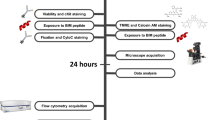
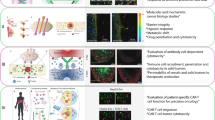
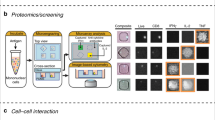
Abstract
Recent advances in microfluidic techniques have enabled researchers to study sensitivities to immune checkpoint therapy, to determine patients’ response to particular antibody treatment. Utilization of this technology is helpful in antibody discovery and in the design of personalized medicine. A variety of microfluidic approaches can provide several functions in processes such as immunologic, genomic, and/or transcriptomic analysis with the aim of improving the efficacy and coverage of immunotherapy, particularly immune checkpoint blockade (ICB). To achieve this requires researchers to overcome the challenges in the current state of the technology. This review looks into the advancements in microfluidic technologies applied to researches on immune checkpoint blockade treatment and its potential shift from proof-of-principle stage to clinical application.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sambi M, Bagheri L, Szewczuk MR. Current challenges in cancer immunotherapy: multimodal approaches to improve efficacy and patient response rates. J Oncol. 2019;2019:4508794.
Reports and Data. Antibodies, Vaccines, Cytokines), By Applications (Lung Cancer, Breast Cancer, Liver Cancer, Head & Neck Cancer), By End Users (Hospitals, Cancer Research Centers, Clinics), And Segment Forecasts, 2016-2026. Available from: https://www.reportsanddata.com/report-detail/cancer-immunotherapy-market. Accessed 1 May 2020.
Chao M, Harris J, Morales RT, Chen W. Microfluidics for immuno-oncology. In: Jiang X, editor. Nanofluidics and microfluidics. 1st edn. Wiley-VCH; 2020. p. 149–76.
Mitchell DA, Sampson JH. Toward effective immunotherapy for the treatment of malignant brain tumors. Neurotherapeutics. 2009;6:527–38.
Nixon NA, Blais N, Ernst S, Kollmannsberger C, Bebb G, Butler M, et al. Current landscape of immunotherapy in the treatment of solid tumours, with future opportunities and challenges. Curr Oncol. 2018;25:e373–84.
Li L, Yan S, Lin B, Shi Q, Lu Y. Single-cell proteomics for cancer immunotherapy. In: Broome AM, editor. Cancer nanotechnology. Elsevier; 2018. p. 185–207.
Ventola CL. Cancer immunotherapy, part 2: efficacy, safety, and other clinical considerations. P T. 2017;42:452–63.
Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8.
Kretschmer A, Schwanbeck R, Valerius T, Rösner T. Antibody isotypes for tumor immunotherapy. Transfus Med Hemother. 2017;44:320–6.
Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2:e192535.
Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med. 2018;50:109.
Kulasinghe A, Wu H, Punyadeera C, Warkiani M. The use of microfluidic technology for cancer applications and liquid biopsy. Micromachines. 2018;9:397.
Sinha N, Subedi N, Tel J. Integrating immunology and microfluidics for single immune cell analysis. Front Immunol. 2018;9:2373.
Lu C, Verbridge S, editors. Microfluidic methods for molecular biology. Cham: Springer; 2016.
Chuah S, Chew V. High-dimensional immuneprofiling in cancer: implications for immunotherapy. J Immunother Cancer. 2020;8:e000363.
Yu Y, Cui J. Present and future of cancer immunotherapy: a tumor microenvironmental perspective. Oncol Lett. 2018;16:4105–13.
Boussommier-Calleja A, Li R, Chen MB, Wong SC, Kamm RD. Microfluidics: a new tool for modeling cancer-immune interactions. Trends Cancer. 2016;2:6–19.
Fares J, Fares MY, Fares Y. Immune checkpoint inhibitors: advances and impact in neuro-oncology. Surg Neurol Int. 2019;10:9.
Bird L. MR1-restricted pan-cancer T cells. Nat Rev Immunol. 2020;20:141.
Zhang J, Shi Z, Xu X, Yu Z, Mi J. The influence of microenvironment on tumor immunotherapy. FEBS J. 2019;286:4160–75.
Jain P, Jain C, Velcheti V. Role of immune-checkpoint inhibitors in lung cancer. Ther Adv Respir Dis. 2018;12:1–13.
