Abstract
MicroRNAs (miRNAs) are recognized as an essential component of the RNA family, exerting multiple and intricate biological functions, particularly in the process of tumorigenesis, proliferation, and metastatic progression. MiRNAs are altered in gastric cancer (GC), showing activity as both tumor suppressors and oncogenes, although their true roles have not been fully understood. This review will focus upon the recent advances of miRNA studies related to the regulatory mechanisms of gastric tumor cell proliferation, apoptosis, and cell cycle. We hope to provide an in-depth insight into the mechanistic role of miRNAs in GC development and progression. In particular, we summarize the latest studies relevant to miRNAs’ impact upon the epithelial-mesenchymal transition, tumor microenvironment, and chemoresistance in GC cells. We expect to elucidate the molecular mechanisms involving miRNAs for better understanding the etiology of GC, and facilitating the development of new treatment regimens for the treatment of GC.
Similar content being viewed by others
Introduction
The World Health Organization has identified cancer as the leading cause of death in 185 countries that were examined [1]. Accordingly, among 36 cancer types, gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of cancer death, respectively, and is much more prevalent in Asian countries compared to non-Asian countries [1]. China contributes to more than 50% of all GC cases worldwide, with approximately 680,000 new cases and 500,000 deaths each year [2]. Surgical resection is the preferred method of initial treatment whenever feasible, with adjuvant chemotherapy as a vital addition to the multidisciplinary approach to treatment. For locoregional recurrence of GC, chemotherapy, with or without external beam radiation therapy, appears to be a reasonable approach to treat patients with unresectable disease. However, the response rates to such approaches are marginal at best, leading to a median overall survival of ~8-17 months [3].
Presently, there is a lack of effective treatment options for patients with GC, translating to a uniformly poor overall survival worldwide. As such, the research focus has gradually switched to the discovery of novel and precise biomarkers that can lead to the development of targeted therapeutics. MicroRNAs (miRNAs) are a class of small nucleic acids and function as the master regulators in the control of gene expression [4]. To date, over 2500 human-specific miRNAs have been identified, with their dysregulation associated with tumor cell proliferation, apoptosis, invasion, and metastatic potential. Furthermore, aberrantly expressed miRNAs are potentially useful biomarkers for GC screening, diagnosis, prognosis, and disease monitoring. This review will summarize the most recent literature on miRNAs and the associated target genes that are specific to GC, highlighting their intrinsic mechanistic role in GC development and progression.
MiRNAs are associated with GC development and progression
Recent studies of miRNAs have shed light on their contributions toward controlling the development and progression of GC. Herein, we will focus on the functional interactions among select miRNAs, their putative target genes, and the relevant signaling pathways that are involved in the key pathophysiological processes of GC.
Helicobacter pylori infection-associated tumorigenesis
Helicobacter pylori (H. pylori) infection is known as a driving cause of significant morbidity in the context of GC. Yang et al. examined the miRNA levels in H. pylori-infected patients with GC, and showed that H. pylori infection was associated with the specific cancer-related signaling pathways regulated by the miRNA-mRNA interaction network [5]. Of interest, miR-155 was reported to be involved in the differentiation of T helper 17 (Th17) and Th1 cells, which contribute to the immunity against H. pylori infection and the infection-associated immunopathology [6]. H. pylori cytotoxin-associated gene A (Cag A) was found to suppress miR-26b, which in turn, upregulated the expression of miR-26b’s putative target gene, karyopherin alpha 2 (KPNA2), a promoter for cancer metastasis [7]. MiR-143-3p, which was found to be the most significantly increased miRNA in H. pylori-positive GC tissues, hindered tumor growth [8]. In addition, miR-155, miR-16, and miR-146a were reported to be upregulated in gastric epithelial cells infected with H. pylori, and increased miR-155 was also found in mucosal tissues from H. pylori-positive patients [9]. These studies demonstrate the concurrent influences of miRNAs on H. pylori-mediated, inflammation-associated tumorigenesis.
Cell proliferation
Numerous studies have directly examined the role of miRNAs in GC growth by targeting cellular signaling pathways and genes. Table 1 shows the miRNAs and their putative targets that are relevant to the GC proliferation and apoptosis. We will focus on the two major signaling pathways that have been extensively studied in the context of GC cell proliferation.
