Abstract
Skin fibrosis is mainly characterized by excessive collagen deposition. Studies have recently identified a number of flavonoids with variable structures that have the potency of inhibiting collagen synthesis and thus attenuating organ fibrosis. In this study, we found that flavones with 5, 7, 3′, 4′ hydroxy substitution reduced collagen expression most efficiently. Among those flavones, luteolin, quercetin, and myricetin were selected for follow-up. In vivo, the three compounds ameliorated skin fibrosis and reduced collagen deposition. Further analysis showed the compounds had significant inhibition on the proliferation, activation and contractile ability of dermal fibroblasts in vitro and in vivo. More importantly, we revealed that luteolin, quercetin, and myricetin selectively downregulated the phosphorylation of Smad2/3 in TGF-β/Smads signaling via binding to activin receptor-like kinase 5 (ALK5) and impairing its catalytic activity. We also found flavones with 5, 7, 3′, 4′ hydroxy substitution showed stronger affinity with ALK5 compared with other flavonoids. Herein, we identified at least in part the underlying molecular basis as well as the critical structures that contribute to the antifibrotic bioactivity of flavones, which might benefit drug design and modification.
Similar content being viewed by others
Introduction
Skin fibrosis is the major manifestation of a series of fibroproliferative disorders such as scleroderma, hypertrophic scar (HS) and keloid1,2. These fibrotic conditions, in most cases, cause cosmetic, functional or psychological impairment and thus have become a significant social and economic burden3,4. For instance, HS is raised and hyperemic, with cicatricial contracture and often leads to debilitated self-esteem and depression5,6. Despite numerous studies on the topic, few satisfactory interventions have been put into clinical practice7. Therefore, it is of great necessity to find out novel therapeutic targets and develop effective management strategies.
Skin fibrosis is mainly characterized by dysregulated activation of dermal fibroblasts that eventually leads to excessive deposition of extracellular matrix (ECM)8. Specifically, substantial collagen expression in the dermis is the most essential and contributes to distorted skin architecture and dysfunction of the skin9,10. Although the underlying mechanisms of the disorder is still elusive, researches have revealed that TGF-β/Smads signaling cascade plays a vital role in the initiation and development of such pathophysiological process11. Aberrant activation of TGF-β/Smads signaling is associated with exuberant behavior of dermal fibroblasts such as abnormal proliferation and transdifferentiation into myofibroblasts12,13,14. Moreover, Smads downstream of TGF-β are one of the most effective mediators in upregulating the expression of collagen in fibroblasts15. Thus, TGF-β/Smads signaling has been a potential therapeutic target for fibrotic disorders of skin.
Flavonoids (from the Latin word flavus meaning yellow, their color in nature) are a class of plant and fungus secondary metabolites. They have been shown to possess a variety of biological characteristics, including antioxidative16 and anti-inflammatory effects17, tumor growth inhibition18, and protection of ischemia/reperfusion injury19. Additionally, flavonoids have been proven to alleviate fibroproliferative disorders including cirrhosis, pulmonary fibrosis, and cardiac fibroblasts20,21,22. Also, our group previously discovered a number of flavonoids such as baicalein and galangin that effectively ameliorate HS formation via TGF-β/Smads-mediated inhibition on the proliferation, activation, contractile ability, and collagen production of dermal fibroblasts23,24. However, we also noticed a number of flavonoids that had no significant impact on fibroplasia. Recently, it has been demonstrated that the bioactivity of flavonoids is predominantly dependent on the backbones and the functional groups of the compounds, but the decisive structural features for the antifibrotic activities of flavonoids are yet to be uncovered.
In this study, the effect of 109 flavones on the expressions of type I and III collagen was at first detected for the sake of screening for potential agents that might ameliorate skin fibrosis. Furthermore, the therapeutic efficacy of the selected candidate compounds was analyzed in two animal models for skin fibrosis as well as in cultured human dermal fibroblasts (HDFs). Lastly, the molecular basis of flavone-induced inhibitory effect on skin fibrosis was studied and the fundamental structure indispensable to antifibrotic property was verified.
Results
Flavones with 5, 7, 3′ and 4′ hydroxy substitution demonstrate significantly stronger inhibition on collagen synthesis than other flavonoids
Chemically, flavonoids have the basic structure of a 15-carbon skeleton that consists of two phenyls (A and B) rings and one heterocyclic (C) ring. This carbon structure can be abbreviated as C6-C3-C6 (Supplementary Figure S1A). Based on their backbones, flavonoids can be divided into several classes: chalkone, isoflavone, flavone, etc. (Supplementary Figure S1B).
