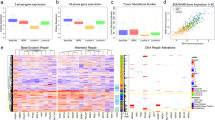
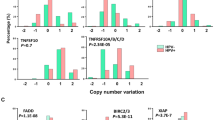
Abstract
Background
Our aim was to evaluate the efficacy and anti-cancer action of a precision medicine approach involving a novel SIRT1-dependent pathway that, when disrupted, leads to the restoration of a functional p53 in human papillomavirus (HPV)-transformed cells.
Methods
The anticancer potential of inhibiting SIRT1 was evaluated by examining the effects of the specific SIRT1 inhibitor EX527 (also known as Selisistat) or genetic silencing, either individually or in conjunction with standard chemotherapeutic agents, on a range of HPV+ cancer cells and a preclinical mouse model of HPV16-induced cancer.
Results
We show that SIRT1 inhibition restores a transcriptionally active K382-acetylated p53 in HPV+ but not HPV- cell lines, which in turn promotes G0/G1 cell cycle arrest and inhibits clonogenicity specifically in HPV+ cells. Additionally, EX527 treatment increases the sensitivity of HPV+ cells to sublethal doses of standard genotoxic agents. The enhanced sensitivity to cisplatin as well as p53 restoration were also observed in an in vivo tumorigenicity assay using syngeneic C3.43 cells harbouring an integrated HPV16 genome, injected subcutaneously into C57BL/6J mice.
Conclusions
Our findings uncover an essential role of SIRT1 in HPV-driven oncogenesis, which may have direct translational implications for the treatment of this type of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included either in this article or in additional files, and the links to public datasets are reported in the manuscript.
References
Berman TA, Schiller JT. Human papillomavirus in cervical cancer and oropharyngeal cancer: one cause, two diseases. Cancer. 2017;123:2219–29.
Lechner M, Liu J, Masterson L, Fenton TR. HPV-associated oropharyngeal cancer: epidemiology, molecular biology and clinical management. Nat Rev Clin Oncol. 2022;19:306–27.
Shewale JB, Gillison ML. Dynamic factors affecting HPV-attributable fraction for head and neck cancers. Curr Opin Virol. 2019;39:33–40.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6:92.
Guo T, Kang SY, Cohen EEW. Current perspectives on recurrent HPV-mediated oropharyngeal cancer. Front Oncol. 2022;12:966899.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72.
Caudell JJ, Gillison ML, Maghami E, Spencer S, Pfister DG, Adkins D, et al. NCCN guidelines® insights: head and neck cancers, version 1.2022. J Natl Compr Canc Netw. 2022;20:224–34.
Harari PM. Open the gates for treatment de-intensification in head and neck cancer. J Clin Oncol. 2019;37:1854–55.
Bates JE, Steuer CE. HPV as a Carcinomic Driver in head and neck cancer: a de-escalated future? Curr Treat Options Oncol. 2022;23:325–32.
Orlandi E, Licitra L. Personalized medicine and the contradictions and limits of first-generation deescalation trials in patients with human papillomavirus-positive oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2018;144:99–100.
McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. 2022;20:95–108.
Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30:F55–70.
Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–60.
Mittal S, Banks L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat Res Rev Mutat Res. 2017;772:23–35.
Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26:158–68.
Hebner C, Beglin M, Laimins LA. Human papillomavirus E6 proteins mediate resistance to interferon-induced growth arrest through inhibition of p53 acetylation. J Virol. 2007;81:12740–7.
Liu TF, McCall CE. Deacetylation by SIRT1 reprograms inflammation and cancer. Genes Cancer. 2013;4:135–47.
Lin Z, Fang D. The Roles of SIRT1 in Cancer. Genes Cancer. 2013;4:s97–104.
Kwon HS, Ott M. The ups and downs of SIRT1. Trends Biochem Sci. 2008;33:517–25.
Gomes AR, Yong JS, Kiew KC, Aydin E, Khongkow M, Laohasinnarong S, et al. Sirtuin1 (SIRT1) in the acetylation of downstream target proteins. Methods Mol Biol. 2016;1436:169–88.
Zhang T, Kraus WL. SIRT1-dependent regulation of chromatin and transcription: linking NAD(+) metabolism and signalling to the control of cellular functions. Biochim Biophys Acta. 2010;1804:1666–75.
Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59.
Lee JT, Gu W. SIRT1: regulator of p53 deacetylation. Genes Cancer. 2013;4:112–7.
