Abstract
Malnutrition increases the risk of non-relapse mortality after allogeneic stem cell transplantation (aHSCT). Here are the results of the ALLONUT clinical trial designed to improve the nutritional outcome of patients receiving aHSCT. ALLONUT is a prospective open label phase 2 clinical trial assessing the efficacy of a close tailored nutritional support and management with traditional and original solutions to improve patients nutritional status following aHSCT. Nutritional status evaluation was performed before transplantation, on Day 0, 30, 100 and one year after transplantation. The study involved 70 patients treated by aHSCT. 10% of patients were moderately or severely malnutrition at baseline and 26.9 were severely malnutrition at D30. Patients’ nutritional status improved thanks to the cooking classes and the personalized outpatient nutrition program. At D100, 23% were still malnutrition, while only 10.8% were severely malnutrition one year after transplantation. The QLQ-C30 show that quality of life (QoL) decreased until D30, and improve to reach the pre-transplant level on D100 before exceeding it on D360. The study confirmed that a close, personalized nutritional program combining traditional and original measures can improve both nutritional status and QoL for patients suffering from moderate or severe malnutrition after aHCST.
Similar content being viewed by others
Introduction
Allogeneic stem cell transplantation (aHSCT) is the best curative option for many hematological malignancies. However, its power is hampered by its toxicity, encompassing an uncontrolled inflammatory state, graft versus host disease (GVHD) and infections [1,2,3,4,5]. In recent years, the development of less toxic conditioning regimen, the improvement of pre and post transplantation anti-tumor therapies and supportive cares have made aHSCT available to more patients especially those with an older age and comorbidities [6, 7]. The constant rise of haploidentical transplantation has made the process available for virtually all patients requiring a transplantation with a survival rate comparable to the one of matched unrelated donors [8]. Malnutrition is a frequent complication of the treatment of hematological malignancies [9,10,11,12,13]. The causes are multifactorial including disease associated inflammation, malabsorption, high dose chemotherapy responsible for mucosal damages, nausea, vomiting, diarrhea, dysbiosis, mucositis, dysgeusia, fever, infections. All these side effects lead to a significant reduction in oral intake leading to weight loss and sarcopenia. For patient receiving transplantation the malnutrition status is aggravated by the effects of the conditioning that includes total body irradiation, high-dose chemotherapy and post transplantation complications such as graft versus host disease, steroids and immune-suppressors use. A decreased oral intake also leads to a lower quality of life and poorer survival [14, 15]. The results of a prospective study are presented here, assessing the efficacy of a close tailored nutritional support and management with traditional (enteral and parenteral nutrition) and original solutions (cooking classes) to improve patients nutritional status following aHSCT. The objectives were to decrease the prevalence of malnutrition at day 100 and improve appetite, quality of life and overall survival after ASCT.
Methods
Study design
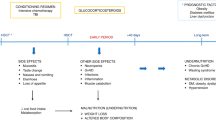
The ALLONUT trial (ClinicalTrials.gov Identifier: NCT03829072) was a prospective phase II trial conducted at the hematology Nice center to assess the efficacy of a combined approach to treat malnutrition after transplantation. The study enrolled adult patients over 18 years old who underwent an aHSCT between March 2019 and January 2022. All patients received enteral or parenteral nutrition during their hospital stay guided by a weekly dietician evaluation, followed by three cooking classes delivered by a Michelin starred chef (Fig. 1). The study was approved by the national ethics committee called Comité de protection des personnes (CPP AU-1469) referenced ID-RCB 2018-A01842-53, and respected the declaration of Helsinki. All patients signed a written informed consent.
Malnutrition
The diagnosis of malnutrition according to the French Health Administration [16] was based on the on the European Society for Clinical Nutrition and Metabolism (ESPEN) and GLIM guidelines [17] and required at least one of the following criteria: BMI < 18.5, a weight loss of more than 5% over the last month or more than 10% over the past 6 months. A moderate malnutrition (MM) was defined as a BMI between 17 and 18.5, a weight loss of more than 5% but less than 10% over the last month or more than 10% but less than 15% over the past 6 months and an albumin level lower than 35 g/L but higher than 30 g/L. The diagnosis of severe malnutrition (SM) required all three following criteria a BMI < 17, a weight loss of more than 10% over the last month or of more than 15% over the past 6 months and an albumin level lower than 30 g/dL.
