Abstract
Background
Apalutamide plus androgen-deprivation therapy (ADT) improved outcomes in metastatic castration-sensitive prostate cancer (mCSPC) and non-metastatic castration-resistant PC (nmCRPC) in the Phase 3 randomised TITAN and SPARTAN studies, respectively, and maintained health-related quality of life (HRQoL). Apalutamide treatment effect by patient age requires assessment.
Methods
Post-hoc analysis assessed patients receiving 240 mg/day apalutamide (525 TITAN and 806 SPARTAN) or placebo (527 TITAN and 401 SPARTAN) with ongoing ADT, stratified by age groups. Prostate-specific antigen declines, radiographic progression-free survival, metastasis-free survival, overall survival (OS), HRQoL and safety were assessed using descriptive statistics, Kaplan-Meier method, Cox proportional-hazards model and mixed-effects model for repeated measures.
Results
Hazard ratios (95% confidence intervals) generally favoured apalutamide plus ADT versus ADT alone across all endpoints regardless of age; e.g., OS values were 0.57 (0.40–0.80), 0.70 (0.54–0.91) and 0.74 (0.40–1.39) (TITAN) and 0.39 (0.19–0.78), 0.89 (0.69–1.16) and 0.81 (0.58–1.15) (SPARTAN) in patients aged <65, 65–79 and ≥80 years. Regardless of age, apalutamide also maintained HRQoL and was tolerated well with a potential trend in rates of adverse events increasing with age. Limitations include post-hoc nature and variability in sample size of age groups.
Conclusions
Apalutamide plus ADT was an effective and well-tolerated option maintaining HRQoL in patients with mCSPC and nmCRPC regardless of age.
Clinical trial registration
TITAN (NCT02489318); SPARTAN (NCT01946204).
Similar content being viewed by others
Introduction
Advanced prostate cancer (PC), such as metastatic castration-sensitive PC (mCSPC) or non-metastatic castration-resistant PC (nmCRPC), generally affects older patients: the median age of patients with mCSPC and nmCRPC enrolled in clinical trials is approximately 68 and 74 years, respectively [1,2,3,4,5,6,7]; real-world populations are even older [8]. Treating older patients with PC is challenged by susceptibility to treatment complications and age-related comorbidities [9]. The International Society of Geriatric Oncology 2019 Guidelines recommend managing PC in older patients based on health status rather than on chronological age [10]. Hormonal interventions (e.g., androgen-deprivation therapy [ADT]) can increase risk of falls, osteoporotic fractures and development of frailty [11, 12], aggravating age-related conditions. Efficacy and safety of androgen receptor inhibitors and ADT combinations in older patients are being investigated [13, 14].
Guidelines highlight the importance of evaluating patient-reported outcomes and tolerability in older patients using well-defined and reliable instruments assessing health-related quality of life (HRQoL) [15, 16]. Functional Assessment of Cancer Therapy-Prostate (FACT-P) is a tool for assessing HRQoL in PC consisting of 39 items measuring physical, functional, emotional and social/family wellbeing, as well as concerns specific to PC [17].
A comprehensive assessment of apalutamide plus ADT in mCSPC and nmCRPC from the TITAN (NCT02489318) and SPARTAN (NCT01946204) placebo-controlled studies, respectively, showed that it improves clinical outcomes (e.g., long-term survival) [2, 7, 18, 19] and maintains HRQoL per FACT-P [20, 21]. Apalutamide treatment effect generally favoured point estimates of radiographic progression-free survival (rPFS) and overall survival (OS) in TITAN and metastasis-free survival (MFS) and OS in SPARTAN regardless of patients’ age, although age stratifications differed. In this post hoc analysis of TITAN and SPARTAN, we performed an in-depth assessment of apalutamide efficacy, tolerability, safety and patient-reported outcomes in advanced disease across uniformly stratified age groups.
