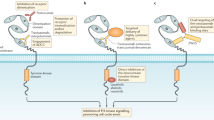
Abstract
The incidence of breast cancer (BC) has been increasing each year, and BC is now the most common malignant tumor in women. Among the numerous BC subtypes, HER2-positive BC can be treated with a variety of strategies based on targeting HER2. Although there has been great progress in the treatment of HER2-positive BC, recurrence, metastasis and drug resistance remain considerable challenges. The dysfunction of ion channels and transporters can affect the development and progression of HER2-positive BC, so these entities are expected to be new therapeutic targets. This review summarizes various ion channels and transporters associated with HER2-positive BC and suggests potential targets for the development of new and effective therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–60.
Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol. 2013;31:3997–4013.
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–74.
Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–8.
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–23.
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–43.
Kreutzfeldt J, Rozeboom B, Dey N, De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10:1045–67.
Tesch ME, Gelmon KA. Targeting HER2 in breast cancer: latest developments on treatment sequencing and the introduction of biosimilars. Drugs. 2020;80:1811–30.
Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer 2020;126:4278–88.
Ma Z, Yuan D, Cheng X, Tuo B, Liu X, Li T. Function of ion transporters in maintaining acid–base homeostasis of the mammary gland and the pathophysiological role in breast cancer. Am J Physiol-Regulatory, Integr Comp Physiol. 2020;318:R98–111.
Wang L, Yule DI. Differential regulation of ion channels function by proteolysis. Biochim Biophys Acta Mol Cell Res 2018;1865:1698–706. 11 Pt B
Zhang M, Li T, Zhu J, Tuo B, Liu X. Physiological and pathophysiological role of ion channels and transporters in the colorectum and colorectal cancer. J Cell Mol Med. 2020;24:9486–94.
Perez-Neut M, Rao VR, Gentile S. hERG1/Kv11.1 activation stimulates transcription of p21waf/cip in breast cancer cells via a calcineurin-dependent mechanism. Oncotarget 2016;7:58893–902.
Britschgi A, Bill A, Brinkhaus H, Rothwell C, Clay I, Duss S, et al. Calcium-activated chloride channel ANO1 promotes breast cancer progression by activating EGFR and CAMK signaling. Proc Natl Acad Sci USA. 2013;110:E1026–34.
Jeong J, VanHouten JN, Dann P, Kim W, Sullivan C, Yu H, et al. PMCA2 regulates HER2 protein kinase localization and signaling and promotes HER2-mediated breast cancer. Proc Natl Acad Sci USA. 2016;113:E282–90.
Lee WJ, Roberts-Thomson SJ, Holman NA, May FJ, Lehrbach GM, Monteith GR. Expression of plasma membrane calcium pump isoform mRNAs in breast cancer cell lines. Cell Signal. 2002;14:1015–22.
Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem Biophys Res Commun. 2005;337:779–83.
Pedersen SF, Stock C. Ion channels and transporters in cancer: pathophysiology, regulation, and clinical potential. Cancer Res. 2013;73:1658–61.
Andersen AP, Moreira JMA, Pedersen SF. Interactions of ion transporters and channels with cancer cell metabolism and the tumour microenvironment. Philos Trans R Soc B: Biol Sci. 2014;369:20130098.
White KA, Grillo-Hill BK, Barber DL. Cancer cell behaviors mediated by dysregulated pH dynamics at a glance. J Cell Sci. 2017;130:663–9.
Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–23.
Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc B: Biol Sci. 2014;369:20130099.
Shenolikar S, Voltz JW, Cunningham R, Weinman EJ. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology (Bethesda). 2004;19:362–9.
Weinman EJ, Hall RA, Friedman PA, Liu-Chen L-Y, Shenolikar S. The association of NHERF adaptor proteins with G protein–coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505.
Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–79.
Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest. 1995;95:2143–9.
Dai JL, Wang L, Sahin AA, Broemeling LD, Schutte M, Pan Y. NHERF (Na+/H+ exchanger regulatory factor) gene mutations in human breast cancer. Oncogene 2004;23:8681–7.