An X, Varadarajan N. Single-cell technologies for profiling T cells to enable monitoring of immunotherapies. Curr Opin Chem Eng. 2018;19:142–52.
Armbrecht L, Müller RS, Nikoloff J, Dittrich PS. Single-cell protein profiling in microchambers with barcoded beads. Microsyst Nanoeng. 2019;5:55.
Jiang X, editor. Nanotechnology and microfluidics. 1st edn. Wiley-VCH; 2020.
Merouane A, Rey-Villamizar N, Lu Y, Liadi I, Romain G, Lu J, et al. Automated profiling of individual cell–cell interactions from high-throughput time-lapse imaging microscopy in nanowell grids (TIMING). Bioinformatics 2015;31:3189–97.
Lu Y, Yang L, Wei W, Shi Q. Microchip-based single-cell functional proteomics for biomedical applications. Lab Chip. 2017;17:1250–63.
Wei W, Shin YS, Ma C, Wang J, Elitas M, Fan R, et al. Microchip platforms for multiplex single-cell functional proteomics with applications to immunology and cancer research. Genome Med. 2013;5:75.
Yang L, Wang Z, Deng Y, Li Y, Wei W, Shi Q. Single-cell, multiplexed protein detection of rare tumor cells based on a beads-on-barcode antibody microarray. Anal Chem. 2016;88:11077–83.
Mathur L, Ballinger M, Utharala R, Merten C. Microfluidics as an enabling technology for personalized cancer therapy. Small. 2020;16:1904321.
Truini A, Alama A, Dal Bello MG, Coco S, Vanni I, Rijavec E, et al. Clinical applications of circulating tumor cells in lung cancer patients by CellSearch system. Front Oncol. 2014;4:242.
Janning M, Kobus F, Babayan A, Wikman H, Velthaus JL, Bergmann S, et al. Determination of pd-l1 expression in circulating tumor cells of NSCLC patients and correlation with response to PD-1/PD-L1 inhibitors. Cancers 2019;11:835.
Nicolazzo C, Raimondi C, Mancini M, Caponnetto S, Gradilone A, Gandini O, et al. Monitoring pd-l1 positive circulating tumor cells in non-small cell lung cancer patients treated with the pd-1 inhibitor nivolumab. Sci Rep. 2016;6:31726.
Kloten V, Lampignano R, Krahn T, Schlange T. Circulating tumor cell PD-L1 expression as biomarker for therapeutic efficacy of immune checkpoint inhibition in NSCLC. Cells. 2019;8:809.
Harb W, Fan A, Tran T, Danila DC, Keys D, Schwartz M, et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl Oncol. 2013;6:528–38.
Po JW, Ma Y, Balakrishna B, Brungs D, Azimi F, de Souza P, et al. Immunomagnetic isolation of circulating melanoma cells and detection of PD-L1 status. PLoS ONE. 2019;14:e0211866.
Jan YJ, Chen JF, Zhu Y, Lu YT, Chen SH, Chung H, et al. NanoVelcro rare-cell assays for detection and characterization of circulating tumor cells. Adv Drug Deliv Rev. 2018;125:78–93.
Winograd P, Hou S, Court CM, Lee YT, Chen PJ, Zhu Y, et al. Hepatocellular carcinoma–circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors. Hepatol. Commun. 2020. https://doi.org/10.1002/hep4.1577.
Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489–96.
Kanwar SS, Dunlay CJ, Simeone DM, Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–900.
Iliescu FS, Vrtačnik D, Neuzil P, Iliescu C. Microfluidic technology for clinical applications of exosomes. Micromachines 2019;10:392.
Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490–5.
Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PD-L1 in tumour immune evasion. Nat Rev Immunol. 2020;20:209–15.
Kim DH, Kim H, Choi YJ, Kim SY, Lee JE, Sung KJ, et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp Mol Med. 2019;51:1–13.
Hwang B, Lee JH, Bang D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med. 2018;50:96.
Zou D, Cui D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol Med. 2018;15:335–53.
Cho H, Kim J, Song H, Sohn KY, Jeona MH, Han KH. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018;143:2936–70.
Chen G, Ning B, Shi T. Single-cell RNA-Seq technologies and related computational data analysis. Front Genet. 2019;10:317.