Methods
TITAN and SPARTAN were multicentre, Phase 3 randomised, double-blind, placebo-controlled studies of apalutamide plus ADT in mCSPC and nmCRPC, respectively, that randomised patients in 1:1 and 2:1 ratio to receive 240 mg/day apalutamide or placebo with concurrent ADT [2, 7]. TITAN was conducted at 260 sites in 23 countries; SPARTAN was conducted at 332 sites in 26 countries. Review boards at all participating institutions approved the studies, which were conducted according to current International Council for Harmonisation Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent.
This post hoc analysis assessed apalutamide treatment effect on efficacy, HRQoL and safety, by patient age: <65, 65–79 and ≥80, or <75 and ≥75 years. Efficacy was assessed in the intent-to-treat (ITT) populations using rPFS and OS in TITAN, MFS and OS in SPARTAN, and best prostate-specific antigen (PSA) decline, defined according to Prostate Cancer Working Group 2 [22] criteria as a decline of ≥50% from baseline or decline to ≤0.2 ng/ml at any time during the studies and confirmed ≥4 weeks later. Patient-reported HRQoL was assessed in the ITT populations using FACT-P total score, FACT-P Physical Wellbeing subscale, and two items from Physical Wellbeing subscale GP1 “I have a lack of energy” and GP5 “I am bothered by side effects of treatment”. Response to FACT-P items were coded 0–4 (from “very much” to “quite a bit”, “sometimes”, “a little bit” and “not at all”); the sum for all responses ranges from 0 to 156, higher scores indicating better HRQoL [17, 23]. Changes from baseline of ≥10 points for FACT-P total,24 ≥ 3 points for FACT-P Physical Wellbeing [24], and ≥1 for GP1 or GP5 were considered clinically meaningful. GP1 and GP5 were considered independent of the FACT-P total score.
Treatment-emergent adverse events (TEAEs), defined as AEs occurring on or after first dose of the study drug through one cycle (30 days in TITAN and 28 days in SPARTAN) after the last study treatment, were assessed in the safety populations (patients who received the study drug). Additional details on study design are described in the Supplementary Methods.
Statistical analysis
rPFS and MFS were analysed at first interim analysis cutoff that was prespecified to be final. OS, best PSA decline, HRQoL and safety were assessed at the final analysis cutoff that analysed crossover patients as a part of the ITT population in the placebo group. The sample size determination is described in Supplementary Methods.
Time-to-event endpoints were assessed using Kaplan–Meier methods and Cox proportional-hazards models. HRQoL was assessed as least squares mean changes from baseline in FACT-P total and item scores using a mixed-effects model for repeated measures at each scheduled visit during a treatment phase that had >10% of patients completing FACT-P. Baseline score, treatment, cycle and treatment-by-cycle interaction were fixed effects, individual patients were included as random effects, and missing data values were assumed to arise randomly. PSA decline, baseline characteristics, TEAEs and concomitant bone-sparing agent use were summarised descriptively.
Results
Patient populations
Between 15 December 2015 and 25 July 2017, 1052 patients were enrolled in TITAN, including 525 and 527 patients in apalutamide and placebo groups (Fig. S1a). The prespecified first interim analysis (final analysis for rPFS) occurred at the cutoff on 23 November 2018, after 22.7 months of median follow-up. The prespecified final analysis occurred at the cutoff on 7 September 2020, after the number of required deaths had occurred within 44.0 months of median follow-up.
Between 4 October 2013 and 15 December 2016, 1207 patients were enrolled in SPARTAN, including 806 and 401 patients in apalutamide and placebo groups (Fig. S1b). The prespecified final analysis for MFS occurred at the cutoff on 19 May 2017, after 20.3 months of median follow-up. The final analysis of OS occurred at the cutoff on 1 February 2020, after the number of required deaths had occurred within 52.0 months of median follow-up.