One of the most evident manifestations of skin fibrosis is excessive collagen deposition. Thus, to screen for antifibrotic flavonoids, type I and III collagen expressions of HDFs treated with a variety of flavones (10 μM) were quantified by qPCR. We found that higher degree of hydroxylation tended to induce stronger inhibition on collagen synthesis and collagen I and III expression were significantly downregulated by flavones containing no less than four hydroxyls compared with vehicle control (Supplementary Figure S2 and Fig. 1a). Both alkylation and glucuronidation of the hydroxyl groups decreased the capacity to suppress Col1a2 and Col3a1 expressions (Supplementary Figure S1C). These results indicated a potential association between the hydroxyl groups of flavones and their antifibrotic activity. Besides, we also found flavones with different backbones had differing antifibrotic potential. Flavones, which are based on the backbone of 2-phenylchromen-4-one, exhibited the most significant inhibition on collagen expression compared with other classes such as chalcone and isoflavone (Supplementary Figure S1D). Based on the findings above, we focused on 39 flavones with 4, 5 or 6 hydroxyls that significantly reduced collagen expression (positive compounds, Supplementary Figure S2). Firstly, our results demonstrated that tetrahydroxyflavones, pentahydroxyflavones, and hexahydroxyflavones instead of hydroxyflavones and bihydroxyflavones had significantly stronger inhibition on Col1a2 and Col3a1 expressions than vehicle in the positive compounds (Fig. 1b). However, there was no noticeable difference among HDFs treated with tetrahydroxyflavones, pentahydroxyflavones, and hexahydroxyflavones as to the fold changes of Col1a2 and Col3a1 expression (Fig. 1b), suggesting that more than four hydroxyls in flavones might be redundant. The findings above led us to the assumption that the antifibrotic activity of flavones could in part depend on the position of the hydroxyl groups. Among 39 positive compounds, we found that the most frequently hydroxylated position was 7 (35/39), followed by 4′ (30/39), 5 (29/39), 3′ (23/39), 3 (19/39) and 8 (10/39) (Fig. 1c). Further comparison among 5, 7, 3′, 4′-hydroxylated flavones (eight compounds), 3, 5, 7, 3′, 4′-hydroxylated flavones (four compounds) and 3, 5, 7, 8, 3′, 4′-hydroxylated flavones (one compound) showed that the existence of hydroxyl in position 3 (Col1a2 0.54 vs. 0.58, P = 0.431, Col3a1 0.43 vs. 0.45, P = 0.792) or 8 (Col1a2 0.54 vs. 0.66, Col3a1 0.43 vs. 0.58) did not enhance the inhibitive effect of the compounds (Fig. 1d). This indicated that the most effective and the key hydroxylation pattern was 5, 7, 3′ and 4′ hydroxylation. And indeed, the flavones with 5, 7, 3′ and 4′ hydroxylation was significantly more efficient on downregulating the expression of both Col1a2 and Col3a1 compared with flavones with other structures (Fig. 1e, f). Therefore, flavones containing 5, 7, 3′, 4′ hydroxy substitution were selected for further analysis.
Flavones with no less than four hydroxyls induce significant inhibition on Col1a2 and Col3a1 expression compared with vehicle in a 109 candidate flavones and b 39 positive compounds. c The frequency of hydroxy substitutions in 39 positive compounds. d The effectiveness of flavones with different hydroxy substitutions in 39 positive compounds. Flavones containing 5, 7, 3′, 4′ hydroxy substitution exhibits significantly stronger inhibition on collagen expression compared with flavones with other structures in e 109 candidate flavones and f 39 positive compounds. Data are the mean ± SD (three independent experiments); **P < 0.01, ***P < 0.001
Luteolin, quercetin or myricetin dose-dependently reduces the expression of type I and III collagen in vitro
To further verify the effect of the positive flavones based on the screening above on collagen expression, luteolin, quercetin, and myricetin that exhibited the strongest inhibitive efficacy were selected for further analysis (Supplementary Figure S3A). Toluidine blue staining of cultured HDFs showed that ECM production was significantly reduced after the cells were treated with luteolin, quercetin or myricetin at 10 μM for 5 days (Supplementary Figure S4A). Furthermore, based on qPCR and western blotting, luteolin, quercetin or myricetin demonstrated specific and dose-dependent downregulation of type I and III collagen expressions in HDFs (Supplementary Figure S3B-D), while the compounds did not induce remarkable changes in other collagen (type II or type X) expressions (Supplementary Figure S4B).
Luteolin, quercetin or myricetin attenuates skin fibrosis in vivo
Given the results above that luteolin, quercetin or myricetin inhibited markedly the expression of collagen in vitro, bleomycin-induced skin fibrosis model and mechanical load-induced HS model, two confirmed animal models of skin fibrosis, were used to investigate the antifibrotic effect of the compounds in vivo. Histological analysis demonstrated that daily injection of luteolin, quercetin or myricetin at 10 μM reduced significantly the dermal thickness in the bleomycin model, and the macroscopic scar area and cross-section area in the loaded HS model (Fig. 2a−d). Next, we quantified collagen density using Picrosirius red staining. This revealed a lower collagen density in mice with luteolin, quercetin or myricetin treatment vs. dimethyl sulfoxide (DMSO) treatment (Fig. 2a−d). These findings identified the in vivo potency of luteolin, quercetin or myricetin to inhibit collagen expression and ameliorate skin fibrosis.
a Representative images of fibrosing skin tissue in bleomycin-induced skin fibrosis model from DMSO-treated mice and drug-treated mice after hematoxylin and eosin (H&E), Masson’s trichrome and Picrosirius red staining. b Quantitative analysis of dermal thickness and collagen density of fibrosing skin from vehicle-treated mice and drug-treated mice in bleomycin-induced skin fibrosis model. c Representative images of macroscopy, H&E and Picrosirius red staining of hypertrophic scar (HS) tissue in mechanical load-induced HS model from DMSO-treated mice and drug-treated mice. d Quantitative study of gross scar, cross-section area, and collagen density of HS tissue from DMSO-treated mice and drug-treated mice in HS model. Data are the mean ± SD (three independent experiments); *P < 0.05, **P < 0.01, ***P < 0.001
Luteolin, quercetin or myricetin suppresses the proliferation, activation and contractile behavior of dermal fibroblasts
As fibroblast proliferation is closely associated with skin fibrosis formation25, we further investigated whether luteolin, quercetin or myricetin regulated fibroblast proliferation. Immunohistochemistry staining and quantification results showed that the agents induced a marked decrease of proliferating cell nuclear antigen (PCNA)-positive cell percentage in the dermis in both in vivo models (Fig. 3a, b). Moreover, in vitro growth of cultured HDFs was significantly retarded after luteolin, quercetin or myricetin treatment as EdU-positive cell percentage dropped significantly in EdU incorporation assay and the Gentian Violet-stained colonies reduced obviously in colony formation assay (Fig. 3c, d).
