Abstract
Taurodontism is a dental anomaly defined by enlargement of the pulp chamber of multirooted teeth with apical displacement of the pulp floor and bifurcation of the roots. Taurodontism can be an isolated trait or part of a syndrome. A study was conducted to document the dental and craniofacial aspects of genetic thin bone disorders in South Africa. Sixty-four individuals with Osteogenesis imperfecta (OI), one individual with Pyle disease and one with Torg-Winchester syndrome respectively, were assessed clinically, radiographically and at a molecular level. Ten patients with OI XI and those with Pyle disease and Torg-Winchester syndrome had taurodontism. Taurodontism has been identified in several genetic disorders necessitating cognizance of the possible existence and implications of this characteristic when managing patients in the dental environment. Further studies should be directed toward identifying the incidence, etiology, and molecular pathways leading to taurodontism and its relationship to genetic syndromes.
Similar content being viewed by others
Introduction
Taurodontism is a developmental anomaly of the teeth. The term was proposed to describe the vertical increase in pulp chamber size, mimicking the shape of bovine teeth.1 It is characterized by enlargement of the pulp chamber of a multirooted tooth with consequent apical displacement of the floor of the pulp as well as the bifurcation of the root.2 This pattern of molar tooth formation has been described in ancient Neanderthals where the tooth resembles that of a cud-chewing animal hence the term “tauro” (bull) and “dont” (tooth).
A taurodont appears as a clinically normal tooth since its roots lie below the alveolar margin. The distinguishing features of affected teeth can only be recognized from diagnostic radiographs.3 Taurodontism is classified into three types, i.e., hypotaurodontism, mesotaurodontism, and hypertaurodontism (Fig. 1A).4 The radiographic characteristics of taurodontism include an extension of the pulp chamber into the elongated body of the tooth, shortened roots and root canals despite a normal sized crown (Fig. 1B).
A Diagrammatic representations of taurodontic teeth: (a) Normal molar, (b) hypotaurodintism, (c) mesotaurodontism, (d) hypertaurodontism; b radiographic image of a molar with features of taurodontism. B Reproduced with permission from Silva et al. 32 available from https://doi.org/10.1590/1981-863720150002000101733.
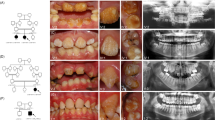
According to Shaw,4 hypotaurodontism (Fig. 2a, b) refers to a moderate enlargement of the pulp chamber at the expense of the roots. In mesotaurodontism (Fig. 2c), the pulp is relatively enlarged with shortened roots, but both roots are still separated from each other. Hypertaurodontism (Fig. 3) refers to pulp chambers that nearly reach the apex of the tooth and then divides into different conically shaped roots.4 Taurodontism may occur either unilaterally or bilaterally (Fig. 3) and affect permanent teeth more often than primary teeth.5,6 These anomalies have no gender predilection. The reported prevalence is variable and ranges between 0.5 and 46%.7 The wide prevalence range is most likely the result of diverse diagnostic criteria used to identify the phenomenon and racial variation.8
a Cropped CBCT image of an individual with phenotypic OI III (OI XI genotype). Intrapulpal calcification (1) and an unusual occlusal anatomy (2) in teeth 17 and 29 are indicative of hypotaurodontism (3); b an individual with Torg-Winchester syndrome. The mandibular molar (L) has 2/3rd of root development completed (1). Large coronal pulp chambers are suggestive of hypotaurodontism and contain several intrapulpal calcifications (2); c An individual with Pyle disease and mesotaurodontism of the first permanent molars (arrows).
Taurodontism is believed to arise from a field effect and all molars are generally involved, the first molar being least affected, and with increased severity in the second and third molars, respectively (Fig. 3).8 Conditions affecting the development of the dentition are numerous and are caused by both environmental and genetic factors.
Taurodontism can occur as either an isolated trait or as a component of a genetic syndrome. In this study we aim to describe the association between taurodontism and genetic disorders of bone in a South African cohort and provide a review of the literature.
Methods
All investigations were undertaken in complete accordance with the Declaration of Helsinki, the Hippocratic Oath and the Singapore Statement on Research Integrity. Formal ethical approval (HREC reference number: 203/2013) was obtained from the University of Cape Town’s ethics committee.