Plummer SJ, Adams L, Simmons JA, Casey G. Localization of a growth suppressor activity in MCF7 breast cancer cells to chromosome 17q24-q25. Oncogene 1997;14:2339–45.
Presneau N, Dewar K, Forgetta V, Provencher D, Mes-Masson A-M, Tonin PN. Loss of heterozygosity and transcriptome analyses of a 1.2 Mb candidate ovarian cancer tumor suppressor locus region at 17q25.1-q25.2. Mol Carcinog. 2005;43:141–54.
Mangia A, Chiriatti A, Bellizzi A, Malfettone A, Stea B, Zito FA, et al. Biological role of NHERF1 protein expression in breast cancer. Histopathology 2009;55:600–8.
Paradiso A, Scarpi E, Malfettone A, Addati T, Giotta F, Simone G, et al. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis. 2013;4:e904.
Bellizzi A, Malfettone A, Cardone RA, Mangia A. NHERF1/EBP50 in breast cancer: clinical perspectives. Breast Care. 2010;5:86–90.
Jeong J, VanHouten JN, Kim W, Dann P, Sullivan C, Choi J, et al. The scaffolding protein NHERF1 regulates the stability and activity of the tyrosine kinase HER2. J Biol Chem. 2017;292:6555–68.
Boedtkjer E, Bunch L, Pedersen SF. Physiology, pharmacology and pathophysiology of the pH regulatory transport proteins NHE1 and NBCn1: similarities, differences, and implications for cancer therapy. Curr Pharm Des. 2012;18:1345–71.
Romero MF, Chen AP, Parker MD, Boron WF. The SLC4 family of bicarbonate (HCO3−) transporters. Mol Asp Med. 2013;34:159–82.
Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–75.
Boedtkjer E, Praetorius J, Füchtbauer E-M, Aalkjaer C. Antibody-independent localization of the electroneutral Na+-HCO3− cotransporter NBCn1 (slc4a7) in mice. Am J Physiol-Cell Physiol. 2008;294:C591–C603.
Boedtkjer E, Moreira JMA, Mele M, Vahl P, Wielenga VT, Christiansen PM, et al. Contribution of Na+,HCO3−-cotransport to cellular pH control in human breast cancer: a role for the breast cancer susceptibility locus NBCn1 (SLC4A7). Int J Cancer. 2013;132:1288–99.
Lee S, Axelsen TV, Jessen N, Pedersen SF, Vahl P, Boedtkjer E. Na+,HCO3−-cotransporter NBCn1 (Slc4a7) accelerates ErbB2-induced breast cancer development and tumor growth in mice. Oncogene 2018;37:5569–84.
Andersen AP, Samsøe-Petersen J, Oernbo EK, Boedtkjer E, Moreira JMA, Kveiborg M, et al. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int J Cancer. 2018;142:2529–42.
Pedersen K, Angelini P-D, Laos S, Bach-Faig A, Cunningham MP, Ferrer-Ramón C, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009;29:3319–31.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–82.
Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–38.
Lauritzen G, Jensen MBF, Boedtkjer E, Dybboe R, Aalkjaer C, Nylandsted J, et al. NBCn1 and NHE1 expression and activity in ΔNErbB2 receptor-expressing MCF-7 breast cancer cells: contributions to pHi regulation and chemotherapy resistance. Exp Cell Res. 2010;316:2538–53.
Lauritzen G, Stock C-M, Lemaire J, Lund SF, Jensen MF, Damsgaard B, et al. The Na+/H+ exchanger NHE1, but not the Na+, HCO3− cotransporter NBCn1, regulates motility of MCF7 breast cancer cells expressing constitutively active ErbB2. Cancer Lett. 2012;317:172–83.
Gorbatenko A, Olesen CW, M⊘rup N, Thiel G, Kallunki T, Valen E, et al. NaErbB2 upregulates the Na+,HCO3−-cotransporter NBCn1/SLC4A7 in human breast cancer cells via Akt, ERK, Src, and Kruppel-like factor 4. FASEB J. 2013;28:350–63.