Representative images of hypertrophic scar (HS) in a fibrosing skin in bleomycin-induced model and b mechanical load-induced HS model from DMSO-treated mice and drug-treated mice stained for proliferating cell nuclear antigen (PCNA) and quantitative analysis of PCNA-positive cells. c Representative immunofluorescence images and quantitative results of EdU incorporation assay in human dermal fibroblasts (HDFs). EdU is shown by red fluorescence and nucleus by blue fluorescence. d Representative images and quantitative results of colony formation assay exhibit an evidently reduced number of HDF colony (purple) after drug treatment. Data are the mean ± SD (three independent experiments); *P < 0.05, ***P < 0.001
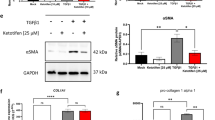
Activated fibroblasts, also called myofibroblasts, play a crucial role in ECM deposition and cicatricial contraction in fibrosing disorders25. Our data demonstrated that the activation of dermal fibroblasts in both animal models was notably diminished with the application of the compounds as a drug-induced decline of α-SMA expression level was shown by immunohistochemistry (Fig. 4a, b). Luteolin, quercetin or myricetin also led to significantly decreased TGF-β1-induced (5 ng/ml) α-SMA expression in cultured HDFs based on immunofluorescence staining (Fig. 4c). Furthermore, HDFs-mediated collagen gel contraction was evidently inhibited by administration of luteolin, quercetin or myricetin at both 5 and 10 μM (Fig. 4d) before TGF-β1 (5 ng/ml) application. In summary, each of the three compounds demonstrated marked inhibition on the exuberant proliferation and activity of dermal fibroblasts in vitro and in vivo.
Representative images and quantitative analysis of fibrosing tissues from in vivo models (a bleomycin-induced model; b mechanical load-induced model) stained for α-SMA show significant suppression induced by luteolin, quercetin or myricetin on fibroblast activation. c Immunofluorescence staining for α-SMA and F-actin in cultured human dermal fibroblasts (HDFs) after incubation with TGF-β1 (5 ng/ml) and DMSO or drugs for 48 or 72 h. α-SMA is shown by red fluorescence and F-actin by green fluorescence. d Representative images and quantitative results of collagen gel contraction assay show that luteolin, quercetin or myricetin significantly inhibited HDF-mediated gel contraction with exogenous TGF-β1 (5 ng/ml) treatment. Data are the mean ± SD (three independent experiments); **P < 0.01, ***P < 0.001
Luteolin, quercetin or myricetin dose-dependently inhibits the phosphorylation of Smad2/3
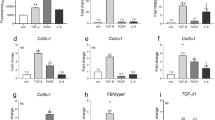
Since luteolin, quercetin, and myricetin exhibited significant potency in ameliorating skin fibrosis formation and fibroblast malfunction, we further looked into the underlying biomolecular mechanisms. It has been reported intensely that the prolonged activation of TGF-β/Smad2/3 pathway contributes critically to collagen deposition and fibroblast malfunctioning14. Interestingly, we found that luteolin, quercetin or myricetin downregulated the level of phosphorylated Smad2/3 (p-Smad2/3) in a dose-dependent manner without having significant impact on total Smad2/3 expression (Fig. 5a). Also, reduction of TGF-β-induced nuclear translocation of Smad3 in HDFs was exhibited by immunofluorescence (Fig. 5d). Moreover, we studied other Smads that modulate BMP signaling and might interact with TGF-β/Smad2/3 signaling, where the total and phosphorylated Smad1/5/9 proteins were not regulated by the compounds (Fig. 5c).
a−c Western blotting and densitometric analysis demonstrate that luteolin, quercetin or myricetin dose-dependently inhibits the expression of phosphorylated Smad2/3 without affecting the other factors in TGF-β/Smads signaling pathway or Smad1/5/9 in BMP signaling. d Representative images of immunofluorescence staining of human dermal fibroblasts exhibit a significant reduction in TGF-β1-induced Smad3 (green) translocation into the nuclei. e Luteolin, quercetin or myricetin has no significant impact on the expression of TGF-β1 based on ELISA assay. f Luteolin, quercetin or myricetin does not induce notable effect on the expression of TGFβRI or TGFβRII according to western blotting. Data are the mean ± SD (three independent experiments); *P < 0.05, **P < 0.01, ***P < 0.001
To investigate the initiation of suppressed Smad2/3 phosphorylation, the downstream and upstream factors of p-Smad2/3 were analyzed. We found that the three compounds did not affect the expression of the pathway inhibitors Smad6/7 (Fig. 5b). Considering that Smad3 forms a complex with Smad4 before translocated to the nuclei and regulating transcription, the protein level of Smad4 in HDFs was also determined by western blotting. However, it was also not obviously affected by luteolin, quercetin or myricetin (Fig. 5a). Lastly, molecules upstream of p-Smad2/3 in TGF-β/Smad2/3 signaling were studied. It was found that neither the secretion of the ligand TGF-β1 (Fig. 5e) nor the expression of the receptors TGFβRI/II was notably altered by luteolin, quercetin or myricetin (Fig. 5f).
In short, we found that luteolin, quercetin or myricetin induced a significant inhibition of Smad2/3 phosphorylation in a dose-dependent manner. However, such inhibition was not attributed to the changes in Smad1/5/9, Smad6/7, TGF-β1 or TGFβRI/II expressions. Thus, the effect of the compounds on p-Smad2/3 downregulation could be attributed to the altered function of the aforementioned molecules.