This study is a sub-study of one conducted by Chetty et al. 9 which examined the dental aspects of various genetic thin bone disorders in a South African cohort. The study consisted of craniofacial and dental examinations of those affected. The cohort was obtained from various centers throughout the country. The age range of the cohort ranged from 3 months to 30 years. This sample consisted of 64 black South African individuals with a clinical diagnosis of osteogenesis imperfecta type III (OI III), one individual with Pyle disease [OMIM 265900] and one with Torg-Winchester syndrome [OMIM 259600]. After molecular studies, it was found that 23 of those clinically diagnosed with OI III, actually had OI XI.9 Radiographic examinations were performed only when necessary. Radiographic images that had previously been obtained by the patient’s physician, for instance, lateral views of the skull, were also available to the authors. Due to limited resources, the imaging facilities used were only available at specific dental centers. All radiographic findings were confirmed by two consultant radiologists from the universities of the Western Cape and Stellenbosch. All radiographs were assessed using the criteria of Shifman and Chanannel2 for taurodontism. Inter-rater agreement was calculated using Stata (Version 15, StataCorp, USA) statistical software.
Results
Of the 66 individuals in the sample, 55 were included in this study due to radiograph availability. This included 15 CBCTs, 20 Panorex, and 20 cephalometric radiographs. All of those included happened to be male. It was found that 12 individuals in our sample showed features of taurodontism (21.8%, n = 55; ICC = 97%). This includes ten individuals with OI XI (18.87%, n = 53), one with Pyle disease (100%, n = 1), and one with Torg-Winchester syndrome (100%, n = 1). None of those with OI III showed signs of taurodontism.
A homozygous mutation was identified in 23 affected persons, thus confirming a diagnosis of OI XI. This homozygous mutation, c.[831dupC]; [831dupC], a frameshift DNA variant which is predicted to alter the protein sequence by substituting a Glycine residue with an Arginine at position 278 of the 65 kDa FK506 Binding protein 10. The introduction of a premature termination codon results in the loss of 211 amino acid residues.
Discussion
This study was the first to assess the prevalence of taurodontism in genetic disorders of the bone in a South African population. The cohort included patients with OI III, OI XI, Pyle Disease, and Torg-Winchester syndrome. Of the 23 individuals with OI XI, ten (43, 48%) showed signs of taurodontism of the permanent dentition. None of those with the OI type III phenotype showed signs of taurodontism. Although our sample size is small, taurodontism may be a sign of an underlying genetic condition of the bone and therefore dentists need to be aware of the implications of taurodontism.
Taurodontism has been associated with several genetic conditions listed in Table 1. Syndromes associated with cleft lip and palate and individuals with non-syndromic cleft lip and palate also show a high incidence of taurodontism.10
Although the term “taurodontism” is well known, there is some confusion concerning the pathogenesis, developmental manifestations, and dental significance of this anomaly. We will briefly overview what is known about the development of taurodontism.
The development of taurodontic teeth
Two primary germ layers, ectoderm and mesoderm together with neural crest cells give rise to teeth. The oral ectoderm gives rise to enamel while the ectomesenchymal tissue contributes to the remaining tooth structure. Several genes are expressed in tooth development and these are associated with signaling molecules as well as epithelial–ectomesenchymal interaction.11,12 The growth of the dental sensory nerves is required for the establishment of the mesenchymal stem cells that provide FGF10 expression; in turn, FGF10 initiates dental tissue formation. The initiation of the position of teeth within the dental arch is associated with early mesenchymal expression of homeobox genes.
Odontogenic tissue is present from the 28th day of embryonic development. The dental laminae undergo induction and proliferate in a constant genetically determined sequence into the underlying ectomesenchymal tissue at locations which correspond to the dental papillae. Fibroblast growth factors (FGF) stimulate mitotic activity of the odontogenic cells and promote the expression of a transcription factor, MSX1 which is required for normal tooth development.11,12
Taurodontism predominantly occurs in molars and for this reason the development of these teeth, especially the roots will be discussed in further detail. During early tooth development, reciprocal and sequential interactions between the epithelium and mesenchyme eventually lead to the formation of root dentin, cementum, and periodontal tissues. The major signaling pathways involved in these processes are the Tgfβ/Bmp, Wnt, Fgf, and Shh pathways, which work together with multiple transcription factors to mediate tissue–tissue interactions that guide root development.13 Transcription factor LHX6/7 is primarily responsible for molar development. The primordia of the permanent molars develop from distal extensions of the dental laminae. Each tooth germ consists of an enamel organ and a dental papilla surrounded by a dental follicle.