Nutritional management strategy
French society of cellular therapy (SFGMTC) recommended nutritional assessment one time before aHSCT and one per week during hospitalization (Fig. 2). After discharge, patients need to be assessed one and three months after aHSCT [18]. It relied on a systematic enteral nutrition starting on day 0. Weekly nutritional assessment allowed for a better adapted nutritional support between the first day of conditioning and day 100 after aHSCT. Oral intakes were calculated and compared to daily calories recommendations for same sex and age in the healthy population. For malnourished patients and patients unable to eat enough to cover their caloric needs, a nutritional intervention was initiated. For most patients the intervention consisted in increasing the daily volume of enteral solution. Several times a week the dietician adapted the nutritional support to the nutritional assessment and the presence of gut acute graft versus host disease (aGVHD). After being discharged from the transplantation unit all patients enrolled in the study benefited from 3 personalized classes with a one-star chef. The chef designed recipes respecting special food guidelines for transplanted patients.
Main and secondary objectives
The main objective was to show an improvement of nutritional status based on French Health Administration guidelines [16] using ALLONUT clinical trial. The secondary objectives was to show an improvement of nutritional status based on Simple Evaluation of Food Intake (SEFI) and Subjective Global Assessment (SGA) scores, brachial circumference determination, oral intake calculation (calories), albumin and prealbumin blood levels; and an improvement of quality of life based on EORTC QLQC30.
Endpoints
The evaluation of pre and post transplantation nutritional status relies on a combinatorial of several parameters including Body Mass Index (BMI), weight loss, sarcopenia, hands grip test, albumin level. French guidelines [16] for the assessment of nutritional status include monitoring of these parameters and calculation of several scores such as the SEFI and SGA scores, brachial circumference determination, oral intake calculation (calories), albumin and prealbumin blood levels.
Nutritional management consisted in a first pre-transplant visit with a dietician specialized in the follow up of hematology patients. The goal of this visit was to perform a first nutritional screening based on the ESPEN guidelines [19, 20] and the French Health Administration Guidelines [16] and French Society for Cell Transplantation and Cell Therapy [18]. During the visits, the dietician collected the following values, weight, height, BMI, brachial circumference and Hands grip test, calculated the weight change between the baseline weight at diagnosis and before transplantation, the SEFI and SGA scores, the dietician also collected the most recent history of nutrients intake, and measured albumin and prealbumin blood levels and made the QLQ C30. During this first visit the patient also received explanations on the trial, especially on the nutritional management that would results from nutritional assessment after each visit.
The primary endpoint was the prevalence of malnutrition (SM + MM) at day 100, while secondary endpoints were oral intake (calories), EVA appetite, SEFI score, SGA score, quality of life, brachial circumference and overall survival at day 30, day 100 and day 360 after aHSCT.
Sample size
We included 70 patients based on the number of Allo-transplanted patients per year in the Nice University Hospital.
Statistical analysis
Continuous variables are described using medians [interquartile ranges] (minimum; maximum) and qualitative variables are described using counts and percentages. Noncontinuous variables were compared by the chi-square test. Mann–Whitney test was used for continuous variables. The median follow-up time was calculated as the median time from initiation of treatment to last follow-up. Overall survival (OS) was calculated from the date of AML diagnosis to the date of death or last follow-up. Survival curves were estimated using the Kaplan–Meier method and were compared with the log-rank test. Statistical tests were considered significant when the 2 tailed P value was <0.05. Confidence intervals (CI) were computed with 95% coverage. All statistical analysis was performed using SPSS v.26 software (IBM SPSS Statistics).
Results
Between March 2019 and January 2022, 70 consecutive patients who received an aHSCT in hematology Nice center were enrolled in the ALLONUT phase II clinical trial. Patient’s characteristics at baseline are described in Table 1. Sex ratio was well balanced, median age was 55 years old (range, 18–73). The main indication for transplantation was acute myeloid leukemia (56.5%). Most patients received a reduced toxicity conditioning. At transplantation mean BMI was 23.9 with normal prealbumin and albumin levels. Median SGA and SEFI scores were also in the normal range at baseline. Most patients (92.5%) developed aGVHD including 39% with grade III-IV aGVHD after aHSCT.
Evolution of nutritional status
At day 0, the prevalence of MM was significantly higher than before aHSCT with 10.3% of MM (p = 0.004) (68 patients evaluable). However, after dietary intervention and cooking classes, the incidence of MM was not different than before transplantation at day 30 (67 patients evaluable), day 100 (57 patients evaluable) and day 360 (44 patients evaluable) after aHSCT. The same results were obtained for SM with a significantly higher incidence of SM at day 0 (p < 0.0001) and no significant difference at day 30, day 100 and day 360 (Table 2).