Demaree B, Weisgerber D, Lan F, Abate AR. An ultrahigh-throughput microfluidic platform for single-cell genome sequencing. J Vis Exp. 2018;135:e57598.
Tang X, Huang Y, Lei J, Luo H, Zhu X. The single-cell sequencing: new developments and medical applications. Cell Biosci. 2019;9:53.
Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, et al. Mapping the mouse cell atlas by microwell-seq. Cell 2018;172:1091–107.
Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods. 2017;14:955–8.
Bratman SV, Yang, SYC, Iafolla MAJ, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer. 2020. https://doi.org/10.1038/s43018-020-0096-5.
Jeanbart L, Swartz M. Engineering opportunities in cancer immunotherapy. PNAS. 2015;112:14467–72.
Thurin M, Cesano A, Marincola F, editors. Biomarkers for immunotherapy of cancer. Humana; 2020.
Jamalian S, Jafernejad M, Aref A. Microfluidics and future of cancer diagnostics. In: Aref A, Barbie D, editors. Ex vivo engineering of the tumor microenvironment. Cancer drug discovery and development. Cham: Humana; 2016.
Briones J, Espulgar W, Koyama S, Yoshikawa H, Park J, Naito Y, et al. A microfluidic platform for single cell fluorometric granzyme B profiling. Theranostics. 2020;10:123–32.
Adler A, Mizrahi R, Spindler M, Adams M, Asensio M, Edgar R, et al. Rare, high-affinity mouse anti-PD-1 antibodies that function in checkpoint blockade, discovered using microfluidics and molecular genomics. mAbs. 2017;9:1270–81.
Wuethrich A, Rajkumar AR, Shanmugasundaram KB, Reza K, Dey S, Howard C, et al. Single droplet detection of immune checkpoints on a multiplexed electrohydrodynamic biosensor. Analyst 2019;144:6914–21.
Reza KK, Sina AII, Wuethrich A, Grewal Y, Howard C, Korbie D, et al. A SERS microfluidic platform for targeting multiple soluble immune checkpoints. Biosens Bioelectron. 2018;126:178–86.
Aref AR, Campisi M, Ivanova E, Portell A, Larios D, Piel BP, et al. 3D microfluidic ex vivo culture of organotypic tumor spheroids to model immune checkpoint blockade. Lab Chip. 2018;18:3129–43.
Balko JM, Sosman JA. A critical need for better cancer immunotherapy models: are organotypic tumor spheroid cultures the answer? Cancer Discov. 2018;8:143–5.
Jeffrey T. Borenstein - microfluidic techniques for cancer therapies. Eur Pharm Rev. Available from: https://www.europeanpharmaceuticalreview.com/article/62865/microfluidic-techniques-for-cancer-therapies. Accessed 1 Aug 2020.
Jenkins RW, Aref AR, Lizotte PH, Ivanova E, Stinson S, Zhou CW, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018;8:196–215.
Cañadas I, Thummalapalli R, Kim JW, Kitajima S, Jenkins RW, Christensen CL, et al. Tumor innate immunity primed by specific interferon-stimulated endogenous retroviruses. Nat Med. 2018;24:1143–50.
Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118:9–16.
Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell. 2018;175:998–1013.
Rashidian M, LaFleur M, Verschoor V, Dongre A, Zhang Y, Nguyen T, et al. Immuno-PET identifies the myeloid compartment as a key contributor to the outcome of the antitumor response under PD-1 blockade. PNAS. 2019;116:16971–80.
Kwon S, Velasquez FC, Rasmussen JC, Greives MR, Turner KD, Morrow JR, et al. Nanotopography-based lymphatic delivery for improved anti-tumor responses to checkpoint blockade immunotherapy. Theranostics. 2019;9:8332–43.
Walsh L, Ryu J, Bock S, Koval M, Mauro T, Ross R, et al. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015;15:2434–41.
Hong X, Sullivan R, Kalinich M, Kwan TT, Giobbie-Hurder A, Pan S, et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. PNAS. 2018;115:2467–72.
Jammes FC, Maerkl SJ. How single-cell immunology is benefiting from microfluidic technologies. Microsyst Nanoeng. 2020;6:45.
Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24:144–53.