Hertwig’s epithelial root sheath (HERS) is formed from the disappearance of the stellate reticulum within the enamel organ, once enamel has formed, and the fusion of the inner and outer enamel epithelia to form the cervical loop. As HERS extends downward, it encloses the dental papilla, thus creating the outline of the root. HERS then stimulates the surrounding dental papilla mesenchyme to differentiate into odontoblasts and secrete the dentin of the root. The number of roots of a tooth is determined by the subdivision, or lack thereof, of HERS.
Several transcription and growth factors such as SHH, DLX2, and Patched2 are expressed by HERS. A delay or failure of HERS to invaginate into the mesenchyme results in the apical displacement of the root furcation and subsequently in the development of taurodontism.11,12
The ectodysplasin A (EDA)/EDA receptor signaling pathway14 plays a critical role in tooth development and in particular, in root development. Edar (the receptor) is expressed in HERS during the development of the root, with mutant mice showing a high incidence of taurodontism and this enhanced susceptibility was being demonstrated in human patients with mutations in EDA-A1.14 This is the first published report that shows a direct role for this pathway during postnatal mouse development and suggests that changes in proliferation and angle of HERS may underlie taurodontism in a range of syndromes.
Dental significance of taurodontism
It is crucial that routine imaging examinations be performed in order to diagnose anomalies such as taurodontism in patients with genetic disorders, as this condition cannot be identified during a routine oral examination. Cone beam CT images may assist the diagnosis of taurodontism and the formulation of a treatment plan.5,15,16
Pulp therapy on taurodontic teeth is challenging. A taurodont tooth displays variation in the size of the pulp chamber which may have varying degrees of obliteration and pulp stones are often present. The configuration of the root canals may also vary with apically positioned canal orifices.5,15
In taurodontism, larger than normal pulpal tissue may result in excessive bleeding during dental treatment and the complete removal of the necrotic pulp tissue may be difficult. Since the roots are short and the pulpal floor is placed apically, extreme care is warranted in order to prevent perforation of the pulp cavity and root canal. Extraction of a taurodont is usually complicated due to dilated roots in the apical third.17
Patients with genetic disorders require a multidisciplinary approach and due to the complexity of the patient’s condition and dental needs, they may require unique oral health care management approaches. Inter-professional communication will aid in obtaining an accurate diagnosis and help optimize the efficiency of their management. In this context, dental health practitioners within a comprehensive craniofacial team have an important role in the achievement of a suitable functional result and consequent improvement in the quality of life of the affected patients.
Although developing molars may have radiographic features similar to taurodontism in children and young adults, recognition of the presence of open apical foramina and incomplete root formation would assist in a definitive diagnosis of the condition.18 In individuals with dentine dysplasia a differential diagnosis of taurodontism may arise at the early stage of dentinogenesis, since the pulp chambers may be large and resemble those which characterizes taurodontism.19 In older individuals and those with a coarse diet, taurodontism may be less obvious due to the deposition of secondary and tertiary dentine. For this reason, clinical caution is recommended when interpreting possible taurodontism in molars with marked occlusal wear.20
Taurodontism in thin bone disorders
Often, taurodontism has a genetic component; for example, in tricho-dento-osseous syndrome, which is associated with DLX3 mutations.21 Mutations in DLX3 are also associated with amelogenesis imperfecta hypoplastic-hypomaturation with taurodontism in humans.22 Of note, the ablation of Smad4, Nfic, or Wnt10a in mice leads to similar phenotypes.23,24 Taurodontism has been well recognized in the relatively common form of OI. Lukinmaa25 documented taurodontism in 3 of 49 persons with OI II and Malmgren and Norgren26 identified taurodontism in 20 of 48 individuals with unspecified forms of OI.
In 73 Danish adults with OI, OI type I, III, and IV, pulp stones and taurodontism were found only in those with mild OI.27 In contrast to this observation, in the South African project, taurodontism was identified in persons with severe phenotypic OI III and genotypic OI XI.9 This observation could reflect genetic and epigenetic factors which are associated with OI XI in this country.
