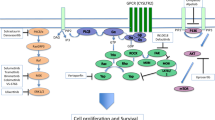
Abstract
Uveal melanoma is a rare malignancy affecting 5.1 patients/million per year with definitive treatment options of enucleation or radiation therapy to the primary tumor. Unfortunately, no FDA-approved systemic therapies exist for patients in the adjuvant or metastatic setting. Molecular profiling over the past decade has helped define uveal melanomas by characteristic mutations: GNAQ, GNA11, BAP1, SF3B1, and EIF1AX mutations. GNAQ/11 mutations are present in over 90% of patients with uveal melanoma and lead to signal transduction through G-protein coupled receptors to downstream growth factors. PKC inhibition has been an active area of investigation targeting this pathway specific to uveal melanoma. Several molecules have been developed and evaluated in clinical trials. Responses have been noted but clinical development has also yielded multiple toxicities and pathways of resistance limiting both breadth and durability of responses leading to combination therapy approaches. PKC inhibition remains an active and encouraging area of research to determine effective therapies for patients with uveal melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye. 2017;31:241–57.
Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44:4651–9.
Pelster MS, Gruschkus SK, Bassett R, Gombos DS, Shephard M, Posada L, et al. Nivolumab and ipilimumab in metastatic uveal melanoma: Results from a single-arm phase II study. J Clin Oncol. 2021;39:599–607.
Bedikian AY, Papadopoulos N, Plager C, Eton O, Ring S. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003;13:303–6.
Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: A phase III, multicenter, randomized trial (SUMIT). J Clin Oncol: Off J Am Soc Clin Oncol. 2018;36:1232–9.
Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21.
Arora S, Velichinskii R, Lesh RW, Ali U, Kubiak M, Bansal P, et al. Existing and emerging biomarkers for immune checkpoint immunotherapy in solid tumors. Adv Ther 2019;36:2638–78.
Zimmer L, Vaubel J, Mohr P, Hauschild A, Utikal J, Simon J, et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS One. 2015;10:e0118564.
Joshua AM, Monzon JG, Mihalcioiu C, Hogg D, Smylie M, Cheng T. A phase 2 study of tremelimumab in patients with advanced uveal melanoma. Melanoma Res. 2015;25:342–7.
Algazi AP, Tsai KK, Shoushtari AN, Munhoz RR, Eroglu Z, Piulats JM, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016;122:3344–53.
Heppt MV, Amaral T, Kahler KC, Heinzerling L, Hassel JC, Meissner M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: A retrospective, multi-center study. J Immunother Cancer. 2019;7:299.
Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206.
Damato BE, Dukes J, Goodall H, Carvajal RD. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers. 2019;11:971.
Shields CL, Dalvin LA, Vichitvejpaisal P, Mazloumi M, Ganguly A, Shields JA. Prognostication of uveal melanoma is simple and highly predictive using The Cancer Genome Atlas (TCGA) classification: A review. Indian J Ophthalmol. 2019;67:1959–63.
Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32:204–20. e15
Harbour JW, Onken MD, Roberson EDO, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3.
Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25:234–9.
Bakhoum MF, Esmaeli B. Molecular characteristics of uveal melanoma: Insights from the Cancer Genome Atlas (TCGA) Project. Cancers. 2019;11:1061.
Ma J, Weng L, Bastian BC, Chen X. Functional characterization of uveal melanoma oncogenes. Oncogene 2021;40:806–20.
Breitkreutz D, Braiman-Wiksman L, Daum N, Denning MF, Tennenbaum T. Protein kinase C family: On the crossroads of cell signaling in skin and tumor epithelium. J Cancer Res Clin Oncol. 2007;133:793–808.
Wu X, Li J, Zhu M, Fletcher JA, Hodi FS. Protein kinase C inhibitor AEB071 targets ocular melanoma harboring GNAQ mutations via effects on the PKC/Erk1/2 and PKC/NF-kappaB pathways. Mol Cancer Ther. 2012;11:1905–14.
Piperno-Neumann S, Larkin J, Carvajal RD, Luke JJ, Schwartz GK, Hodi FS, et al. Genomic profiling of metastatic uveal melanoma and clinical results of a phase I study of the protein kinase C inhibitor AEB071. Mol Cancer Ther. 2020;19:1031–9.
Musi E, Ambrosini G, de Stanchina E, Schwartz GK. The phosphoinositide 3-kinase alpha selective inhibitor BYL719 enhances the effect of the protein kinase C inhibitor AEB071 in GNAQ/GNA11-mutant uveal melanoma cells. Mol Cancer Ther. 2014;13:1044–53.
Wu X, Zhu M, Fletcher JA, Giobbie-Hurder A, Hodi FS. The protein kinase C inhibitor enzastaurin exhibits antitumor activity against uveal melanoma. PLoS One. 2012;7:e29622.
Chen X, Wu Q, Tan L, Porter D, Jager MJ, Emery C, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene. 2014;33:4724–34.
Sagoo MS, Harbour JW, Stebbing J, Bowcock AM. Combined PKC and MEK inhibition for treating metastatic uveal melanoma. Oncogene. 2014;33:4722–3.
Croce M, Ferrini S, Pfeffer U, Gangemi R. Targeted therapy of uveal melanoma: Recent failures and new perspectives. Cancers. 2019;11:846.
Li Y, Shi J, Yang J, Ge S, Zhang J, Jia R, et al. Uveal melanoma: Progress in molecular biology and therapeutics. Ther Adv Med Oncol. 2020;12:1758835920965852.
SMR Congress 2017 abstracts. Pigment Cell & Melanoma Research. 2018;31:125-230.
Kapiteijn E, Carlino M, Boni V, Loirat D, Speetjens F, Park J, et al. Abstract CT068: A Phase I trial of LXS196, a novel PKC inhibitor for metastatic uveal melanoma. Cancer Research. 2019;79(13 Supplement):CT068.