Langsfeld ES, Bodily JM, Laimins LA. The deacetylase Sirtuin 1 regulates human papillomavirus replication by modulating histone acetylation and recruitment of DNA damage factors NBS1 and Rad51 to viral genomes. PLoS Pathog. 2015;11:e1005181.
Das D, Smith N, Wang X, Morgan IM. The deacetylase SIRT1 regulates the replication properties of human papillomavirus 16 E1 and E2. J Virol. 2017;91:e00102–17.
Velez-Perez A, Wang XI, Li M, Zhang S. SIRT1 overexpression in cervical squamous intraepithelial lesions and invasive squamous cell carcinoma. Hum Pathol. 2017;59:102–7.
So D, Shin HW, Kim J, Lee M, Myeong J, Chun YS, et al. Cervical cancer is addicted to SIRT1 disarming the AIM2 antiviral defense. Oncogene. 2018;37:5191–204.
Solomon JM, Pasupuleti R, Xu L, McDonagh T, Curtis R, DiStefano PS, et al. Inhibition of SIRT1 catalytic activity increases p53 acetylation but does not alter cell survival following DNA damage. Mol Cell Biol. 2006;26:28–38.
Yi J, Luo J. SIRT1 and p53, effect on cancer, senescence and beyond. Biochim Biophys Acta. 2010;1804:1684–9.
Atkins KM, Thomas LL, Barroso-González J, Thomas L, Auclair S, Yin J, et al. The multifunctional sorting protein PACS-2 regulates SIRT1-mediated deacetylation of p53 to modulate p21-dependent cell-cycle arrest. Cell Rep. 2014;8:1545–57.
Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, et al. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–54.
Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–26.
Talis AL, Huibregtse JM, Howley PM. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem. 1998;273:6439–45.
Feltkamp MCW, Smits HL, Vierboom MPM, Minnaar RP, de Jongh BM, Drijfhout JW, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. J. Immunol. 1993;23:2242–9.
Smith KA, Meisenburg BL, Tam VL, Pagarian RR, Wong R, Joea DK, et al. Lymph node targeted immunotherapy mediates potent immunity resulting in regression of isolated or disseminated HPV transformed tumors. Clin Cancer Res. 2009;15:6167–76.
Accardi L, Paolini F, Mandarino A, Percario Z, Di Bonito P, Di Carlo V, et al. In vivo antitumor effect of an intracellular single-chain antibody fragment against the E7 oncoprotein of human papillomavirus 16. Int J Cancer. 2014;134:2742–7.
Yang R, Klimentová J, Göckel-Krzikalla E, Ly R, Gmelin N, Hotz-Wagenblatt A, et al. Combined transcriptome and proteome analysis of immortalized human keratinocytes expressing human papillomavirus 16 (HPV16) oncogenes reveals novel key factors and networks in HPV induced carcinogenesis. mSphere. 2019;4:e00129–19.
Lo Cigno I, Calati F, Borgogna C, Zevini A, Albertini S, Martuscelli L, et al. Human papillomavirus E7 oncoprotein subverts host innate immunity via SUV39H1-mediated epigenetic silencing of immune sensor genes. J Virol. 2020;94:e01812–9.
Lo Cigno I, De Andrea M, Borgogna C, Albertini S, Landini MM, Peretti A, et al. The nuclear DNA sensor IFI16 acts as a restriction factor for human papillomavirus replication through epigenetic modifications of the viral promoters. J Virol. 2015;89:7506–20.
Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, et al. Rapid and automated tetrazolium-based colourimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–21.
Itahana K, Itahana Y, Dimri GP. Colourimetric detection of senescence-associated β galactosidase. Methods Mol Biol. 2013;965:143–56.
Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82.
Gertz M, Fischer F, Nguyen GT, Lakshminarasimhan M, Schutkowski M, Weyand M, et al. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc Natl Acad Sci USA. 2013;110:E2772–81.
Meissner JD. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J Gen Virol. 1999;80:1725–33.
Abdulkarim B, Sabri S, Deutsch E, Chagraoui H, Maggiorella L, Thierry J, et al. Antiviral agent Cidofovir restores p53 function and enhances the radiosensitivity in HPV-associated cancers. Oncogene. 2002;21:2334–46.