In TITAN, respectively, 331 (31%), 628 (60%) and 93 (9.0%) patients were aged <65, 65–79 and ≥80 years, and 806 (77%) and 246 (23%) patients were <75 and ≥75 years. In SPARTAN, 149 (12%), 741 (61%) and 317 (26%) patients were <65, 65–79 and ≥80 years, and 625 (52%) and 582 (48%) were <75 and ≥75 years. TITAN and SPARTAN patients had broadly similar baseline characteristics across age groups, except that older SPARTAN patients had higher rates of Eastern Cooperative Oncology Group performance status (ECOG PS) of 1, higher median PSA levels and longer median duration of prior ADT for localised disease than younger patients (Tables S1, S2).
PSA declines
Irrespective of age, rates of confirmed deep PSA declines to ≤0.2 ng/ml were higher with apalutamide plus ADT than ADT alone in both studies (Table 1). In TITAN patients aged <65, 65–79 and ≥80 years, 60%, 71% and 67% from apalutamide and 29%, 33% and 32% from placebo groups achieved PSA ≤ 0.2 ng/ml during the study. Respective numbers in SPARTAN were 48%, 40% and 26% of apalutamide-treated and no placebo-treated patients. In both TITAN and SPARTAN, patients across all age groups treated with apalutamide plus ADT had substantially lower median PSA nadir and longer median time to achieve it versus ADT alone (Table 1).
Efficacy outcomes
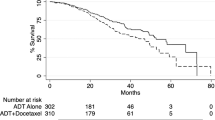
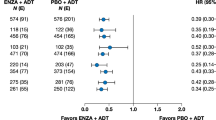
In general, patients in all age groups derived benefit with apalutamide across both studies (Fig. 1 and Figs. S2–S4).
In TITAN patients aged <65, 65–79 and ≥80 years, hazard ratios (HRs) (95% confidence interval [CI]) for rPFS were 0.45 (0.31–0.66), 0.51 (0.39–0.68) and 0.55 (0.25–1.21), respectively, favouring apalutamide (Fig. 1a). In respective age groups of SPARTAN patients, HRs (95% CI) for MFS were 0.14 (0.08–0.27), 0.29 (0.23–0.37) and 0.43 (0.28–0.65), also favouring apalutamide. Respective numbers for OS in TITAN were 0.57 (0.4–0.8), 0.70 (0.54–0.91) and 0.74 (0.40–1.39), and those in SPARTAN were 0.39 (0.19–0.78), 0.89 (0.69–1.16) and 0.81 (0.58–1.15) (Fig. 1b); median OS was increased with apalutamide (Fig. S2). PC-specific survival also favoured apalutamide in both studies (Table S3). Results in patients stratified by age <75 versus ≥75 years were similar, consistently favouring apalutamide irrespective of age (Figs. S3, S4). Notably, in the TITAN study, patients aged ≥75 with ECOG PS 1 showed HRs (95% CI) of 0.44 (0.22–0.9) for rPFS and 0.49 (0.26–0.93) for OS. In the SPARTAN study, patients aged ≥75 with ECOG PS 1 demonstrated HRs (95% CI) of 0.42 (0.25–0.71) for MFS and 0.74 (0.49–1.14) for OS. Additionally, in SPARTAN patients aged ≥75 with PSA doubling time (PSADT) ≤ 6 months, HRs (95% CI) for MFS and OS were 0.46 (0.32–0.66) and 0.78 (0.57–1.06), respectively. These findings further support the favourable effects of apalutamide in these patient populations.
Apalutamide safety profile
In patients aged <65, 65–79 and ≥80 years from both treatment groups, TEAEs occurred in 97%, 97% and 100% in TITAN and in 96%, 96% and 97% in SPARTAN, with similar rates across treatment groups (Table 2). TEAEs leading to discontinuation or death and TEAEs of interest were generally increased with age in both studies regardless of treatment (Tables 2, S4). There was a suggestion of a potential trend towards a greater difference in serious TEAEs and TEAEs leading to discontinuation or death among older adults receiving apalutamide (Table 2).