Flavones with 5, 7, 3′, 4′ hydroxy substitution binds to the catalytic region of ALK5
During the transduction of TGF-β/signaling, Smad2/3 binds to the L45 loop of ALK5, and is then phosphorylated by the kinase26. Increasing evidence has shown that a variety of small molecule compounds may bind to ALK5 and impair its interaction with Smads27,28. Thus, we next observed the binding affinities of the selected positive compounds and a proportion of negative compounds with ALK5.
The first class of compounds (eight representative compounds) has the same flavone core with 5, 7, 3′, 4′ hydroxy substitution (positive compounds). According to the docking results, this class of flavones can form several hydrogen bonds with the catalytic site of ALK5 (Fig. 6a). As seen in Fig. 6c, luteolin (5, 7, 3′, 4′-Tetrahydroxyflavone), quercetin (3, 5, 7, 3′, 4′-Pentahydroxyflavone), and myricetin (3, 5, 7, 3′, 4′, 5′-Hexahydroxyflavone) can both form two strong hydrogen bonds with the amino acid residues Lys232 and His283 of ALK5. In addition, two H−π interactions can form between the flavone core and the Val219, Leu340 of the ALK5, respectively (Fig. 6c). For the other five flavones in this class, they can almost form these interactions, such as hydrogen bonds between the compounds with the Lys232 and His283, H−π interactions between the flavone core and the Val219 and Leu340 (Supplementary Figure S5A). Due to these ligand−protein interactions, this class of flavones can bind efficiently to the ALK5 protein.
a Schematic of computational docking simulation of 5, 7, 3′, 4′-hydroxylated flavones interaction with the ATP-binding site of ALK5. Amino acid residues interacting with the compounds are shown (yellow) with the hydrogen bonds indicated by dashed lines. b Mean docking score of 5, 7, 3′, 4′ hydroxyl-containing flavones vs. flavones with other structures. c Docking simulation of luteolin, quercetin, and myricetin binding to the catalytic site of ALK5. Amino acid residues interacting with the compounds are indicated and hydrogen bonds are represented with straight dashed lines. **P < 0.01
For the second class of flavones, they have the same flavone core as the first class of flavones but without 5, 7, 3′, 4′ hydroxy substitution. Five representative compounds of this class were not able to form a hydrogen bond with the His283 of the ALK5 protein (Supplementary Figure S5B). In addition, the two H−π interactions were not seen between the carbonyl group of the flavone core and the Asp351 of ALK5 (Supplementary Figure S5B). Furthermore, the docking scores of positive compounds were significantly lower than the scores of the second class of flavones (−8.74 kcal/mol vs. −6.82 kcal/mol, Fig. 6b). Therefore, the first class of the flavones with 5, 7, 3′,4′ hydroxy substitution has stronger affinity with the ALK5 protein than the second class of flavones.
Luteolin, quercetin or myricetin inhibits ALK5 serine/threonine kinase activity
In order to further validate the interaction between flavones containing 5, 7, 3′, 4′ hydroxy substitution and ALK5, we performed the LanthaScreenTM Eu Kinase Binding Assay to detect competitive binding to the kinase. This assay showed a decreasing trend of the emission ratio induced by luteolin, quercetin or myricetin in a dose-dependent manner, indicating the competitive binding of the compounds with ALK5 (Fig. 7a). IC50 of the compounds approximated to 4 μM. Additionally, the enzymatic activity of ALK5 was significantly restrained by application of luteolin, quercetin or myricetin as ALK5-mediated production of p-Smad3 was notably decreased by the compounds in kinase activity assay (Fig. 7b).
a Dose−response curve of LanthaScreenTM Eu kinase binding assay demonstrated a dose-dependent decrease in emission ratios induced by luteolin, quercetin or myricetin. Luteolin, quercetin or myricetin inhibited ALK5 activity with IC50 values of 1.062, 1.480, and 4.083 μM, respectively. b In vitro phosphorylation of GST-Smad3 by ALK5 was significantly reduced by luteolin, quercetin or myricetin. Data are the mean ± SD (three independent experiments)
Discussion
Excessive collagen deposition is one of the most significant features shared by fibrotic diseases in skin29. In light of this, we used Col1a2 and Col3a1 as targets to perform a screen of a variety of flavonoids for candidate compounds that might be extensively effective on these diseases. It turned out that the number and position of the hydroxyl group in the compounds were major determinants of their antifibrotic property. Specifically, flavone with hydroxyls in positions 5, 7, 3′ and 4′ exhibited the most potent activity in downregulating the expression of Col1a2 and Col3a1. Thus, luteolin, quercetin, and myricetin with such structure and the most evident effect were then selected for follow-up. In vivo and in vitro studies showed efficient properties of the compounds in attenuating skin fibrosis, especially with respect to diminishing collagen production. These results indicate the potential use of the selected novel flavones in a wide range of disorders that manifest skin fibroproliferation, including HS, scleroderma and graft versus host disease.