Gorbatenko A, Olesen CW, Loebl N, Sigurdsson HH, Bianchi C, Pedraz-Cuesta E, et al. Oncogenic p95HER2 regulates Na+–HCO3− cotransporter NBCn1 mRNA stability in breast cancer cells via 3′UTR-dependent processes. Biochem J. 2016;473:4027–44.
Baenke F, Dubuis S, Brault C, Weigelt B, Dankworth B, Griffiths B, et al. Functional screening identifies MCT4 as a key regulator of breast cancer cell metabolism and survival. J Pathol. 2015;237:152–65.
Kronblad Å, Jirström K, Rydén L, Nordenskjöld B, Landberg G. Hypoxia inducible factor-1α is a prognostic marker in premenopausal patients with intermediate to highly differentiated breast cancer but not a predictive marker for tamoxifen response. Int J Cancer. 2006;118:2609–16.
Brennan DJ, Jirstrom K, Kronblad A, Millikan RC, Landberg G, Duffy MJ, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. 2006;12:6421–31.
Kaya AO, Gunel N, Benekli M, Akyurek N, Buyukberber S, Tatli H, et al. Hypoxia inducible factor-1 alpha and carbonic anhydrase IX overexpression are associated with poor survival in breast cancer patients. J BUON 2012;17:663–8.
Pedemonte N, Galietta LJ. Structure and function of TMEM16 proteins (anoctamins). Physiol Rev. 2014;94:419–59.
Hartzell HC, Yu K, Xiao Q, Chien L-T, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl− channels. J Physiol. 2009;587:2127–39.
Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008;455:1210–5.
Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 2008;134:1019–29.
Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008;322:590–4.
Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: are they all chloride channels? Acta Pharmacol Sin. 2011;32:685–92.
West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165:107–13.
Huang X, Gollin SM, Raja S, Godfrey TE. High-resolution mapping of the 11q13 amplicon and identification of a gene, TAOS1, that is amplified and overexpressed in oral cancer cells. Proc Natl Acad Sci USA. 2002;99:11369–74.
Carneiro A, Isinger A, Karlsson A, Johansson J, Jonsson G, Bendahl PO, et al. Prognostic impact of array-based genomic profiles in esophageal squamous cell cancer. BMC Cancer. 2008;8:98.
Carles A, Millon R, Cromer A, Ganguli G, Lemaire F, Young J, et al. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene 2006;25:1821–31.
Ubby I, Bussani E, Colonna A, Stacul G, Locatelli M, Scudieri P, et al. TMEM16A alternative splicing coordination in breast cancer. Mol Cancer. 2013;12:75.
Ayoub C, Wasylyk C, Li Y, Thomas E, Marisa L, Robe A, et al. ANO1 amplification and expression in HNSCC with a high propensity for future distant metastasis and its functions in HNSCC cell lines. Br J Cancer. 2010;103:715–26.
Duvvuri U, Shiwarski DJ, Xiao D, Bertrand C, Huang X, Edinger RS, et al. TMEM16A induces MAPK and contributes directly to tumorigenesis and cancer progression. Cancer Res. 2012;72:3270–81.
Ruiz C, Martins JR, Rudin F, Schneider S, Dietsche T, Fischer CA, et al. Enhanced expression of ANO1 in head and neck squamous cell carcinoma causes cell migration and correlates with poor prognosis. PLoS ONE. 2012;7:e43265.
Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca2+-activated Cl− channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012;326:41–51.
St-Pierre Y, Wu H, Guan S, Sun M, Yu Z, Zhao L, et al. Ano1/TMEM16A overexpression is associated with good prognosis in PR-positive or HER2-negative breast cancer patients following tamoxifen treatment. PLoS ONE. 2015;10:e0126128.
Keam SJ. Trastuzumab deruxtecan: first approval. Drugs 2020;80:501–8.