TITAN and SPARTAN patients from all three age groups were treated for longer with apalutamide than with placebo (Table 2). Exposure-adjusted rates of TEAEs increased with age in both studies regardless of treatment (Table 2); however, TEAEs of interest, including skin rash, falls and fractures, were generally higher in apalutamide- than placebo-treated patients. The use of concomitant bone-sparing medications was similar regardless of treatment, and was observed in 33%, 29% and 41.9% of TITAN and in 32.9%, 31.4% and 39.3% of SPARTAN patients aged <65, 65–79 and ≥80 years, respectively (Table S5). Among patients who had fractures, 10/16, 27/57 and 3/7 TITAN and 9/20, 49/106 and 26/49 SPARTAN patients aged <65, 65–79 and ≥80 years received concomitant bone-sparing medications (Table S5).
Health-related quality of life
HRQoL in TITAN and SPARTAN was similar across treatment and age groups (Fig. 2).
a FACT-P total score. b FACT-P Physical Wellbeing question GP5: “I am bothered by side effects of treatment”. Bars show standard error, dotted horizontal lines show clinically meaningful change from baseline at visits that had >10% of patients completing FACT-P. APA apalutamide, FACT-P Functional Assessment of Cancer Therapy-Prostate, LS least squares, PBO placebo, PRO patient-reported outcome.
Apalutamide- and placebo-treated patients across age groups maintained FACT-P total score at similar levels over the course of the study for up to 5.4 and 3 years, respectively, except the oldest SPARTAN placebo-treated patients, who reported lower FACT-P than patients receiving apalutamide (Fig. 2a). Regardless of age, apalutamide-treated patients reported not being bothered by treatment side effects over time or not any more than placebo-treated patients (Fig. 2b). The overall physical wellbeing and energy levels remained consistent over time in all age groups of apalutamide-treated patients from both studies and were comparable with those in placebo-treated patients (Fig. S5). Similar results for FACT-P total score were observed in patients aged <75 or ≥75 years (Fig. S6). TITAN and SPARTAN patients aged ≥75 years with ECOG PS of 1 at baseline or SPARTAN patients with PSADT ≤ 6 months have maintained general HRQoL per FACT-P total score (data not shown).
Discussion
In this post hoc analysis of TITAN and SPARTAN, efficacy generally favoured apalutamide plus ADT versus ADT alone across all age groups. The safety profile of apalutamide was consistent with previous reports [2, 7, 18, 19], and patients from all age groups tolerated apalutamide treatment well. There was a suggestion of a potential trend towards a greater difference in serious TEAEs and TEAEs leading to discontinuation or death among older adults receiving apalutamide.
Apalutamide showed greater disease control per PSA kinetics, regardless of age, than placebo. Thus, TITAN patients across all age groups achieved lower median PSA nadir with apalutamide plus ADT versus ADT alone, and the majority achieved deep PSA decline to ≤0.2 ng/ml, consistent with the overall population [25]. SPARTAN patients also achieved lower PSA nadir with apalutamide plus ADT versus ADT alone regardless of age. A large proportion of apalutamide-treated and no placebo-treated SPARTAN patients achieved deep PSA decline to ≤0.2 ng/ml across all age groups, consistent with the overall population [26]. In both studies, time to achieve PSA nadir with apalutamide plus ADT was longer than that with ADT alone, consistent with lower PSA nadir and the overall populations [25, 26]. Notably, PSA nadir values and rate of PSA ≤ 0.2 ng/ml decreased with age in SPARTAN. Approximately 50% of younger (<65 years) and a quarter of octogenarian (≥80 years) apalutamide-treated patients achieved PSA ≤ 0.2 ng/ml at any time during the study, whereas in the overall population this rate was 38% [26]. Diminishing PSA control may be associated with more aggressive disease in SPARTAN patients aged 65–79 and ≥80 years who had longer time on prior ADT and higher PSA levels at baseline than younger patients.