In the rare genetic thin bone disturbances identified in the project, taurodontism was present in Pyle disease [OMIM 265900]. This condition is a rare autosomal recessive skeletal dysplasia in which both metaphyseal expansion and under-modeling of the tubular bones are significant features. This condition is associated with homozygous mutations in SFRP4, a gene that encodes soluble Wnt inhibitor responsible for differential regulation of Wnt and bone morphogenetic protein.28
Taurodontism was also recognized in the Torg-Winchester syndrome [OMIM 259600] in which the wrists and ankles are affected and there is progressive dissolution of the carpals and tarsals.29 Two separate mutations in the MMP2 gene result in complete loss of matrix metalloproteinase activity.30,31 This disorder probably represents a continuous clinical spectrum which results from intragenic heterogeneity in the MMP2 gene and the eponym “Torg-Winchester syndrome” is now widely accepted.
Conclusion
Taurodontism has been considered to be a primary feature of some syndromes, but it has become evident from recent reports which suggest the presence of this feature in several genetic syndromes. Our study revealed features of taurodontism in a South African cohort of patients with OI XI, Pyle disease, and Torg-Winchester syndrome. Recognition of taurodontism in several separate genetic disorders have led to an increased awareness, necessitating cognizance of the possible existence of this characteristic when managing patients in the dental environment. Further studies could be directed toward identifying the incidence of taurodontism in affected individuals worldwide. The etiology of this abnormality is worthy of investigation and the possibility that general disturbance during development of molar teeth plays a role.
Knowledge of the genetic contribution to conditions affecting the oral and craniofacial complex will allow practitioners to identify their patients risk factors. Understanding the genetics of each syndrome highlights the potential value of translation into clinical care with improved patient outcomes. Interventions could be targeted more specifically, and timing and duration of treatment could be objectively employed.
Providers of oral health care are faced with rapid changes in knowledge related to etiology, diagnosis, and treatment of conditions affecting the oral craniofacial health of the population. Advances in genomics are also impacting upon health in dentistry and the understanding of rare genetic diseases such as taurodontism is crucial in this context.
References
Keith, A. Problems relating to the teeth of the earlier forms of prehistoric man. Proc. R. Soc. Med. 6, 103–124 (1913).
Shifman, A. & Chanannel, I. Prevalence of taurodontism found in radiographic dental examination of 1,200 young adult Israeli patients. Community Dent. Oral. Epidemiol. 6, 200–203 (1978).
Bharti, R., Chandra, A., Tikku, A. P. & Wadhwani, K. K. “Taurodontism” an endodontic challenge: a case report. J. Oral. Sci. 51, 471–474 (2009).
Shaw, J. Taurodont teeth in South African Races. https://www-ncbi-nlm-nih-gov.ezproxy.uwc.ac.za/pmc/articles/PMC1249989/ (1928).
Rao, A. & Arathi, R. Taurodontism of deciduous and permanent molars: report of two cases. J. Indian Soc. Pedod. Prev. Dent. 24, 42 (2006).
Surendar, M. N., Pandey, R. K. & Khanna, R. Bilateral taurodontism in primary dentition with hypodontia. https://casereports-bmj-com.ezproxy.uwc.ac.za/content/2013/bcr-2012-008259.short (2013).
Bafna, Y., Kambalimath, H. V., Khandelwal, V. & Nayak, P. Taurodontism in deciduous molars. Case Rep. 2013, bcr2013010079 (2013).
MacDonald, D. Taurodontism. Oral. Radiol. 36, 129–132 (2020).
Chetty, M., Roberts, T., Shaik, S. & Beighton, P. Dentinogenesis imperfecta in Osteogenesis imperfecta type XI in South Africa: a genotype–phenotype correlation. BDJ Open. 5 https://doi.org/10.1038/s41405-019-0014-z (2019).
Weckwerth, G. M., Santos, C., Brozoski, D. T., Centurion, B. S. & Pagin, O. Taurodontism, root dilaceration, and tooth transposition: a radiographic study of a population with nonsyndromic cleft lip and/or palate. https://journals-sagepub-com.ezproxy.uwc.ac.za/doi/full/10.1597/14-299 (2016).
Thesleff, I. The genetic basis of tooth development and dental defects. Am. J. Med Genet A. 140, 2530–2535 (2006).
Thesleff, I. Epithelial-mesenchymal signalling regulating tooth morphogenesis. J. Cell Sci. 116, 1647–1648 (2003).
Li, J., Parada, C. & Chai, Y. Cellular and molecular mechanisms of tooth root development. Dev. Camb. Engl. 144, 374–384 (2017).