PI3K/Akt/mTOR signaling pathway
The activation of the phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathway is important for regulating gene expression in a variety of human cancers. This pathway is also involved in cell cycle regulation, apoptosis, transcription, translation, metabolism, and angiogenesis. For instance, elevated miR-21 in GC was reported to target the 15-hydroxyprostaglandin dehydrogenase (15-PGDH) gene and the phosphatase and tensin homolog (PTEN) gene, to promote GC proliferation. In doing so, miR-21 exerts its oncogenic effect through the prostaglandin E2 (PGE2)/PI3K/Akt/Wnt/β-catenin axis, resulting in GC cell proliferation [10,11,12]. In addition, Akt and mTOR were reported to be targeted by miR-495 directly; the overexpression of miR-495 could inhibit the growth and induce the apoptosis of GC cells, with the blockade of the PI3K/Akt/mTOR signaling, which in turn, altered the expression of Bax, caspase-3/-9, and cyclin D1 [13]. MiR-495 was also shown to accelerate the death of GC cells through noncanonical beclin 1-independent autophagy induced by the Akt/mTOR pathway [14]. Furthermore, tripartite motif-containing 24 (TRIM24) elicited tumor-stimulating effects through the regulation of the PI3K/Akt and Wnt/β-catenin signaling pathways, and these effects seemed to be negated by the overexpression of miR-511 [15] (Fig. 1). A recent study also reported that GC tumor-derived exosomes containing enriched miR-107 could enter myeloid-derived suppressor cells (MDSCs) and downregulate the PTEN gene, leading to the activation of the PI3K pathway in MDSCs [16].
15-PGDH 15-hydroxyprostaglandin dehydrogenase, β-cat β-catenin, Akt protein kinase B, APC adenomatous polyposis coli, DIXDC1 disheveled–axin domain containing 1, GFs growth factors, GSK-3 glycogen synthase kinase-3, mTOR mammalian target of rapamycin, PI3K phosphoinositide 3-kinases, PGE2 prostaglandin E2, PTEN phosphatase and tensin homolog, SFRP1 secreted frizzled-related protein 1, SOX5 SRY-box transcription factor 5, SOX9 SRY-box transcription factor 9, STRA6 stimulated by retinoic acid 6, SUFU suppressor of fused homolog, TRIM24 tripartite motif-containing 24.
Wnt/β-catenin signaling pathway
The Wnt/β-catenin signaling pathway is involved in numerous physiological processes, including cell cycle regulation and tumorigenicity. MiR-154 was reported to inhibit the activation of the Disheveled-Axin domain containing 1 (DIXDC1)/Wnt signaling, which then mitigated the growth of GC cells [17]. It is reported that miR-511 could suppress the PI3K/Akt and Wnt/β-catenin signaling by directly targeting TRIM24, while the ectopic expression of miR-511 significantly inhibited GC cell proliferation, with the reduced expression of p-Akt, β-catenin, cyclin D1, and c-Myc [15]. Other studies have shown that miR-338-3p, miR-520f-3p, and miR-873 were downregulated in GC cells, with the forced expression of these miRNAs able to suppress GC progression via the downregulation of SRY-box transcription factor 5 (SOX5), SRY-box transcription factor 9 (SOX9), and stimulated by retinoic acid 6 (STRA6) to further block Wnt/β-catenin signaling [18,19,20]. In addition, miR-27a, miR-194, miR-192, and miR-215 were reported to be upregulated in GC cells, and their ectopic expression could promote the tumor cell proliferation and cancer development [21,22,23]. The mechanistic studies demonstrated that these oncogenic miRNAs could repress the negative regulators of the Wnt signaling cascade, including secreted frizzled-related protein 1 (SFRP1), suppressor of fused homolog (SUFU), and adenomatous polyposis coli (APC), with the translocation of β-catenin into the nucleus, as shown in Fig. 1.
Apoptosis
Li et al. showed that miR-3174 inhibited mitochondria-dependent apoptosis and autophagic cell death, with the high expression of miR-3174 shown to be related to the resistance of cisplatin (DDP) [24]. Others have shown the effect of miR-1284 in modulating multidrug resistance (MDR) and accelerating drug-induced apoptosis, further preventing cells from entering the S phase of the cell cycle [25]. The mechanistic studies demonstrated that these phenotypes resulted from miR-1284 directly targeting the gene of eukaryotic translation initiation factor 4A1 (EIF4A1), and indirectly suppressing the gene expression of Jun and matrix metallopeptidase 12 (MMP-12) and facilitating the gene expression of Myc [25]. MiR-30a’s downregulation was recently identified in DDP-resistant SGC-7901 cells and decreased DDP-induced apoptosis. Of note, the downregulation of LC3-II by miR-30a was able to inhibit chemoresistance-associated autophagy and increase the total apoptotic rate in chemoresistant cells [26].