Regardless of patient age, apalutamide also showed a clinical benefit consistent with previous findings [2, 7, 18, 19]. Apalutamide treatment effect on rPFS and OS in TITAN was seen across all age groups but was most pronounced in patients aged <65 and 65–79 years. The ≥80 year subgroup had seemingly less pronounced benefits but had a relatively small number of patients. With the dichotomous cutoff of 75 years, the subgroup of TITAN patients aged ≥75 years was larger, and rPFS and OS benefits from apalutamide were reassuringly consistent with those in the younger subgroup. In SPARTAN patients, treatment effect of apalutamide for MFS was observed regardless of age. The OS benefit was most pronounced in the youngest patients (<65 years), likely owing to less aggressive disease or decreased frailty as reflected by low PSA levels and low ECOG PS score at baseline, and also reflecting longer apalutamide exposure. Nevertheless, OS favoured apalutamide in older SPARTAN patients.
The overall safety profile of apalutamide in TITAN and SPARTAN was consistent with previous reports [18, 19]. Rash, known to be more common in apalutamide-treated than placebo-treated patients [18, 19], increased with age, consistent with the age-related susceptibility to toxic complications [27]. Rates of falls and fractures also increased with age in both treatment groups of TITAN and SPARTAN, consistent with age- and ADT-related falls and osteoporosis reported previously [11, 12, 27]. Falls and fractures were more frequent with apalutamide than with ADT, supporting our previous finding that older age was one of the independent predictors of falls in apalutamide-treated SPARTAN patients [28]. Falls and fractures were also shown to be associated with enzalutamide and age in nmCRPC [13]. A prevention programme should be in place to address falls and fractures in patients with advanced PC during oncologic treatments. Interestingly, the use of bone-sparing medications in TITAN and SPARTAN was <40% and did not consistently increase with age. Only ≈40–50% of TITAN and SPARTAN patients aged >65 years who had fractures received bone-sparing medications, suggesting inadequate addressing of bone-related syndromes in older patients with advanced disease. It should be recognised that older adults with cancer are at higher risk for falls than the general geriatric population [29, 30]. Current guidelines recommend denosumab or zoledronic acid mainly in mCRPC [30]; however, they should also be considered in patients with primary or developing osteopenia or osteoporosis regardless of prostate disease state.
Despite an increase in several types of AEs with addition of apalutamide to ADT, patients of all ages reported minimal side effect bother and no increase in fatigue. In all age groups, there were no clinically meaningful differences in overall HRQoL between apalutamide- and placebo-treated patients. Among patients aged >80 years with nmCRPC, there was a notable trend toward longer maintenance of HRQoL with apalutamide plus ADT versus ADT alone.
Our analysis supports early treatment of patients with advanced disease with apalutamide, regardless of age. PSA decline and long-term survival in TITAN and SPARTAN favoured apalutamide across all age groups but became less pronounced with older age in SPARTAN, likely due to higher median age in SPARTAN (74 years [7]) than in TITAN (69 years [2]). Age-related toxicity and syndromes known in older patients [27] may explain the increased susceptibility of older TITAN and SPARTAN patients to rash and predispose them to falls and fractures, also emphasising the need to address these concurrently.
Limitations of this analysis include its post hoc nature and small size of some subgroups. Furthermore, there is variability in group sizes and potential imbalances in co-morbidities, which were not accounted for in this analysis. Prospective studies with larger populations of older patients are needed. Despite the limitations, our analysis supports apalutamide benefit for older patients with advanced PC.
Conclusions
This post hoc analysis of TITAN and SPARTAN patients with mCSPC and nmCRPC demonstrates a benefit with apalutamide regardless of patient age. The tolerability of apalutamide varied across age groups, with more notable differences in older adults. Known AEs associated with apalutamide should be considered in the context of age and addressed accordingly with individualised treatment based on disease stage, physiological status and patient preference.