Canonici A, Gijsen M, Mullooly M, Bennett R, Bouguern N, Pedersen K, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 2013;4:1592–605.
Kulkarni S, Bill A, Godse NR, Khan NI, Kass JI, Steehler K, et al. TMEM16A/ANO1 suppression improves response to antibody-mediated targeted therapy of EGFR and HER2/ERBB2. Genes, Chromosomes Cancer. 2017;56:460–71.
Fujimoto M, Inoue T, Kito H, Niwa S, Suzuki T, Muraki K, et al. Transcriptional repression of HER2 by ANO1 Cl− channel inhibition in human breast cancer cells with resistance to trastuzumab. Biochem Biophys Res Commun. 2017;482:188–94.
Matsuba S, Niwa S, Muraki K, Kanatsuka S, Nakazono Y, Hatano N, et al. Downregulation of Ca2+-activated Cl− channel TMEM16A by the inhibition of histone deacetylase in TMEM16A-expressing cancer cells. J Pharmacol Exp Ther. 2014;351:510–8.
Fujimoto M, Kito H, Kajikuri J, Ohya S. Transcriptional repression of human epidermal growth factor receptor 2 by ClC-3 Cl−/H+ transporter inhibition in human breast cancer cells. Cancer Sci. 2018;109:2781–91.
Bailey ST, Miron PL, Choi YJ, Kochupurakkal B, Maulik G, Rodig SJ, et al. NF-κB activation-induced anti-apoptosis renders HER2-positive cells drug resistant and accelerates tumor growth. Mol Cancer Res. 2014;12:408–20.
Liu JUN, Liu YU, Ren Y, Kang LI, Zhang L. Transmembrane protein with unknown function 16A overexpression promotes glioma formation through the nuclear factor-κB signaling pathway. Mol Med Rep. 2014;9:1068–74.
Korn SJ, Trapani JG. Potassium channels. IEEE Trans NanoBioscience. 2005;4:21–33.
Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International union of pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev. 2005;57:463–72.
Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol. 2014;206:151–62.
Lastraioli E. Focus on triple-negative breast cancer: potassium channel expression and clinical correlates. Front Pharmacol. 2020;11:725.
Iorio J, Meattini I, Bianchi S, Bernini M, Maragna V, Dominici L, et al. hERG1 channel expression associates with molecular subtypes and prognosis in breast cancer. Cancer Cell Int. 2018;18:93.
Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–8.
Perez-Neut M, Shum A, Cuevas BD, Miller R, Gentile S. Stimulation of hERG1 channel activity promotes a calcium-dependent degradation of cyclin E2, but not cyclin E1, in breast cancer cells. Oncotarget 2015;6:1631–9.
Fukushiro-Lopes DF, Hegel AD, Rao V, Wyatt D, Baker A, Breuer EK, et al. Preclinical study of a Kv11.1 potassium channel activator as antineoplastic approach for breast cancer. Oncotarget 2018;9:3321–37.
Lansu K, Gentile S. Potassium channel activation inhibits proliferation of breast cancer cells by activating a senescence program. Cell Death Dis. 2013;4:e652.
Breuer E-K, Fukushiro-Lopes D, Dalheim A, Burnette M, Zartman J, Kaja S, et al. Potassium channel activity controls breast cancer metastasis by affecting β-catenin signaling. Cell Death Dis. 2019;10:180.
Assiri AA, Mourad N, Shao M, Kiel P, Liu W, Skaar TC, et al. MicroRNA 362-3p reduces hERG-related current and inhibits breast cancer cells proliferation. Cancer Genomics Proteomics. 2019;16:433–42.
Steudel FA, Mohr CJ, Stegen B, Nguyen HY, Barnert A, Steinle M, et al. SK4 channels modulate Ca2+ signalling and cell cycle progression in murine breast cancer. Mol Oncol. 2017;11:1172–88.