Evolution of calories uptake
The food intake measured by calories intake calculation demonstrated an improvement over time with a significantly lower calories intake at day 0 (1653 kcal vs 1045 kcal, p = 0.002) and at day 30 (1653 kcal vs 1498 kcal, p = 0.02). The oral intake recovered during the trial to reach the pre-aHSCT from day 100. The SGA score followed the same pattern with a significantly higher median SGA score at day 0 (7) than at baseline (2) (p = 0.009). The median SGA score gradually decreased at day 30 (p = 0.025), day 100 (p = not significant) and 360 (p = not significant) following aHSCT and was not different from the pre-aHSCT median SGA from day 100 (Fig. 3). The oral intake correlated with the improvement of albumin and prealbumin levels after aHSCT. Albumin and prealbumin levels were significantly lower at day 0 than at baseline with a mean albumin level of 42.2 g/L before aHSCT that decreased to 32.5 g/L at day 0 (p < 0.0001). There was no difference in albumin concentration between baseline and day 30, day 100 and day 360 following aHSCT. Regarding mean serum prealbumin level, it was significantly lower at day 0 (0.2 g/L) than before aHSCT (0.3 g/L) (p < 0.0001) (Fig. 4). Similar to the serum albumin level, the prealbumin increased at day 30 (0.4 g/L), day 100 (0.3 g/L) and day 360 (0.3 g/L) (data not shown).
Evaluation of appetite
The evaluation of appetite using the SEFI score revealed an improvement of the score over time after aHSCT. At day 0, patients had a significantly lower appetite as compared to pre-transplantation baseline (5 vs 10, p < 0.0001). SEFI score stay significantly different from baseline at day 30 (p < 0.0001) and appetite was restored from day 100 with no significant difference observed at day 100 and day 360 (Fig. 5).
Evaluation of brachial circumference and hand grip test
Muscle mass and function evaluation through brachial circumference (BC) and hand grip test revealed no difference for the mean BC at day 0 with a mean BC of 30 cm at baseline versus 39 cm at day 0, 28.1 cm at day 30, 28.4 cm at day 100 and 28.9 cm at day 100. There was also no difference for mean hand grip test result between pre and post aHSCT results (data not shown).
Evaluation of quality of life and survival
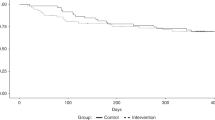
The quality of life was significantly worse only at day 0 than before aHSCT (p = 0.002). Figure 6 showed evolution of quality of life based on question 1. (How would you rate your overall health during the past week?) and question 2 (How would you rate your overall quality of life during the past week?) of QLQC30. Quality of life was restored from day 30 in the study. One-year overall survival was 65% (Fig. 7).
Discussion
Malnutrition has been the focus of recent investigations as it has been shown to correlate with weight loss, non-relapse mortality and therefore overall survival after aHSCT. Several factors contributed to malnutrition before, during and after transplantation. In the pre aHSCT period, chemotherapy, inflammation caused by the underlying disease and infections lead to a decreased balance of calories intake and a catabolic state [21,22,23]. In the study, 20% of the patients already suffered from malnutrition at baseline. Conditioning regimen and severe aGVHD (especially gut aGVHD) are risk factors for the onset of severe malnutrition. In the clinical trial, most patients displayed symptoms of aGVHD, among whom 39% had severe grade III-IV aGVHD.
Despite the current effort in standardizing the evaluation of malnutrition for aHSCT, a unique scoring system has yet to be validated. Several systems have been proposed to better assess the severity of malnutrition. An early and dynamic evaluation of nutritional status in cancer patients is provided by the Patient Generated Subjective Global Assessment (PG-SGA), that is recommended by the Academy of Nutrition and Dietetics [24]. The higher the score, the worse the nutritional status is. Serum albumin and prealbumin levels are commonly used as biomarkers to both diagnose and follow patients’ nutritional status after aHSCT and holds a predictive value for those suffering from gut aGVHD. In the study, SGA and biomarkers including serum albumin and pre-albumin levels moved in parallel after aHSCT showing an initial alteration followed by a recovery from day 30. The impact of malnutrition on quality of life was assessed using the QLQC30 and showed a decrease in quality of life in parallel with the level of malnutrition. A recent prospective study on 36 aHSCT recipients reported a link between malnutrition and quality of life [25]. In this prospective cohort a severe malnutrition at the time of discharge after aHSCT was correlated with a worse quality of life. At day 360, transplanted patients had better quality of life than before aHSCT partly due to complete remission status and free of treatment for hematologic malignancy.