Our investigation also discovered, at least in part, the molecular basis of flavone-induced antifibrotic effect. Analysis on fibrosis-associated signaling pathway revealed an inhibitory effect of the compounds on Smad2/3 phosphorylation, but without affecting the expression of other factors in the cascade or Smad1/5/9 in ALK1-mediated signaling. It took us to the assumption that luteolin, quercetin or myricetin might impede directly the function of the factors in signal transduction. Taking into account the increasing evidence that a variety of small molecule compounds have direct interaction with the catalytic site of kinases30,31, we finally performed molecular docking study, kinase binding assay and ALK5 activity assay that revealed the competitive binding of the eight representative flavones with hydroxyls in positions 5, 7, 3′ and 4′ to the catalytic site of ALK5. Hence, the present study not only confirmed the probable therapeutic effect of flavonoids in skin fibrosis, but also revealed a marked affinity between the compounds and the kinase ALK5. Thus, our findings could as well facilitate the verification of the versatile health benefits of flavonoids. For example, flavonoids are shown to have antitumor properties but few reports focus on the association between such properties and ALK5-mediated signaling32,33. Since ALK5 is linked with various oncogenic mechanisms including tumor angiogenesis34,35 and epithelial-to-mesenchymal transition36,37, it would be fascinating to investigate in future study whether the inhibitory effect of flavones on cancer is attributed to their binding to ALK5.
Moreover, it is of great importance that these compounds were shown to have a preference for interfering with TGF-β/Smad2/3 signaling other than other antagonistic pathways such as ALK1/Smad1/5/9 signaling, since ALK1 downregulated collagen synthesis in fibrosis-associated cells, including myofibroblasts and fibroblasts, by functioning adversely against ALK5-induced transduction38,39. Such specificity could enhance the antifibrotic efficacy of the compounds. In addition, one of the obstacles that hinders the clinical use of small molecule drugs is the uncertainty of side effect. It is promising that the specificity of the selected flavonoids might minimalize undesirable side effect and attain satisfactory outcomes.
The present study also emphasizes the significance of hydroxyls in flavonoids on their antifibrotic effect. It is widely acknowledged that the arrangement of the functional groups in flavonoids is critical to their biological activities such as antioxidation, hepatoprotective activities and antibacterial activities32,40,41. For instance, the configuration and number of hydroxyls in flavonoids is found to have profound impact on scavenging free radicals42. However, there have scarcely been studies concerning the association between the chemical structures of flavonoids and their antifibrotic activity. Herein, we for the first time identified the decisive functional group and configuration for the antifibrotic activity of flavonoids. 5, 7, 3′ and 4′ hydroxy substitution in flavones plays pivotal roles in inducing such bioactivity as 4′-hydroxyl forms a hydrogen bond with the amino acid residue His283 of the catalytic site of ALK5 and two H−π bonds are formed between the backbone and Val219 and Leu340. However, flavones without 5, 7, 3′, 4′ hydroxy substitution fail to form such bonds with the kinase and thus demonstrate significantly poorer affinities with ALK5. These results explain at least in part the divergent potency of flavonoids in alleviating collagen production that is predominantly regulated by ALK5/Smad2/3 signaling. Also, the findings could be constructive to the modification of the compounds in the future if necessary.
In conclusion, this study discovered a class of flavones that effectively diminishes collagen production and attenuates skin fibrosis via binding to ALK5 and thus downregulates TGF-β/Smads signaling, suggesting potential therapeutic agents for fibroproliferative disorders. More importantly, we verify that hydroxyls in positions 5, 7, 3′ and 4′ are decisive to the antifibrotic effect, which will be constructive to drug design and modification in the future.
Materials and methods
Cell culture
HDFs were obtained from 12 patients who had undergone scar resection 6−12 months after severe trauma or burn (Supplementary Table S1). None of the patients had received any treatment for the scar before surgical excision. All skin tissues were obtained with written informed consent according to the Declaration of Helsinki Principles. HDFs were maintained in Dulbecco’s Modified Eagle Medium (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA), 100 U/ml penicillin, and 100 mg/l streptomycin. HDFs were incubated at 37 °C in a humidified atmosphere with 5% CO2. Primary fibroblasts of passages 4–6 were used.
Real-time PCR
The total RNA of cells was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and subjected to reverse transcription with Oligo (dT) and M-MLV Reverse Transcriptase (Thermo Fisher Scientific). Quantitative PCR amplification was performed with the ViiA™ 7 Real-Time PCR System (Applied Biosystems, Foster City, CA) by using the SYBR quantitative PCR SuperMix W/ROX (Invitrogen, Carlsbad, CA, USA). The primers used are listed in Supplementary Table S2.
Western blotting
Cultured HDFs were lysed with radio-immunoprecipitation assay lysis buffer (Beyotime, Jiangsu, China). Protein concentrations were determined using a micro bicinchoninic acid assay (Thermo Fisher Scientific). Total proteins were separated on acrylamide gels, and immunodetected with the primary antibodies that included the following: Col1a2, Col3a1 (Genetex, Biozol, Eching, Germany, 1:1000), α-SMA, Smad6, Smad7, TGF-β receptor I (TGFβRI), TGF-β receptor II (TGFβRII) (Abcam, Cambridge, UK, 1:1000), Smad2, p-Smad2, Smad3, p-Smad3, Smad4, Smad1/5/9, p-Smad1/5/9 (Cell Signaling Technology, Beverly, MA, 1:1000). Immunoreactive bands were quantitatively analyzed with ImageJ software.
EdU incorporation assay
Cell proliferation was detected using Click-iT® EdU Alexa Fluor® 488 Imaging Kit (Invitrogen, Carlsbad, CA, USA). In short, HDFs were incubated with EdU for 2 h, fixed in 4% paraformaldehyde (PFA), and incubated with a Click-iT EdU reaction cocktail for 30 min. Immunofluorescence signals were captured using a Zeiss 710 laser-scanning microscope (Zeiss, Thornwood, NY, USA).