Ninck S, Reisser C, Dyckhoff G, Helmke B, Bauer H, Herold-Mende C. Expression profiles of angiogenic growth factors in squamous cell carcinomas of the head and neck. Int J Cancer. 2003;106:34–44.
Scheffner M, Münger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci USA. 1991;88:5523–7.
Srivastava S, Tong YA, Devadas K, Zou ZQ, Chen Y, Pirollo KF, et al. The status of the p53 gene in human papilloma virus positive or negative cervical carcinoma cell lines. Carcinogenesis. 1992;13:1273–5.
Piboonniyom SO, Duensing S, Swilling NW, Hasskarl J, Hinds PW, Münger K. Abrogation of the retinoblastoma tumour suppressor checkpoint during keratinocyte immortalization is not sufficient for induction of centrosome-mediated genomic instability. Cancer Res. 2003;63:476–83.
Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16:93–105.
Hall AH, Alexander KA. RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol. 2003;77:6066–9.
Olthof NC, Huebbers CU, Kolligs J, Henfling M, Ramaekers FC, Cornet I, et al. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int J Cancer. 2015;136:E207–18.
Rose PG. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;341:708.
Dell’Omo G, Crescenti D, Vantaggiato C, Parravicini C, Borroni AP, Rizzi N, et al. Inhibition of SIRT1 deacetylase and p53 activation uncouples the anti-inflammatory and chemopreventive actions of NSAIDs. Br J Cancer. 2019;120:537–46.
Kong X, Yu D, Wang Z, Li S. Relationship between p53 status and the bioeffect of ionizing radiation. Oncol Lett. 2021;22:661.
Acknowledgements
We thank Marcello Arsura for critically reviewing the manuscript. NOKE6/E7 cells were kindly provided by Martina Niebler and Frank Rösl, German Cancer Research Center (DKFZ)-Heidelberg and C3.43 cells by Martin Kast, University of Southern California in Los Angeles. We are also grateful to Michela Salvo from the Histology Research Core Facility at University of Piemonte Orientale (Novara, Italy) for technical support in tissue processing and histological analysis, Nausicaa Clemente from the Department of Health Sciences at University of Piemonte Orientale (Novara, Italy) for technical support to perform in vivo experiments, and Valeria Caneparo at CAAD, Center for Translational Research and Autoimmune and Allergic Disease, for assistance in SA-β-gal analysis.
Funding
This work was supported by the Italian Ministry for University and Research-MIUR (20178ALPCM-PRIN 2017 to MG), the AGING Project–Department of Excellence–DIMET, University of Piemonte Orientale, Associazione Italiana per la Ricerca sul Cancro-AIRC (25767-IG 2021 to MG), Next Generation EU—PNRR M6C2—Investimento 2.1 Valorizzazione e potenziamento della ricerca biomedica del SSN (PNRR-MAD-2022-12376570 to MG), and by the Italian Ministry of Foreign Affairs and International Cooperation (PGRBR22GR03 to AV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
ILC: Conceptualisation, data curation, investigation, methodology, project administration, software, validation, writing–original draft. FC: Conceptualisation, data curation, investigation, methodology, writing–original draft. CG: Data curation, investigation, methodology. CB: Formal analysis, project administration, supervision, writing–review and editing. AV: Resources, validation, writing–review and editing. RB: Investigation, validation, writing–review and editing. MG: Conceptualisation, supervision, funding acquisition, writing–original draft, writing–review and editing.
Corresponding author
Ethics declarations
Competing interests
ILC, FC and MG report the Italian Patent No. 102020000023281 issued on October 2nd, 2020 (“SIRT1 INHIBITOR OR ANTAGONIST FOR USE IN PREVENTING AND/OR TREATING AN HPV-INDUCED TUMOUR”). This patent is pending for the international patent application approval (PCT/IB2021/059055). All the other authors declare no competing financial interests.
Ethics approval
The mice used for the in vivo tumorigenicity assays were housed in our animal facilities in accordance with “The Guide for the Care and Use of Laboratory Animals”, and the experimentation was approved by the Italian Ministry of Health (Agreement No. 219/2020-PR).
Consent for publication
This manuscript has not been previously published and is not under consideration for publication elsewhere.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lo Cigno, I., Calati, F., Girone, C. et al. SIRT1 is an actionable target to restore p53 function in HPV-associated cancer therapy. Br J Cancer 129, 1863–1874 (2023). https://doi.org/10.1038/s41416-023-02465-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02465-x