Current management of malnutrition varies between countries and even between transplant centers in a same country. To homogenize nutritional management of cancer patients, the French Health agency published its guidelines [18]. These recommendations are based on the ESPEN guidelines and stratify malnourished patients into MM or SM. A recently reported European multicenter retrospective study has reported the current practices in nutrition in 28 transplant centers from Austria, Germany and Switzerland [26]. The authors reported that all centers had national nutritional guidelines for aHSCT recipients. Seventy-five percent of centers used parenteral nutrition (PN) based on a drop of oral intake or body weight and adapted the dose of PN to the remaining oral intake. The Francophone Society for Bone Marrow Transplantation and Cellular Therapy also provides nutritional guidelines for transplantation centers [18]. Based on that PN could increase catheter infections and favorize gut dysbiosis increasing risk of gut aGVHD, frontline enteral nutrition (EN) was used for all patients at day 1 after aHSCT with a dose adapted three times a week to the remaining oral intake based on dietitian evaluation.
Malnutrition most often persists and can aggravates after aHSCT related to loss of appetite, dysgeusia, aGVHD and many restrictions for cooking. All patients received a personalized cooking classes with a one-star chef. He designed 4 to 5 courses meal respecting patients’ restrictions after aHSCT. The SEFI score was assessed at baseline, day 0, day 30, day 100 and day 360 after aHSCT and showed a restoration of appetite from day 100 after aHSCT when they have completed all the nutritional program.
The study reports an original approach to improve the nutritional state after aHSCT combining both the screening of malnutrition, a tailored nutritional support adapted several times a week based on clinical and biological tests, but also using cooking classes to improve appetite and food intake. In this study, there was a decrease of malnutrition at day 30 compared to the literature. This decrease of prevalence of malnutrition at day 30 was associated with a faster than usual improvement for recovery of appetite and quality of life. Even if it was not clearly observed in the study due to the small number population, improvement in nutritional status suggests that it could have an impact on immune recovery and GVHD rates after HSCT. The absence of a control group and the size of the sample limits the power of this study and the approach requires a validation in larger multicentric prospective clinical trial. The ALLONUT program could be implemented in all transplant units to be evaluated in a larger real-life cohort. Cooking classes will be edited as a cooking book to become available for all transplanted patients in France.
Conclusion
The ALLONUT clinical trial combined an original, never reported dual approach to tackle malnutrition after aHSCT, using three times a week dietitian adapted nutritional support to improve calories intake and cooking classes with a one-star chef. We have assumed that the use of this personalized nutrition program could induce an early restoration of malnutrition, appetite and quality of life.
Data availability
Dataset is available on demand to corresponding author.
References
McDonald GB, Shulman HM, Sullivan KM, Spencer GD. Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology. 1986;90:460–77.
Couriel D, Caldera H, Champlin R, Komanduri K. Acute graft-versus-host disease: pathophysiology, clinical manifestations, and management. Cancer. 2004;101:1936–46.
Fredricks DN. The gut microbiota and graft-versus-host disease. J Clin Invest. 2019;129:1808–17.
Bassim CW, Fassil H, Dobbin M, Steinberg SM, Baird K, Cole K, et al. Malnutrition in patients with chronic GVHD. Bone Marrow Transpl. 2014;49:1300–6.
Bassim CW, Fassil H, Mays JW, Edwards D, Baird K, Steinberg SM, et al. Oral Disease Profiles in Chronic Graft versus Host Disease. J Dent Res. 2015;94:547–54.
Blaise D, Fürst S, Crocchiolo R, El-Cheikh J, Granata A, Harbi S, et al. Haploidentical T Cell-Replete Transplantation with Post-Transplantation Cyclophosphamide for Patients in or above the Sixth Decade of Age Compared with Allogeneic Hematopoietic Stem Cell Transplantation from an Human Leukocyte Antigen-Matched Related or Unrelated Donor. Biol Blood Marrow Transpl. 2016;22:119–24.
Sophie S, Yves B, Frédéric B. Current Status and Perspectives of Allogeneic Hematopoietic Stem Cell Transplantation in Elderly Patients with Acute Myeloid Leukemia. Stem Cells Transl Med. 2022;11:461–77.