Data availability
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
References
Armstrong AJ, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, Azad A, et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–86.
Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl J Med. 2019;381:13–24.
Davis ID, Martin AJ, Stockler MR, Begbie S, Chi KN, Chowdhury S, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N. Engl J Med. 2019;381:121–31.
Fizazi K, Shore N, Tammela TL, Ulys A, Vjaters E, Polyakov S, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl J Med. 2019;380:1235–46.
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore N, Ferreira U, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl J Med. 2018;378:2465–74.
Smith MR, Hussain M, Saad F, Fizazi K, Sternberg CN, Crawford ED, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N. Engl J Med. 2022;386:1132–42.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl J Med. 2018;378:1408–18.
National Cancer Institute. Cancer stat facts: prostate cancer. https://seer.cancer.gov/statfacts/html/prost.html. Accessed November 15, 2022 (2022).
Caffo O, Maines F, Rizzo M, Kinspergher S, Veccia A. Metastatic castration-resistant prostate cancer in very elderly patients: challenges and solutions. Clin Inter Aging. 2017;12:19–28.
Boyle HJ, Alibhai S, Decoster L, Efstathiou E, Fizazi K, Mottet N, et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur J Cancer. 2019;116:116–36.
Wallander M, Axelsson KF, Lundh D, Lorentzon M. Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures – a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos Int. 2019;30:115–25.
Winters-Stone KM, Moe E, Graff JN, Dieckmann NF, Stoyles S, Borsch C, et al. Falls and frailty in prostate cancer survivors: current, past, and never users of androgen deprivation therapy. J Am Geriatr Soc. 2017;65:1414–9.
De Giorgi U, Hussain M, Shore N, Fizazi K, Tombal B, Penson D, et al. Consistent survival benefit of enzalutamide plus androgen deprivation therapy in men with nonmetastatic castration-resistant prostate cancer: PROSPER subgroup analysis by age and region. Eur J Cancer. 2021;159:237–46.
Graff JN, Baciarello G, Armstrong AJ, Higano CS, Iversen P, Flaig TW, et al. Efficacy and safety of enzalutamide in patients 75 years or older with chemotherapy-naive metastatic castration-resistant prostate cancer: results from PREVAIL. Ann Oncol. 2016;27:286–94.
Scotte F, Bossi P, Carola E, Cudennec T, Dielenseger P, Gomes F, et al. Addressing the quality of life needs of older patients with cancer: a SIOG consensus paper and practical guide. Ann Oncol. 2018;29:1718–26.
U.S. Department of Health and Human Services, Food and Drug Administration, Oncology Center of Excellence (OCE), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Core patient-reported outcomes in cancer clinical trials. Draft guidance for industry. https://www.fda.gov/media/149994/download. Accessed November 15, 2022 (2021).
Esper P, Mo F, Chodak G, Sinner M, Cella D, Pienta KJ. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology. 1997;50:920–8.
Chi KN, Chowdhury S, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39:2294–303.
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–8.
Agarwal N, McQuarrie K, Bjartell A, Chowdhury S, Pereira de Santana Gomes AJ, Chung BH, et al. Health-related quality of life after apalutamide treatment in patients with metastatic castration-sensitive prostate cancer (TITAN): a randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2019;20:1518–30.
Oudard S, Hadaschik B, Saad F, Cella D, Basch E, Graff JN, et al. Health-related quality of life at the SPARTAN final analysis of apalutamide for nonmetastatic castration-resistant prostate cancer patients receiving androgen deprivation therapy. Eur Urol Focus. 2022;8:958–67.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59.
FACIT.org. FACT-P (Version 4) https://www.facit.org/_files/ugd/626819_bcdd612dbf734297a32172aa2873d7f4.pdf. Accessed April 21 (2023).