Colony formation assay
Colony formation assay of HDFs was performed using monolayer culture43. Cells were cultured in six-well plates at a density of 500 per well, and treated with 5 or 10 μM drugs or DMSO. After culture for 14 days, surviving colonies were fixed with 4% PFA and stained with Gentian Violet (Sigma-Aldrich, St. Louis, MO, USA) for 5 min.
Immunofluorescence cell staining
HDFs were fixed with 4% PFA and blocked with 5% goat serum in phosphate-buffered saline with 0.1% TritonX-100 (PBST) (0.1% TritonX-100 in phosphate-buffered saline) for 1 h. For F-actin staining, cells were incubated with Alexa Fluor® 488 Phalloidin (Cytoskeleton, Inc., Denver, CO, USA, 1:200) for 1 h at room temperature. For α-SMA staining, cells were incubated with anti-α-SMA (Abcam, Cambridge, UK, 1:200) overnight at 4 °C, followed by an Alexa Fluor® 555-conjugated secondary antibody. For Smad3 staining, cells were incubated with primary antibodies against Smad3 (Cell Signaling Technology, Beverly, MA, 1:200) overnight at 4 °C, followed by an Alexa Fluor® 488-conjugated secondary antibody. Fluorescence was analyzed using a Zeiss 710 laser-scanning microscope (Zeiss, Thornwood, NY, USA).
Collagen gel contraction assay
Collagen gel contraction assay was performed according to previous publication44. Briefly, 6×104 HDFs and 500 μl of collagen suspension (Shengyou, Hangzhou, China) were plated per well in a 24-well plate. The plates were incubated at 37 °C for 10 min to allow collagen polymerization. After collagen gel polymerization, DMSO, luteolin, quercetin or myricetin (5 or 10 μM) were added for 1 h before TGF-β1 (5 ng/ml) treatment. Gels were detached from well and photographed at time 24 h.
Bleomycin-induced skin fibrosis model and mechanical load-induced hypertrophic scar model
Adult female BALB/c mice aged 6 weeks were purchased from the Shanghai Slac Laboratory Animal (Slac, Shanghai, China). All procedures were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University.
Skin fibrosis was induced by daily intradermal injection of bleomycin sulfate (100 μl, 1 mg/ml in vehicle) into the back skin of mice for 4 weeks as described45. A total of 40 mice were equally randomized into five groups: (1) Control group, where mice were injected with vehicle (100 μl sterile saline plus 1% DMSO) intradermally; (2) Bleomycin group, where mice were treated with 100 μl bleomycin sulfate; (3–5) Treatment groups, where mice were treated with 100 μl bleomycin plus luteolin, quercetin or myricetin, respectively (Supplementary Figure S6).
A mouse HS model was generated as previously published46. A total of 40 mice were made full-thickness incisions and divided into five groups. We established a 2-cm full-thickness incision at the dorsal midline of each mouse and then reapproximated them with 6-0 nylon sutures. Four days later, the sutures were removed, and the loading devices were fixed upon the incisions. In control group, the wound served as an internal control, without loading devices activated. In loaded group, the wound was loaded with proper function. Each of them was injected intradermally with vehicle (100 μl sterile saline plus 1% DMSO) once a day. In loaded-and-drug groups, mice with incisions that loaded and functioned well received local intradermal injection of luteolin, quercetin or myricetin, respectively. The loading devices were distracted by 4 mm every other day to maintain tension on the incisions continuously. Half of the mice in each group were sacrificed on day 14 for the sake of scar harvest while the other half on day 21 (Supplementary Figure S6).
Histology and immunohistochemistry
Skin samples were fixed with 4% PFA, embedded in paraffin, and cut into 5-µm-thick sections. After deparaffinizing in xylene and rehydrating using an alcohol series, the sections were stained with hematoxylin and eosin (H&E), Masson’s trichrome (Trichrome stain LG solution, HT10316, Sigma-Aldrich, USA) and Picrosirius red (Fluka, Buchs, Switzerland).
For immunohistochemical staining, the sections were detected with primary antibodies against PCNA (Abcam, Cambridge, UK, 1:100) and α-SMA (Abcam, Cambridge, UK, 1:200) overnight at 4 °C. After incubation with the appropriate secondary antibodies at room temperature for 30 min, the sections were developed with diaminobenzidine and counterstained with hematoxylin. The number of positive cells was calculated with the Image Pro-Plus software.
Molecular docking study
Three-dimensional structure of ALK5 was obtained from the Protein Data Bank (PDB code: 2WOT, 1.85 Å). The co-crystallized structure was prepared using QuickPrep module of MOE version 2016.08. The binding site for ALK5 has been well characterized based on structural information derived from the cro-crystallized structures (PDB codes: 2WOT/2WOU). The 2D structures of the two classes of flavones were built using the Builder module in MOE and converted to 3D through the energy minimization. MOE Dock module was used for docking simulation of the two classes of flavones and predicting their binding affinity with the ALK5 protein structure. The binding site of the cro-crystallized ligand was chosen as the binding pocket for docking. The GBVI/WSA dG score was used to evaluate the binding of the two classes of flavones with ALK5 protein. The binding mode and the ligand−protein interactions were analyzed in MOE after the refinement minimization.