IDEAYA Biosciences. Darovasertib (IDE196) Investor Day April 2021. [PDF] https://filecache.investorroom.com/mr5ir_ideayabio/154/20210416_IDE196%20Investor%20Day_vFF.pdf.
Frey CR, Wagle M-C, Vaidya K, Hambleton J, Lackner M, Mounir Z. Abstract 5337: Analysis of drug combinations with the PKC inhibitor IDE196 support dual MEK and PKC inhibition as a rational combination in metastatic uveal melanoma. Cancer Research. 2020;80(16 Supplement):5337-.
Wagle M-C, Ravindran N, Pankajakshan D, Lackner M, Mounir Z. Abstract 1343: Preclinical evaluation of a PKC and MET inhibitor combination in metastatic uveal melanoma. Cancer Research. 2021;81(13 Supplement):1343-.
Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, et al. Co-targeting HGF/cMET signaling with MEK inhibitors in metastatic uveal melanoma. Mol Cancer Ther. 2017;16:516–28.
IDEAYA Biosciences. Darovasertib (IDE196) Investor Day August 2021. [PDF] https://ir.ideayabio.com/image/20210416_IDE196+Investor+Day_vFF.pdf.
Faller DV. Response to “selective PKCdelta inhibitor B106 elicits uveal melanoma growth inhibitory effects independent of activated PKC isoforms”. ACS Chem Biol. 2019;14:131.
Heijkants R, Teunisse A, de Vries J, Ovaa H, Jochemsen A. Selective PKCdelta inhibitor B106 elicits uveal melanoma growth inhibitory effects independent of activated PKC isoforms. ACS Chem Biol. 2019;14:132–6.
Balasubramanya R, Selvarajan SK, Cox M, Joshi G, Deshmukh S, Mitchell DG, et al. Imaging of ocular melanoma metastasis. Br J Radiol. 2016;89:20160092.
Park JJ, Diefenbach RJ, Byrne N, Long GV, Scolyer RA, Gray ES, et al. Circulating tumor DNA reflects uveal melanoma responses to protein kinase C inhibition. Cancers. 2021;13:1740.
Carita G, Frisch-Dit-Leitz E, Dahmani A, Raymondie C, Cassoux N, Piperno-Neumann S, et al. Dual inhibition of protein kinase C and p53-MDM2 or PKC and mTORC1 are novel efficient therapeutic approaches for uveal melanoma. Oncotarget. 2016;7:33542–56.
Chen X, Wu Q, Depeille P, Chen P, Thornton S, Kalirai H, et al. RasGRP3 mediates MAPK pathway activation in GNAQ mutant uveal melanoma. Cancer Cell. 2017;31:685–96. e6
Paradis JS, Acosta M, Saddawi-Konefka R, Kishore A, Gomes F, Arang N, et al. Synthetic lethal screens reveal cotargeting FAK and MEK as a multimodal precision therapy for GNAQ-driven uveal melanoma. Clin Cancer Res. 2021;27:3190–200.
Harbour JW. Therapeutic escape in Galphaq-mutant uveal melanoma: It’s a FAK. Clin Cancer Res. 2021;27:2967–9.
Yoo JH, Shi DS, Grossmann AH, Sorensen LK, Tong Z, Mleynek TM, et al. ARF6 is an actionable node that orchestrates oncogenic GNAQ signaling in uveal melanoma. Cancer Cell. 2016;29:889–904.
Cerne JZ, Hartig SM, Hamilton MP, Chew SA, Mitsiades N, Poulaki V, et al. Protein kinase C inhibitors sensitize GNAQ mutant uveal melanoma cells to ionizing radiation. Invest Ophthalmol Vis Sci. 2014;55:2130–9.
Funding
No financial assistance was received in support of the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors acquired the content and interpreted it, as well as drafted/revised the manuscript, approved the final version, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
CL has no competing financial interest to disclose. MM has the following competing interests: Research Grants—Paid to Institution—Ascentage Pharma Group, Bicycle Therapeutics, Dragonfly Therapeutics, Epizyme, Exelixis, Genentech, GlaxoSmithKline, IDEAYA Biosciences, Ikena Oncology, Infinity Pharmaceuticals, Jacobio Pharmaceuticals, Moderna, NBE Therapeutics, Novartis, Oncorus, Plexxikon, Prelude Therapeutics, Regeneron, Sapience Therapeutics, Seattle Genetics, Tizona Therapeutics, TMUNITY Therapeutics, TopAlliance Biosciences, Bayer, BioMed Valley Discoveries, EMD Serono, MedImmune, Nektar Therapeutics, Pfizer, Teneobio, Arvinas, Tempest Therapeutics, Arcus Biosciences, Synthorax, Alpine Immune, Scholar Rock, BioNTech, Erasca, Kechow Pharma, Mereo BioPharma, Foghorn Therapeutics, Pyramid Biosciences, PACT pharma, ImmVira Pharma, Kinnate Biopharma, Metabomed. Consulting/Advisory Role—Paid to Institution—Array BioPharma, AstraZeneca, MedPage Today, Pfizer, Regeneron Pharmaceuticals, Astellas Pharma, BicycleTx Limited, Castle Biosciences, Ideaya Biosciences, iTeos.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lietman, C.D., McKean, M. Targeting GNAQ/11 through PKC inhibition in uveal melanoma. Cancer Gene Ther 29, 1809–1813 (2022). https://doi.org/10.1038/s41417-022-00437-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-022-00437-6
This article is cited by
-
Transactivation of Met signaling by oncogenic Gnaq drives the evolution of melanoma in Hgf-Cdk4 mice
Cancer Gene Therapy (2024)