Goda AA, Siddique AB, Mohyeldin M, Ayoub NM, El Sayed KA. The Maxi-K (BK) channel antagonist penitrem A as a novel breast cancer-targeted therapeutic. Mar Drugs. 2018;16:157.
Marino A, Battaglini M, De Pasquale D, Degl’Innocenti A, Ciofani G. Ultrasound-activated piezoelectric nanoparticles inhibit proliferation of breast cancer cells. Sci Rep. 2018;8:6257.
Lu C, Ma Z, Cheng X, Wu H, Tuo B, Liu X, et al. Pathological role of ion channels and transporters in the development and progression of triple-negative breast cancer. Cancer Cell Int. 2020;20:377.
Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–73.
Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29.
Makena MR, Rao R. Subtype specific targeting of calcium signaling in breast cancer. Cell Calcium. 2020;85:102109.
Peters AA, Simpson PT, Bassett JJ, Lee JM, Da Silva L, Reid LE, et al. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor–negative breast cancer. Mol Cancer Ther. 2012;11:2158–68.
Kaemmerer E, Turner D, Peters AA, Roberts-Thomson SJ, Monteith GR. An automated epifluorescence microscopy imaging assay for the identification of phospho-AKT level modulators in breast cancer cells. J Pharmacol Toxicol Methods. 2018;92:13–9.
Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol Cancer Ther. 2002;1:707–17.
O’Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–502.
Yang ZY, Di MY, Yuan JQ, Shen WX, Zheng DY, Chen JZ, et al. The prognostic value of phosphorylated Akt in breast cancer: a systematic review. Sci Rep. 2015;5:7758.
Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95.
Sumoza-Toledo A, Espinoza-Gabriel MI, Montiel-Condado D. Evaluation of the TRPM2 channel as a biomarker in breast cancer using public databases analysis. Bol Med Hosp Infant Mex. 2016;73:397–404.
Putney JW Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–9.
Baldi C, Vazquez G, Boland R. Capacitative calcium influx in human epithelial breast cancer and non-tumorigenic cells occurs through Ca2+ entry pathways with different permeabilities to divalent cations. J Cell Biochem. 2003;88:1265–72.
Reese DM, Slamon DJ. HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells. 1997;15:1–8.
Riese DJ 2nd, van Raaij TM, Plowman GD, Andrews GC, Stern DF. The cellular response to neuregulins is governed by complex interactions of the erbB receptor family. Mol Cell Biol. 1995;15:5770–6.
Holmes WE, Sliwkowski MX, Akita RW, Henzel WJ, Lee J, Park JW, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–10.
Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–61.
Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21.
Keizer J, Li YX, Stojilković S, Rinzel J. InsP3-induced Ca2+ excitability of the endoplasmic reticulum. Mol Biol Cell. 1995;6:945–51.
Li YX, Keizer J, Stojilković SS, Rinzel J. Ca2+ excitability of the ER membrane: an explanation for IP3-induced Ca2+ oscillations. Am J Physiol. 1995;269:C1079–92.
van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337.
Liao JY, Li LL, Wei Q, Yue JC. Heregulinbeta activates store-operated Ca2+ channels through c-erbB2 receptor level-dependent pathway in human breast cancer cells. Arch Biochem Biophys. 2007;458:244–52.
Pera E, Kaemmerer E, Milevskiy MJG, Yapa K, O’Donnell JS, Brown MA, et al. The voltage gated Ca2+-channel Cav3.2 and therapeutic responses in breast cancer. Cancer Cell Int. 2016;16:24.
Chovancova B, Liskova V, Miklikova S, Hudecova S, Babula P, Penesova A, et al. Calcium signaling affects migration and proliferation differently in individual cancer cells due to nifedipine treatment. Biochem Pharmacol. 2020;171:113695.
Wang L, Zhang Y, Wu X, Yu G. Aquaporins: new targets for cancer therapy. Technol Cancer Res Treat. 2016;15:821–8.
Agre P. The aquaporin water channels. Proc Am Thorac Soc. 2006;3:5–13.