LanthaScreenTM Eu kinase binding assay for ALK5
As a verified method to detect kinase inhibitor, LanthaScreenTM Eu kinase binding assay was used to analyze the interaction of the selected flavonoids with ALK547. Kinase buffer consisting of 50 mM 4-(2-hydroxyethyl)-1- piperazineethanesulfonic acid (pH 7.5), 10 mM MgCl2, 1 mM ethyleneglycoltetraacetic acid, and 0.01% Brij-35 was used, in which 1% DMSO was added to form compound dilution buffer. A mixture of fourfold serially diluted compounds (5 μl), kinase/antibody solution (5 μl of GST-ALK5 (15 nM) and Eu-anti-GST antibody (6 nM); Invitrogen, Carlsbad, CA, USA) and kinase tracer 178 (5 μl of 30 nM; Invitrogen, Carlsbad, CA, USA) was added to each assay well in a low-volume 384-well plate (Corning Part #3676) for 1-h incubation at room temperature. Fluorescence resonance energy transfer signal was read using a Tecan Infinite F-500 plate reader. The emission ratio was defined as the acceptor/tracer emission (665 nm) divided by the antibody/donor emission (615 nm). IC50 value was determined based on the sigmoidal dose−response curve that was generated using GraphPad Prism software.
ALK5 kinase activity assay
The in vitro ALK5 kinase activity assay was performed as previously described48. ALK5 were obtained from Invitrogen (Carlsbad, CA, USA). Briefly, a mixture of ALK5 (2 μg) and bacterially expressed GST-Smad3 (50 mg/ml) in kinase buffer (pH 7.4; 5 mM Tris, 1 mM MgCl2, and 0.1 mM CaCl2) containing 50 mM ATP was treated with or without the selected flavonoids, and was then incubated for 45 min at room temperature. Phospho-Smad3 and GST-Smad3 in the products were detected by western blotting.
Statistical analysis
To determine significant differences between groups in the case of count data, χ2 test was performed. In the case of continuous data, differences between the two groups were analyzed through the Student’s t test. Continuous data were reported as mean ± standard error (SD). The statistical analyses were performed with the statistical software package SPSS 23.0 (IBM, USA). P values less than 0.05 were considered statistically significant.
References
Rinkevich, Y. et al. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348, aaa2151 (2015).
Abignano, G. & Del, G. F. Quantitating skin fibrosis: innovative strategies and their clinical implications. Curr. Rheumatol. Rep. 16, 404 (2014).
Babalola, O., Mamalis, A., Lev-Tov, H. & Jagdeo, J. The role of microRNAs in skin fibrosis. Arch. Dermatol. Res. 305, 763–776 (2013).
Yamaguchi, Y. et al. A peptide derived from endostatin ameliorates organ fibrosis. Sci. Transl. Med. 4, 136ra71 (2012).
Friedstat, J. S. & Hultman, C. S. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann. Plast. Surg. 72, S198–S201 (2014).
Kwak, I. S., Choi, Y. H., Jang, Y. C. & Lee, Y. K. Immunohistochemical analysis of neuropeptides (protein gene product 9.5, substance P and calcitonin gene-related peptide) in hypertrophic burn scar with pain and itching. Burns 40, 1661–1667 (2014).
Babalola, O., Mamalis, A., Lev-Tov, H. & Jagdeo, J. NADPH oxidase enzymes in skin fibrosis: molecular targets and therapeutic agents. Arch. Dermatol. Res. 306, 313–330 (2014).
Canady, J., Karrer, S., Fleck, M. & Bosserhoff, A. K. Fibrosing connective tissue disorders of the skin: molecular similarities and distinctions. J. Dermatol. Sci. 70, 151–158 (2013).
Jinnin, M. Mechanisms of skin fibrosis in systemic sclerosis. J. Dermatol. 37, 11–25 (2010).
Chizzolini, C., Brembilla, N. C., Montanari, E. & Truchetet, M. E. Fibrosis and immune dysregulation in systemic sclerosis. Autoimmun. Rev. 10, 276–281 (2011).
Beanes, S. R., Dang, C., Soo, C. & Ting, K. Skin repair and scar formation: the central role of TGF-beta. Expert Rev. Mol. Med. 5, 1–22 (2003).
Xiao, L. et al. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front. Biosci. (LandmarkEd) 17, 2667–2674 (2012).
Carthy, J. M. et al. Tamoxifen inhibits TGF-beta-mediated activation of myofibroblasts by blocking non-Smad signaling through ERK1/2. J. Cell. Physiol. 230, 3084–3092 (2015).
Desmouliere, A., Geinoz, A., Gabbiani, F. & Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 122, 103–111 (1993).
Yano, H. et al. Smad, but not MAPK, pathway mediates the expression of type I collagen in radiation induced fibrosis. Biochem. Biophys. Res. Commun. 418, 457–463 (2012).
Atala, E., Fuentes, J., Wehrhahn, M. J. & Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 234, 479–485 (2017).
Zhang, Y. F. et al. Quercetin promotes neuronal and behavioral recovery by suppressing inflammatory response and apoptosis in a rat model of intracerebral hemorrhage. Neurochem. Res. 40, 195–203 (2015).
Wang, Y. et al. Baicalein upregulates DDIT4 expression which mediates mTOR inhibition and growth inhibition in cancer cells. Cancer Lett. 358, 170–179 (2015).
Wu, K., Li, H., Tian, J. & Lei, W. Protective effect of baicalein on renal ischemia/reperfusion injury in the rat. Ren. Fail. 37, 285–291 (2015).
Heeba, G. H. & Mahmoud, M. E. Therapeutic potential of morin against liver fibrosis in rats: modulation of oxidative stress, cytokine production and nuclear factor kappa B. Environ. Toxicol. Pharmacol. 37, 662–671 (2014).
Balta, C. et al. Chrysin attenuates liver fibrosis and hepatic stellate cell activation through TGF-beta/Smad signaling pathway. Chem. Biol. Interact. 240, 94–101 (2015).
Zhang, L. et al. Baicalin inhibits hypoxia-induced pulmonary artery smooth muscle cell proliferation via the AKT/HIF-1alpha/p27-associated pathway. Int. J. Mol. Sci. 15, 8153–8168 (2014).