Beer TM, Miller K, Tombal B, Cella D, Phung, Holmstrom S, et al. The association between health-related quality-of-life scores and clinical outcomes in metastatic castration-resistant prostate cancer patients: exploratory analyses of AFFIRM and PREVAIL studies. Eur J Cancer. 2017;87:21–29.
Chowdhury C, Bjartell A, Agarwal N, Chung BH, Given RW, Pereira de Santana Gomes AJ, et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann Oncol. https://doi.org/10.1016/j.annonc.2023.1002.1009. (2023).
Saad F, Small EJ, Feng FY, Graff JN, Olmos D, Hadaschik BA, et al. Deep prostate-specific antigen response following addition of apalutamide to ongoing androgen deprivation therapy and long-term clinical benefit in SPARTAN. Eur Urol. 2022;81:184–92.
National Comprehensive Cancer Network. Older adult oncology. Version 2.2022-July 12, 2022. https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed November 15, 2022 (2022).
Pollock YY, Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik B, et al. Clinical characteristics associated with falls in patients with non-metastatic castration-resistant prostate cancer treated with apalutamide. Prostate Cancer Prostatic Dis. 2023;26:156–61.
Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29:1458–64.
National Comprehensive Cancer Network. Prostate cancer. Version 1.2023-September 16, 2022. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed November 15, 2022 (2022).
Acknowledgements
The authors would like to thank the patients who participated in TITAN and SPARTAN and their families, as well as the investigators, study coordinators, study teams and nurses. TITAN and SPARTAN were funded by Janssen Research & Development. Writing assistance was provided by Larissa Belova, PhD, of Parexel, and was funded by Janssen Global Services, LLC.
Previous presentation: The results of this analysis were presented at 2021 ESMO Annual Meeting, September 16-21, 2021, Virtual (Shen J, Chowdhury S, Agarwal N, Karsh LI, Oudard S, Gartrell BA, et al. 618 P - Apalutamide (APA) for advanced prostate cancer in older patients (pts): Combined analysis of TITAN & SPARTAN. Ann Oncol 32 [Suppl 5], S626–S677 [2021]).
Funding
TITAN and SPARTAN were funded by Janssen Research & Development. The sponsor was involved in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. All the authors had access to the data, drafted the manuscript with input from the sponsor (Janssen), reviewed and approved the manuscript before submission, and made the decision to submit the manuscript for publication. Janssen Global Services, LLC provided funding for editorial assistance.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the conception and design, analysis and interpretation of data, drafting of the manuscript and critical revision of the manuscript. SD Brookman-May, B Rooney, A Bhaumik, SA McCarthy, KB Bevans, SD Mundle participated in acquisition of data. A Bhaumik performed statistical analysis.
Corresponding author
Ethics declarations
Competing interests
JS reports a consulting role for AstraZeneca, Bayer, Kite and Sanofi, and institutional research funding from Ambrx, Arcus, Arvinas, BMS, Exelixis, Lilly, MacroGenics and Merck. SC reports consultancy to Astellas Pharma, Bayer, Beigene, Clovis, Janssen-Cilag, Johnson & Johnson, Novartis and Sanofi; research funding (self) from Clovis; and honoraria from Astellas Pharma, Bayer, Beigene, Clovis, Janssen-Cilag, Johnson & Johnson, Novartis and Sanofi. NA reports consultancy to Astellas, AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics and Seattle Genetics; and research funding to institution from Astellas, AstraZeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, GlaxoSmithKline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda and Tracon. LIK reports consulting or advisory role for 3D Biopsy, Astellas, AstraZeneca, Bayer, Dendreon, Ferring, Janssen, Pfizer and Vaxiion; speakers’ bureau for