Abstract
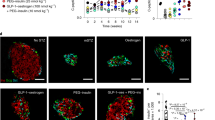
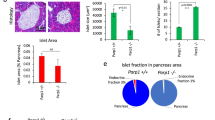
We reported that inactivation of menin (the protein product of MEN1) increases activity of Dnmt1 and mediates DNA hypermethylation in the development of multiple endocrine neoplasia type 1 (MEN1) syndrome. We have developed a RCAS-TVA-based somatic gene transfer system that enables tissue-specific delivery of Dnmt1 to individual β-cells of the pancreas in a RIP-TVA mouse model. In the present study, we mediated Dnmt1 expression in islet β-cells in RIP-TVA mice by utilizing the RCAS-TVA system to test if the upregulation of Dnmt1 can promote β-cell proliferation. In vitro, we demonstrated that upregulation of Dnmt1 increased β-cell proliferation. In vivo, our results showed that the levels of serum insulin were increased in the RIP-TVA mice with RCASBP-Dnmt1 infection compared with wild-type control mice with RCASBP-Dnmt1 infection. Furthermore, we confirmed that mRNA and protein expression of Dnmt1 as well as Dnmt1 enzyme activity were upregulated in the RIP-TVA mice with RCASBP-Dnmt1 infection compared with wild-type control mice with RCASBP-Dnmt1 infection. Finally, we demonstrated that upregulation of Dnmt1 resulted in hyperplasia through β-cell proliferation. We conclude that the upregulation of Dnmt1 promotes islet β-cell proliferation and targeting Dnmt1 may be a promising therapy for patients suffering from pancreatic neuroendocrine tumors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7.
Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–94.
Marx SJ, Agarwal SK, Kester MB, Heppner C, Kim YS, Emmert-Buck MR, et al. Germline and somatic mutation of the gene for multiple endocrine neoplasia type 1 (MEN1). J Intern Med. 1998;243:447–53.
Agarwal SK, Lee Burns A, Sukhodolets KE, Kennedy PA, Obungu VH, Hickman AB, et al. Molecular pathology of the MEN1 gene. Ann N Y Acad Sci. 2004;1014:189–98.
Mutch MG, Dilley WG, Sanjurjo F, DeBenedetti MK, Doherty GM, Wells SA Jr, et al. Germline mutations in the multiple endocrine neoplasia type 1 gene: evidence for frequent splicing defects. Hum Mutat. 1999;13:175–85.
Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32.
Francis J, Lin W, Rozenblatt-Rosen O, Meyerson M. The menin tumor suppressor protein is phosphorylated in response to DNA damage. PLoS ONE. 2011;6:e16119.
Busygina V, Kottemann MC, Scott KL, Plon SE, Bale AE. Multiple endocrine neoplasia type 1 interacts with fork head transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–403.
Agarwal SK, Novotny EA, Crabtree JS, Weitzman JB, Yaniv M, Burns AL, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci Usa. 2003;100:10770–5.
La P, Yang Y, Karnik SK, Silva AC, Schnepp RW, Kim SK, et al. Menin-mediated caspase 8 expression in suppressing multiple endocrine neoplasia type 1. J Biol Chem. 2007;282:31332–40.
Hussein N, Casse H, Fontanière S, Morera AM, Asensio MJ, Bakeli S, et al. Reconstituted expression of menin in Men1-deficient mouse Leydig tumor cells induces cell cycle arrest and apoptosis. Eur J Cancer. 2007;43:402–14.
Farnham PJ. Insights from genomic profiling of transcription factors. Nat Rev Genet. 2009;10:605–16.
Kobayashi Y, Absher DM, Gulzar ZG, Young SR, McKenney JK, Peehl DM, et al. DNA methylation profiling reveals novel biomarkers and important roles for DNA methyltransferases in prostate cancer. Genome Res. 2011;21:1017–27.
Jin B, Ernst J, Tiedemann RL, Xu H, Sureshchandra S, Kellis M, et al. Linking DNA methyltransferases to epigenetic marks and nucleosome structure genome-wide in human tumor cells. Cell Rep. 2012;2:1411–24.
Bestor TH. The DNA methyltransferases of mammals. Hum Mol Genet. 2000;9:2395–402.
Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92.
Lindberg D, Akerström G, Westin G. Evaluation of CDKN2C/p18, CDKN1B/p27 and CDKN2B/p15 mRNA expression, and CpG methylation status in sporadic and MEN1-associated pancreatic endocrine tumours. Clin Endocrinol (Oxf). 2008;68:271–7.
Juhlin CC, Kiss NB, Villablanca A, Haglund F, Nordenström J, Höög A, et al. Frequent promoter hypermethylation of the APC and RASSF1A tumour suppressors in parathyroid tumours. PLoS ONE. 2010;5:e9472.
Yuan Z, Claros CS, Suzuki M, Maggi EC, Kaner KD, Kinstlinger N, et al. Loss of MEN1 activates DNMT1 implicating DNA hypermethylation as a driver of MEN1 tumorigenesis. Oncotarget. 2016;7:12633–50.