Fons Romero, J. M. et al. The impact of the eda pathway on tooth root development. J. Dent. Res. 96, 1290–1297 (2017).
Tsesis, I., Shifman, A. & Kaufman, A. Y. Taurodontism: an endodontic challenge. Report of a case. J. Endod. 29, 353–355 (2003).
Borges, A. H. et al. Using cone beam computed tomography images to diagnose multiple taurodontisms. Gen. Dent. 62, e20–22 (2014).
Yeh, S. C. & Hsu T. Endodontic treatment in taurodontism with Klinefelter’s syndrome: a case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. https://doi.org/10.1016/s1079-2104(99)70094-6 (1999).
Mohan, R. P. S., Verma, S., Agarwal, N. & Singh, U. Taurodontism. Case Rep. 2013, bcr2012008490 (2013).
O Carroll, M. K., Duncan, W. K. & Perkins, T. M. Dentin dysplasia: review of the literature and a proposed subclassification based on radiographic findings. Oral. Surg. Oral. Med Oral. Pathol. 72, 119–125 (1991).
Jafarzadeh, H., Azarpazhooh, A. & Mayhall, J. T. Taurodontism: a review of the condition and endodontic treatment challenges. Int Endod. J. 41, 375–388 (2008).
Whitehouse, L. L. E. et al. Novel DLX3 variants in amelogenesis imperfecta with attenuated tricho-dento-osseous syndrome. Oral. Dis. 25, 182–191 (2019).
Dong, J. et al. DLX3 mutation associated with autosomal dominant amelogenesis imperfecta with taurodontism. Am. J. Med Genet A. 133A, 138–141 (2005).
Li, J. et al. BMP-SHH signaling network controls epithelial stem cell fate via regulation of its niche in the developing tooth. Dev. Cell. 33, 125–135 (2015).
Liu, Y. et al. An Nfic-hedgehog signaling cascade regulates tooth root. Dev. Dev. 142, 3374–3382 (2015).
Lukinmaa, P. L., Ranta, H., Ranta, K. & Kaitila, I. Dental findings in osteogenesis imperfecta: I. Occurrence and expression of type I dentinogenesis imperfecta. J. Craniofac Genet Dev. Biol. 7, 115–125 (1987).
Malmgren, B. & Norgren, S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol. Scand. 60, 65–71 (2002).
Thuesen, K. J. et al. The dental perspective on osteogenesis imperfecta in a Danish adult population. BMC Oral. Health 18, 1–7 (2018).
Galada, C., Shah, H., Shukla, A. & Girisha, K. M. A novel sequence variant in SFRP4 causing Pyle disease. J. Hum. Genet. 62, 575–576 (2017).
Vanatka, R., Rouzier, C., Lambert, J. C., Leroux, C. & Coussement, A. Winchester syndrome: the progression of radiological findings over a 23-year period. Skelet. Radiol. 40, 347–351 (2011).
Zankl, A., Bonafé, L., Calcaterra, V., Di Rocco, M. & Superti-Furga, A. Winchester syndrome caused by a homozygous mutation affecting the active site of matrix metalloproteinase 2. Clin. Genet. 67, 261–266 (2005).
Zankl, A. et al. Torg syndrome is caused by inactivating mutations in MMP2 and is allelic to NAO and Winchester syndrome. J. Bone Min. Res. 22, 329–333 (2007).
Silva, E. J. N. L., Senna, P. M., Coutinho Filho, T. S. & Krebs, R. L. Endodontic treatment of a mandibular hypertaurodontic second molar. Rev. Gaúch Odontol. 63, 203–206 (2015).
Crawford, P. J. & Aldred, M. J. Amelogenesis imperfecta with taurodontism and the tricho-dento-osseous syndrome: separate conditions or a spectrum of disease? Clin. Genet. 38, 44–50 (1990).
de Moraes, M. E. L. et al. Dental anomalies in patients with Down syndrome. Braz. Dent. J. 18, 346–350 (2007).
Dagrus, K., Manjunatha, B. S., Shah, V. & Shah, S. N. Concomitant presence of ectodermal dysplasia with taurodontism: a rare entity. https://imsear.searo.who.int/jspui/handle/123456789/188467 (2018).
Zheng, J. et al. Tooth defects of EEC and AEC syndrome caused by heterozygous TP63 mutations in three Chinese families and genotype-phenotype correlation analyses of TP63-related disorders. Mol. Genet Genom. Med. 7, e704 (2019).