Li C, Wang W. Molecular biology of aquaporins. Adv Exp Med Biol. 2017;969:1–34.
Mobasheri A, Barrett-Jolley R. Aquaporin water channels in the mammary gland: from physiology to pathophysiology and neoplasia. J Mammary Gland Biol Neoplasia. 2014;19:91–102.
Liu S, Zhang S, Jiang H, Yang Y, Jiang Y. Co-expression of AQP3 and AQP5 in esophageal squamous cell carcinoma correlates with aggressive tumor progression and poor prognosis. Med Oncol. 2013;30:636.
Niu D, Kondo T, Nakazawa T, Yamane T, Mochizuki K, Kawasaki T, et al. Expression of aquaporin3 in human neoplastic tissues. Histopathology 2012;61:543–51.
Niu D, Kondo T, Nakazawa T, Kawasaki T, Yamane T, Mochizuki K, et al. Differential expression of aquaporins and its diagnostic utility in thyroid cancer. PLoS ONE. 2012;7:e40770.
Machida Y, Ueda Y, Shimasaki M, Sato K, Sagawa M, Katsuda S, et al. Relationship of aquaporin 1, 3, and 5 expression in lung cancer cells to cellular differentiation, invasive growth, and metastasis potential. Hum Pathol. 2011;42:669–78.
Dorward HS, Du A, Bruhn MA, Wrin J, Pei JV, Evdokiou A, et al. Pharmacological blockade of aquaporin-1 water channel by AqB013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J Exp Clin Cancer Res. 2016;35:36.
Watanabe T, Fujii T, Oya T, Horikawa N, Tabuchi Y, Takahashi Y, et al. Involvement of aquaporin-5 in differentiation of human gastric cancer cells. J Physiol Sci. 2009;59:113–22.
Chen R, Shi Y, Amiduo R, Tuokan T, Suzuk L. Expression and prognostic value of aquaporin 1, 3 in cervical carcinoma in women of Uygur ethnicity from Xinjiang, China. PLoS ONE. 2014;9:e98576.
Kasa P, Farran B, Prasad GLV, Nagaraju GP. Aquaporins in female specific cancers. Gene 2019;700:60–4.
Rodrigues C, Milkovic L, Bujak IT, Tomljanovic M, Soveral G, Cipak Gasparovic A. Lipid profile and aquaporin expression under oxidative stress in breast cancer cells of different malignancies. Oxid Med Cell Longev. 2019;2019:2061830.
Kang S, Chae YS, Lee SJ, Kang BW, Kim JG, Kim WW, et al. Aquaporin 3 expression predicts survival in patients with HER2-positive early breast cancer. Anticancer Res. 2015;35:2775–82.
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH, Park HY, et al. AQP5 expression predicts survival in patients with early breast cancer. Ann Surg Oncol. 2014;21:375–83.
Acknowledgements
We thank Guorong Wen, Hai Jin, Jiaxing An, and Jiaxing Zhu for suggestions for the paper and support with daily experiments.
Funding
This research was supported by the National Natural Science Foundation of China (81860103 and 82070536 to X.M.L., 82160505 to T.L.L., and 82073087 to B.G.T.), 15851 Talent Projects of Zunyi City (2018 to T.L.L.), Zunyi Science and Technology Project [Zunshi Kehe Hz Zi (2019) grant no. 107], Guizhou Province Science Plan Program (Qian Ke He Foundation-ZK [2021] General 461), and Doctor Foundation of Affiliated Hospital of Zunyi Medical University (grant no. 201718).
Author information
Authors and Affiliations
Contributions
Z.X.Z. and C.M.Z. participated in writing the manuscript. Z.Y.M. and H.W. collected the data. B.G.T. and X.M.C. assembled the data. X.M.L. and T.L.L. participated in designing the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Zhang, C., Ma, Z. et al. Pathophysiological role of ion channels and transporters in HER2-positive breast cancer. Cancer Gene Ther 29, 1097–1104 (2022). https://doi.org/10.1038/s41417-021-00407-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-021-00407-4