Astellas, Bayer, Janssen, Pfizer, and Clovis; honoraria from Astellas, Bayer, Janssen, Pfizer and Dendreon; stock or other ownership from Swan Valley Medical; research funding from Astellas, AstraZeneca, Bayer, BioXcel Therapeutics, Bristol Myers Squibb, BU Optics, CUSP, Dendreon, Epizyme, Exact Sciences, Ferring, FKD, Genentech/Roche, GenomeDX, Genomic Health, Janssen, Merck, Myovant, Nucleix, OncoCell MDx, Pfizer, Pharmtech/Very, Precision Med and QED; and travel, accommodations and expenses from Astellas, Bayer, Dendreon, Janssen and Pfizer. SO reports advisory roles for Astellas Pharma, Bayer, Bristol Myers Squibb, Eisai, Janssen, Merck Sharp & Dohme, Novartis, Pfizer and Sanofi; compensation for travel from Bayer, Bristol Myers Squibb, Eisai, Merck Sharp & Dohme, Novartis and Pfizer; honoraria from Astellas Pharma, Bayer, Bristol Myers Squibb, Eisai, Janssen, Merck Sharp & Dohme, Novartis, Pfizer and Sanofi; and research funding from Ipsen and Sanofi. BAG reports consulting fees from Blue Earth, Immunogen, Janssen, Pfizer and Sanofi. Susan Feyerabend has nothing to disclose. FS reports advisory roles for Astellas Pharma, AstraZeneca/MedImmune, Bayer, Janssen Oncology and Sanofi; honoraria from AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bayer, Janssen Oncology and Sanofi; and research funding grants provided to his institution from Astellas Pharma, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen Oncology, Pfizer and Sanofi. CMP reports consulting or advisory role for Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Dendreon, Janssen Oncology, Merck, Sun Pharma, Pfizer and Tolmar; speakers’ bureau for Astellas Pharma, Bayer, Dendreon, Janssen Oncology, Merck, Myovant Sciences, Pfizer, Sun Pharma and Tolmar; honoraria from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Dendreon, Janssen, Merck, Myovant Sciences, Pfizer, Sun Pharma and Tolmar; and research funding from Advantagene, Astellas Pharma, AstraZeneca, Bayer, Dendreon, Innocrin Pharma, Janssen Oncology, Merck and Pfizer. KNC reports consultancy to Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Daiichi Sankyo, Essa, Janssen, Novartis, Merck, Pfizer, Point Pharma, Roche and Sanofi; research funding to institution from Astellas, Janssen and Sanofi; and honoraria from Astellas, AstraZeneca, Bayer, Janssen, Merck and Pfizer. EJS reports advisory roles for Fortis and Janssen Oncology; compensation for travel from Janssen; stock and interest in Fortis and Harpoon Therapeutics; honoraria from Janssen; and research funding from Janssen and Merck Sharp & Dohme. MRS reports advisory roles for Amgen, Bayer, Janssen Oncology, Lilly, Novartis and Pfizer; compensation for travel from Amgen, Bayer, Janssen and Lilly; and research funding from Bayer, Gilead Sciences and Janssen Oncology. JNG reports an advisory role for Exelixis; compensation for travel from Bayer, Clovis Oncology and Merck Sharp & Dohme; patents, royalties and intellectual property with Oncoresponse: Exceptional Responders; honoraria from Astellas, Bayer, Janssen Oncology and Medivation; and research funding from Bristol Myers Squibb, Janssen Oncology, Medivation, Merck Sharp & Dohme and Sanofi. SDBM, BR, AB, SAMC, KBB and SDM are employees of Janssen Research & Development and may hold stock in Johnson & Johnson. AB also reports stock ownership of AbbVie.
Ethics approval and consent to participate
Review boards at all participating institutions approved the TITAN and SPARTAN studies, which were conducted in according to current International Council for Harmonisation Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shen, J., Chowdhury, S., Agarwal, N. et al. Apalutamide efficacy, safety and wellbeing in older patients with advanced prostate cancer from Phase 3 randomised clinical studies TITAN and SPARTAN. Br J Cancer 130, 73–81 (2024). https://doi.org/10.1038/s41416-023-02492-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02492-8