Du YC, Lewis BC, Hanahan D, Varmus H. Assessing tumor progression factors by somatic gene transfer into a mouse model: Bcl-xL promotes islet tumor cell invasion. PLoS Biol. 2007;5:e276.
Du YC, Klimstra DS, Varmus H. Activation of PyMT in beta cells induces irreversible hyperplasia, but oncogene-dependent acinar cell carcinomas when activated in pancreatic progenitors. PLoS ONE. 2009;4:e6932.
Zhang G, Chi Y, Du YN. Identification and characterization of metastatic factors by gene transfer into the Novel RIP-Tag; RIP-tva Murine Model. J. Vis. Exp. 2017;Oct 16.
Shen HC, He M, Powell A, Adem A, Lorang D, Heller C, et al. Recapitulation of pancreatic neuroendocrine tumors in human multiple endocrine neoplasia type I syndrome via Pdx1-directed inactivation of Men1. Cancer Res. 2009;69:1858–66.
Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–70.
Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22.
Bird A. DNA methylation patterns and epigenetic memory. Genes & Dev. 2002;16:6–21.
Koh KP, Rao A. DNA methylation and methylcytosine oxidation in cell fate decisions. Curr Opin Cell Biol. 2013;25:152–61.
Ma T, Li H, Sun M, Yuan Y, Sun LP. DNMT1 overexpression predicting gastric carcinogenesis, subsequent progression and prognosis: a meta and bioinformatic analysis. Oncotarget. 2017;8:96396–408.
Denis H, Ndlovu MN, Fuks F. Regulation of mammalian DNA methyltransferases: a route to new mechanisms. EMBO Rep. 2011;12:647–56.
Gokul G, Khosla S. DNA methylation and cancer. Subcell Biochem. 2013;61:597–625.
Ngollo M, Dagdemir A, Karsli-Ceppioglu S, Judes G, Pajon A, Penault-Llorca F, et al. Epigenetic modifications in prostate cancer. Epigenomics. 2014;6:415–26.
Weisenberger DJ. Characterizing DNA methylation alterations from the cancer genome atlas. J Clin Invest. 2014;124:17–23.
Etoh T, Kanai Y, Ushijima S, Nakagawa T, Nakanishi Y, Sasako M, et al. Increased DNA methyltransferase 1 (DNMT1) protein expression correlates significantly with poorer tumor differentiation and frequent DNA hypermethylation of multiple CpG islands in gastric cancers. Am J Pathol. 2004;164:689–99.
Peng DF, Kanai Y, Sawada M, Ushijima S, Hiraoka N, Kitazawa S, et al. DNA methylation of multiple tumor-related genes in association with overexpression of DNA methyltransferase 1 (DNMT1) during multistage carcinogenesis of the pancreas. Carcinogenesis. 2006;27:1160–8.
Qadir XV, Han C, Lu D, Zhang J, Wu T. miR-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am J Pathol. 2014;184:2355–64.
Zhao H, Zhang LE, Guo S, Yuan T, Xia B, Zhang L, et al. Overexpression of DNA methyltransferase 1 as a negative independent prognostic factor in primary gastrointestinal diffuse large B-cell lymphoma treated with CHOP-like regimen and rituximab. Oncol Lett. 2015;9:2307–12.
Rahman MM, Qian ZR, Wang EL, Yoshimoto K, Nakasono M, Sultana R, et al. DNA methyltransferases 1, 3a, and 3b overexpression and clinical significance in gastroenteropancreatic neuroendocrine tumors. Hum Pathol. 2010;41:1069–78.
Li B, Ge Z, Song S, Zhang S, Yan H, Huang B, Zhang Y. Decreased expression of SOX7 is correlated with poor prognosis in lung adenocarcinoma patients. Pathol Oncol Res. 2012;18:1039–45.
Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y. SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer. 2008;98:824–31.
Liu H, Du L, Wen Z, Yang Y, Li J, Dong Z, et al. Sex determining region Y-box 2 inhibits the proliferation of colorectal adenocarcinoma cells through the mTOR signaling pathway. Int J Mol Med. 2013;32:59–66.
Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat. Genet. 2012 Oct;44:1111-6.
Li J, Han C, ZHENG L, GUO M. Epigenetic regulation of Wnt signaling pathway gene SRY-related HMG-box 17 in papillary thyroid carcinoma. Chin Med J (Engl). 2012;125:3526–31.
Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/b-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–56. https://doi.org/10.18632/oncotarget.667
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yuan, Z., Gardiner, J., Maggi, E.C. et al. Tissue-specific induced DNA methyltransferase 1 (Dnmt1) in endocrine pancreas by RCAS-TVA-based somatic gene transfer system promotes β-cell proliferation. Cancer Gene Ther 26, 94–102 (2019). https://doi.org/10.1038/s41417-018-0046-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41417-018-0046-x