Ghosh, S., Setty, S., Sivakumar, A. & Pai, K. Report of a new syndrome: Focus on differential diagnosis and review of Ellis-van Creveld, Curry-Hall, acrofacial dysostosis, and orofacial digital syndromes. Oral. Surg. Oral. Med Oral. Pathol. Oral. Radio. Endod. 103, 670–676 (2007).
Peña-Cardelles, J.-F., Domínguez-Medina, D. A., Cano-Durán, J. A., Ortega-Concepción, D. & Cebrián, J.-L. Oral manifestations of ellis-van creveld syndrome. A rare case report. J. Clin. Exp. Dent. 11, e290–e295 (2019).
Shaik, S., Raviraj, J., Dirasantchu, S. & Venkata, S. S. Ellis–van Creveld syndrome with unusual oral and dental findings: a rare clinical entity. Dent. Res J. 13, 193–197 (2016).
McGovern, E. et al. Oral features and dental health in Hurler Syndrome following hematopoietic stem cell transplantation. Int J. Paediatr. Dent. 20, 322–329 (2010).
Tsai, S. J. & O’Donnell, D. Dental findings in an adult with Lowe’s syndrome. Spec. Care Dent. 17, 207–210 (1997).
Jayashankara, C., Shivanna, A., Sridhara, K. & Kumar, P. Taurodontism: a dental rarity. J. Oral. Maxillofac. Pathol. 17, 478 (2013).
Havle, A., Shedge, S., Malashetti, S. & Jain, V. Oro-facial-digital syndrome type II with otolaryngological manifestations. J. Oral. Maxillofac. Pathol. 19, 266 (2015).
Passarelli, P. C. et al. Orofacial signs and dental abnormalities in patients with Mulvihill-Smith syndrome: a literature review on this rare progeroid pathology. Medicine (Baltimore) 97, e0656 (2018).
Feller, L. et al. Report of a black South African child with oculodentodigital dysplasia and a novel GJA1 gene mutation. Am. J. Med. Genet. A. 146A, 1350–1353 (2008).
Merametdjian, L., Prud’Homme, T., Le Caignec, C., Isidor, B. & Lopez-Cazaux, S. Oro-dental phenotype in patients with RUNX2 duplication. Eur. J. Med Genet. 62, 85–89 (2019).
Bougeard, G., Hadj-Rabia, S., Faivre, L., Sarafan-Vasseur, N. & Frébourg, T. The Rapp-Hodgkin syndrome results from mutations of the TP63 gene. Eur. J. Hum. Genet. 11, 700–704 (2003).
Scott, J., Adams, C., Beetstra, S. & Zarate, Y. A. SATB2-associated syndrome (SAS) and associated dental findings. Spec. Care Dent. 39, 220–224 (2019).
Tomona, N., Smith, A. C. M., Guadagnini, J. P. & Hart, T. C. Craniofacial and dental phenotype of Smith–Magenis syndrome. Am. J. Med. Genet. A. 140A, 2556–2561 (2006).
Johnston, N. J. & Franklin, D. L. Dental findings of a child with Wolf-Hirschhorn syndrome. Int J Paediatr Dent. 16, 139–42 (2006).
Jaspers, M. T. & Witkop, C. J. Taurodontism, an isolated trait associated with syndromes and X-chromosomal aneuploidy. Am. J. Hum. Genet. 32, 396–413 (1980).
Acknowledgements
The authors would like to acknowledge the essential input of Prof. Tina Roberts.
Author information
Authors and Affiliations
Contributions
M.C.: contributed to conception, design, data acquisition and interpretation, drafted and critically revised the manuscript. I.A.R.: contributed to the interpretation, drafting, and critically revised the manuscript. P.B.: contributed to conception, design, and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Investigations were undertaken with ethical approval in accordance with the Declaration of Helsinki as updated in the version promulgated in June 2013 and the Singapore Statement on Research Integrity. Ethics approval was received from the University of Cape Town (Faculty of Health Sciences: Human Research Ethics Committee) (no. 203/2013). Informed consent to publish clinical information and pictures was obtained from the affected persons, with a signature from a health professional as a witness.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chetty, M., Roomaney, I.A. & Beighton, P. Taurodontism in dental genetics. BDJ Open 7, 25 (2021). https://doi.org/10.1038/s41405-021-00081-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-021-00081-6