Zhang, Y. F. et al. Baicalein attenuates hypertrophic scar formation via inhibition of the transforming growth factor-beta/Smad2/3 signalling pathway. Br. J. Dermatol. 174, 120–130 (2016).
Zhang, Y. F. et al. Galangin inhibits hypertrophic scar formation via ALK5/Smad2/3 signaling pathway. Mol. Cell. Biochem. 413, 109–118 (2016).
Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Invest. Dermatol. 127, 526–537 (2007).
Moustakas, A. Smad signalling network. J. Cell Sci. 115, 3355–3356 (2002).
Lin, A., Cai, Z., Hu, G. & Li, Q. Identification of ALK5 inhibitor via structure-based virtual screening and ADMET prediction. J. Recept. Signal. Transduct. Res. 35, 559–564 (2015).
Huynh, Q. K. et al. Screening and identification of a novel class of TGF-beta type 1 receptor kinase inhibitor. J. Biomol. Screen. 16, 724–733 (2011).
Kudo, H. et al. Decreased interleukin-20 expression in scleroderma skin contributes to cutaneous fibrosis. Arthritis Rheumatol. 66, 1636–1647 (2014).
Patel, H. M. et al. Design, synthesis and evaluation of small molecule imidazo[2,1-b][1,3,4]thiadiazoles as inhibitors of transforming growth factor-beta type-I receptor kinase (ALK5). Eur. J. Med. Chem. 93, 599–613 (2015).
Sapitro, J. et al. Suppression of transforming growth factor-beta effects in rabbit subconjunctival fibroblasts by activin receptor-like kinase 5 inhibitor. Mol. Vis. 16, 1880–1892 (2010).
Kumar, S. & Pandey, A. K. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal 2013, 162750 (2013).
Cassidy, A., Huang, T., Rice, M. S., Rimm, E. B. & Tworoger, S. S. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am. J. Clin. Nutr. 100, 1344–1351 (2014).
Safina, A., Vandette, E. & Bakin, A. V. ALK5 promotes tumor angiogenesis by upregulating matrix metalloproteinase-9 in tumor cells. Oncogene 26, 2407–2422 (2007).
Imaizumi, N., Monnier, Y., Hegi, M., Mirimanoff, R. O. & Ruegg, C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS ONE 5, e11084 (2010).
Park, C. Y. et al. An novel inhibitor of TGF-beta type I receptor, IN-1130, blocks breast cancer lung metastasis through inhibition of epithelial-mesenchymal transition. Cancer Lett. 351, 72–80 (2014).
Pang, L. et al. TGF-beta1/Smad signaling pathway regulates epithelial-to-mesenchymal transition in esophageal squamous cell carcinoma: in vitro and clinical analyses of cell lines and nomadic Kazakh patients from northwest Xinjiang, China. PLoS ONE 9, e112300 (2014).
Goumans, M. J. et al. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell 12, 817–828 (2003).
Munoz-Felix, J. M., Gonzalez-Nunez, M. & Lopez-Novoa, J. M. ALK1-Smad1/5 signaling pathway in fibrosis development: friend or foe? Cytokine Growth Factor Rev. 24, 523–537 (2013).
Lago, J. H. et al. Structure−activity association of flavonoids in lung diseases. Molecules 19, 3570–3595 (2014).
Xie, Y., Yang, W., Tang, F., Chen, X. & Ren, L. Antibacterial activities of flavonoids: structure−activity relationship and mechanism. Curr. Med. Chem. 22, 132–149 (2015).
Kumar, S., Mishra, A. & Pandey, A. K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 13, 120 (2013).
Jin, H. et al. Epigenetic silencing of a Ca(2+)-regulated Ras GTPase-activating protein RASAL defines a new mechanism of Ras activation in human cancers. Proc. Natl. Acad. Sci. USA 104, 12353–12358 (2007).
Woeller, C. F., O’Loughlin, C. W., Roztocil, E., Feldon, S. E. & Phipps, R. P. Salinomycin and other polyether ionophores are a new class of antiscarring agent. J. Biol. Chem. 290, 3563–3575 (2015).
Yamamoto, T. et al. Animal model of sclerotic skin. I: local injections of bleomycin induce sclerotic skin mimicking scleroderma. J. Invest. Dermatol. 112, 456–462 (1999).
Aarabi, S. et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21, 3250–3261 (2007).
Lebakken, C. S. et al. Development and applications of a broad-coverage, TR-FRET-based kinase binding assay platform. J. Biomol. Screen. 14, 924–935 (2009).
Wrighton, K. H., Lin, X., Yu, P. B. & Feng, X. H. Transforming growth factor {beta} can stimulate Smad1 phosphorylation independently of bone morphogenic protein receptors. J. Biol. Chem. 284, 9755–9763 (2009).
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (No. 81601698), National Natural Science Foundation of China (No. 81620108019) and Shanghai Sailing Program (grant No.16YF1406700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by E. Candi
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, Y., Wang, J., Zhou, S. et al. Flavones hydroxylated at 5, 7, 3′ and 4′ ameliorate skin fibrosis via inhibiting activin receptor-like kinase 5 kinase activity. Cell Death Dis 10, 124 (2019). https://doi.org/10.1038/s41419-019-1333-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-019-1333-7
This article is cited by
-
Salvianolic Acid B Attenuates Hypertrophic Scar Formation In Vivo and In Vitro
Aesthetic Plastic Surgery (2023)
-
Alpinumisoflavone ameliorates choroidal neovascularisation and fibrosis in age-related macular degeneration in in vitro and in vivo models
Scientific Reports (2022)