Gooptu M, Romee R, St Martin A, Arora M, Al Malki M, Antin JH, et al. HLA-haploidentical vs matched unrelated donor transplants with posttransplant cyclophosphamide-based prophylaxis. Blood. 2021;138:273–82.
Iestra JA, Fibbe WE, Zwinderman AH, Romijn JA, Kromhout D. Parenteral nutrition following intensive cytotoxic therapy: an exploratory study on the need for parenteral nutrition after various treatment approaches for haematological malignancies. Bone Marrow Transpl. 1999;23:933–9.
Iestra JA, Fibbe WE, Zwinderman AH, van Staveren WA, Kromhout D. Body weight recovery, eating difficulties and compliance with dietary advice in the first year after stem cell transplantation: a prospective study. Bone Marrow Transpl. 2002;29:417–24.
Wang B, Yan X, Cai J, Wang Y, Liu P. Nutritional assessment with different tools in leukemia patients after hematopoietic stem cell transplantation. Chin J Cancer Res. 2013;25:762–9.
Liu P, Wang B, Yan X, Cai J, Wang Y. Comprehensive evaluation of nutritional status before and after hematopoietic stem cell transplantation in 170 patients with hematological diseases. Chin J Cancer Res. 2016;28:626–33.
Hadjibabaie M, Iravani M, Taghizadeh M, Ataie-Jafari A, Shamshiri AR, Mousavi SA, et al. Evaluation of nutritional status in patients undergoing hematopoietic SCT. Bone Marrow Transpl. 2008;42:469–73.
Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9.
Nourissat A, Vasson MP, Merrouche Y, Bouteloup C, Goutte M, Mille D, et al. Relationship between nutritional status and quality of life in patients with cancer. Eur J Cancer. 2008;44:1238–42.
Diagnostic de la dénutrition de l’enfant et de l’adulte. Haute Autorité de Santé. 2019. https://has-sante.fr/jcms/p_3118872/fr/diagnostic-de-la-denutrition-de-l-enfant-et-de-l-adulte.
Cederholm T, Jensen GL, Correia MITD, Gonzalez MC, Fukushima R, Higashiguchi T, et al. GLIM criteria for the diagnosis of malnutrition – A consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9.
Bay J-O, Dendoncker C, Angeli M, Biot T, Chikhi M, Combal C, et al. Prise en charge nutritionnelle des patients hospitalisés pour allogreffe de CSH : recommandations de la Société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC). Bull du Cancer. 2016;103:S201–S206.
Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36:1187–96.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Brotelle T, Cabrespine A, Combal C, Tournilhac O, Hermet E, Ravinet A, et al. Prévalence de la dénutrition à distance d’une allogreffe de cellules souches hématopoïétiques chez l’adulte. Nutr Clin Métabolisme. 2016;30:233–4.
Cayón-Blanco M, García-Figueras-Mateos C, Báez-Rivas M, Martín-Chacón E, García-García-Doncel L. Evaluation of nutritional status of onco-hematological patients undergoing hematopoietic stem cell transplantation. Clin Nutr. 2018;37:S194.
Ferreira ÉE, Guerra DC, Baluz K, de Resende Furtado W, da Silva Bouzas LF. Nutritional status of patients submitted to transplantation of allogeneic hematopoietic stem cells: a retrospective study. Rev Brasileira Hematologia Hemoterapia. 2014;36:414–9.
Jager-Wittenaar H, Ottery FD. Assessing nutritional status in cancer: role of the Patient-Generated Subjective Global Assessment. Curr Opin Clin Nutr Metab Care. 2017;20:322–9.
Cioce M, Botti S, Lohmeyer FM, Galli E, Magini M, Giraldi A, et al. Nutritional status and quality of life in adults undergoing allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2022;116:266–75.
Toenges R, Greinix H, Lawitschka A, Halter J, Baumgartner A, Simon A, et al. Current practice in nutrition after allogeneic hematopoietic stem cell transplantation - Results from a survey among hematopoietic stem cell transplant centers. Clin Nutr. 2021;40:1571–7.
Author information
Authors and Affiliations
Contributions
SE collected data; SE, ML, SB, AS, EC, RS, GR, AJ, JC, SS and TC edited the manuscript; SE, JC and TC designed the study; SE, ML and TC wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Estran, S., Loschi, M., Benachour, S. et al. Improving nutritional status after allogeneic stem cell transplantation: results of phase 2 ALLONUT clinical trial. Bone Marrow Transplant (2024). https://doi.org/10.1038/s41409-024-02271-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-024-02271-w