sung YJ, Park WS, Nam SW, et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005;24:141–7.
Hopkins-Donaldson S, Ziegler A, Kurtz S, Bigosch C, Kandioler D, Ludwig C, et al. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003;10:356–64.
Martinez R, Setien F, Voelter C, Casado S, Quesada MP, Schackert G, et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis. 2007;28:1264–8.
Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–35.
Li M, Wu XM, Gao J, Yang F, Zhang CL, Ke K, et al. Mutations in the P10 region of procaspase-8 lead to chemotherapy resistance in acute myeloid leukemia by impairing procaspase-8 dimerization. Cell Death Dis. 2018;9:516.
Shalini S, Dorstyn L, Wilson C, Puccini J, Ho L, Kumar S. Impaired antioxidant defence and accumulation of oxidative stress in caspase-2-deficient mice. Cell Death Differ. 2012;19:1370–80.
Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B, et al. Caspase-2 deficiency enhances aging-related traits in mice. Mech Ageing Dev. 2007;128:213–21.
Janzen V, Fleming HE, Riedt T, Karlsson G, Riese MJ, Lo Celso C, et al. Hematopoietic stem cell responsiveness to exogenous signals is limited by caspase-3. Cell Stem Cell. 2008;2:584–94.
Carlile GW, Smith DH, Wiedmann M. Caspase-3 has a nonapoptotic function in erythroid maturation. Blood. 2004;103:4310–6.
Kozuma Y, Yuki S, Ninomiya H, Nagasawa T, Kojima H. Caspase activation is involved in early megakaryocyte differentiation but not in platelet production from megakaryocytes. Leukemia. 2009;23:1080–6.
White MJ, McArthur K, Metcalf D, Lane RM, Cambier JC, Herold MJ, et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell. 2014;159:1549–62.
Kim M, Hwang S, Park K, Kim SY, Lee YK, Lee DS. Increased expression of interferon signaling genes in the bone marrow microenvironment of myelodysplastic syndromes. PLoS ONE. 2015;10:e0120602.
Acknowledgements
The authors thank the staff of the Department of Comparative Medicine of Loyola University Medical Center for animal care services. We appreciate laboratory technical support in the form of FACS sorting and analysis and assistance kindly rendered by Patricia Simms.
Funding
This work was supported by NIH grants R01 HL133560-01, R01 CA223194-01 and 5F30HL162475-02 through Loyola University Chicago, as well as Loyola program development funds to JZ.
Author information
Authors and Affiliations
Contributions
SL, KJ, LZ, WL, RM, AR, PH, KB, PB, AK, HJ, ZW and JZ conducted the experiments and analyzed the data. SL, KJ and JZ drafted the first version of the paper. PB and JZ wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Dr. Gemma Kelly
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Joshi, K., Zhang, L. et al. Caspase 8 deletion causes infection/inflammation-induced bone marrow failure and MDS-like disease in mice. Cell Death Dis 15, 278 (2024). https://doi.org/10.1038/s41419-024-06660-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06660-3