Armbrecht L, Gabernet G, Kurth F, Hiss JA, Schneider G, Dittrich PS. Characterisation of anticancer peptides at the single-cell level. Lab Chip. 2017;17:2933–40.
Buder-Bakhaya K, Hassel J. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment—a review from the melanoma perspective and beyond. Front Immunol. 2018;9:1474.
Ma S, Murphy T, Lu C. Microfluidics for genome-wide studies involving next generation sequencing. Biomicrofluidics. 2017;11:021501.
Matula K, Rivello F, Huck W. Single‐cell analysis using droplet microfluidics. Adv Biosys. 2020;4:1900188.
H PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35.
Adriani G, Pavesi A, Tan AT, Bertoletti A, Theiry JP, Kamm RD. Microfluidic models for adoptive cell-mediated cancer immunotherapies. Drug Discov Today. 2016;21:1472–8.
Yuki K, Cheng N, Nakano M, Kuo CJ. Organoid models of tumor immunology. Trends Immunol. 2020;41:652–64.
Zhao Z, Zheng L, Chen W, Weng W, Song J, Ji J. Delivery strategies of cancer immunotherapy: recent advances and future perspectives. J Hematol Oncol. 2019;12:126.
Sackmann E, Fulton A, Beebe D. The present and future role of microfluidics in biomedical research. Nature. 2014;507:181–9.
Seah YFS, Hu H, Merten CA. Microfluidic single-cell technology in immunology and antibody screening. Mol Asp Med. 2018;59:47–61.
Cote B, Rao D, Alany RG, Kwon GS, Alani AWG. Lymphatic changes in cancer and drug delivery to the lymphatics in solid tumors. Adv Drug Deliv Rev. 2019;144:16–34.
Wuethrich A, Howard CB, Trau M. Geometric optimisation of electrohydrodynamic fluid flows for enhanced biosensing. Microchem J. 2018;137:231–7.
Joshi P, Lee MY. High content imaging (HCI) on miniaturized three-dimensional (3D) cell cultures. Biosensors. 2015;5:768–90.
Bankó P, Lee SY, Nagygyörgy V, Zrínyi M, Chae CH, Cho DH, et al. Technologies for circulating tumor cell separation from whole blood. J Hematol Oncol. 2019;12:48.
Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374–94.
Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med. 2013;5:179ra47.
Wang L, Balasubramanian P, Chen AP, Kummar S, Evrard YA, Kinders RJ. Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells. Semin Oncol. 2016;43:464–75.
Li X, Li Y, Shao W, Li Z, Zhao R, Ye Z. Strategies for enrichment of circulating tumor cells. Transl Cancer Res. 2020;9:3.
Rao Q, Zhang Q, Zheng C, Dai W, Zhang B, Zanetti CI, et al. Detection of circulating tumour cells in patients with epithelial ovarian cancer by a microfluidic system. Int J Clin Exp Pathol. 2017;10:9599–606.
Lu YT, Delijani K, Mecum A, Goldkorn A. Current status of liquid biopsies for the detection and management of prostate cancer. Cancer Manag Res. 2019;11:5271–91.
Contreras-Naranjo JC, Wu HJ, Ugaz VM. Microfluidics for exosome isolation and analysis: enabling liquid biopsy for personalized medicine. Lab Chip. 2017;17:3558–77.
Yang F, Liao X, Tian Y, Li G. Exosome separation using microfluidic systems: size‐based, immunoaffinity‐based and dynamic methodologies. Biotechnol J. 2017;12:1600699.
Jia Y, Ni Z, Sun H, Wang C. Microfluidic approaches toward the isolation and detection of exosome nanovesicles. IEEE Access. 2019;7:45080–98.
Kordasht HK, Hasanzadeh M. Biomedical analysis of exosomes using biosensing methods: recent progress. Anal Methods. 2020;12:2795–811.
Acknowledgements
We acknowledge the financial support provided by the Core Research for Evolutionary Science and Technology (CREST) Program with JST CREST Grant Number JPMJCR16G2, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Briones, J., Espulgar, W., Koyama, S. et al. The future of microfluidics in immune checkpoint blockade. Cancer Gene Ther 28, 895–910 (2021). https://doi.org/10.1038/s41417-020-00248-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-020-00248-7