Cell cycle
Many oncogenic and tumor suppressor miRNAs have been reported to be involved in cell cycle regulation. Table 2 is the summary of select miRNAs and their putative target genes that have been reported to contribute to the cell cycle progression in GC cells. For instance, the downregulation of miR-31 has been found in several human cancer cell lines (MGC-803, MKN-45, AGS, and SGC-7901), but not in the N87 cell line. Functionally, miR-31 suppresses tumor cell proliferation, induces apoptosis, blocks G1 transition, and reduces migration and invasion in both SGC-7901 and MGC-803 cells via the inhibition of expression for the E2F transcription factor 2 (E2F2) gene [27]. Clinical and pathological characteristics show that low miR-329 expression, along with high expression of its target gene, histone lysine demethylase 1 A (KDM1A), likely contribute to GC progression. Forced expression of miR-329 showed a comparable phenotype as that of KDM1A silencing in inhibiting BGC-823 cell viability, facilitating G1 arrest, reducing colony formation, and promoting tumor cell apoptosis [28]. The inhibition of miR-106a accelerated GC cell apoptosis with a visible sub-G1 peak as a reliable indicator of apoptosis, which was further confirmed in AGS and N87 cells transfected with miR-106a antisense oligonucleotides [29]. The tumor suppressor miR-101 induced a more significant accumulation of sub-G1 phase cells, with both early and late apoptotic cells after 72 h of miR-101 mimic transfection in MKN-45 cells. The overexpression of miR-101 induced the cleavage of poly(ADP-ribose) polymerase (PARP) and suppressed migratory and invasive abilities, as well as the epithelial-mesenchymal transition (EMT), and directly inhibited the expression of the zinc finger E-box-binding homeobox 1 (ZEB1) gene [30]. Furthermore, miR-126 could function as a putative tumor suppressor in GC, potently inhibiting the cell growth as a result of cell cycle arrest in the G0/G1 phase by synergistically targeting Crk and a disintegrin and metallopeptidase domain 9 (ADAM9) [31, 32]. The overexpression of miR-214 has been identified in GC cells, and its downregulation can induce G1 cell cycle arrest by upregulating PTEN in BGC-823 and MKN-45 cells [33]. A similar phenotype was observed in SGC-7901 and U87 cells after the transfection of miR-383, which targets cyclin E2 [34].
EMT
EMT is a process associated with tumor initiation, progression, invasion, metastasis, and resistance to drug therapy [35]. During this process, E-cadherin can sustain key intracellular binding structures, such as desmosomes and claudins, and switch to N-cadherin [36]. The downregulation of E-cadherin can be mediated by putative miRNAs and EMT-inducing TFs, such as snail1 (snail), snail2 (slug), Twist, ZEB1, and ZEB2 [36].
MiR-25 exhibits inhibitory effects on human diffuse-type GC, with the inhibition of miR-25 leading to increased collagen type I alpha 2 chain (COL1A2), as well as the attenuation of E-cadherin gene expression [37]. In addition, miR-25 was shown to suppress p53 gene expression and sensitize c-Src activation, revealing its role in intestinal-type GC [37]. MiR-30a’s overexpression increased E-cadherin levels, but decreased N-cadherin levels in SGC-7901 cells, with the activity of mitigating MDR and modulating EMT in GC cells [38]. Moreover, fibroblast-like morphology may be shifted to a more epithelial-like phenotype with miR-30a overexpression in DDP-resistant SGC-7901 cells, increasing DDP sensitivity and inducing a concomitant reduction in both snail and vimentin levels [39]. The restored function of miR-216a resulted in a reduction of GC liver metastatic lesions in nude mice, and was also notable for suppressing EMT via the Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway [40]. Furthermore, the alteration of several miRNAs, such as miR-181a-5p [41], miR-302b [42], miR-223 [43], and miR-181b [44], also displayed a variety of effects on EMT, cell proliferation, and migration in GC. A study examining GC stem cells additionally confirmed that miR-196a-5p serves as a regulator of EMT and invasion, with one of the target genes identified as SMAD family member 4 (Smad4) [45].
MiRNAs and the GC tumor microenvironment
The tumor microenvironment (TME) is defined as a complex milieu within the tumor mass itself, surrounded by fibroblasts, blood vessels, immune and inflammatory cells, adipose cells, neuroendocrine cells, and the extracellular matrix [46]. Each component in the TME has a contributing role and function, as it relates to the tumor development and progression. Herein, we will summarize how miRNAs are intimately involved with the regulation of the TME for GC.
Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) have emerged as one of the key participants involved in the reactive stromal generation that regulates a tumor-promoting environment in cancer [47]. We have progressed our current understanding of CAF’s oncogenic functions, learning that the dysregulation of miRNAs in stromal cells has a significant influence on this important tumor milieu, likely contributing to the transformation of CAFs to promote cancer progression. For instance, miR-149 expression negatively regulates CAFs, mediating the crosstalk with tumor cells through the PGE2/interleukin-6 (IL-6) signaling [48]. Another miRNA, miR-106b, has been shown to be upregulated in CAFs, promoting cell migration and invasion by targeting the PTEN gene [49]. The low expression levels of miR-200b and miR-200c have been demonstrated to correlate with an overall poor prognosis for patients with GC [50]. A recent study reported that miR-200b downregulation was associated with the transformation of CAFs in GC. Specifically, miR-200b promoter methylation was observed in GC patients with high expression of alpha-smooth muscle actin (α-SMA), which was one of the specific markers of CAFs [51]. Functional studies further demonstrated that CAFs could promote tumor invasion by epigenetically altering miR-200b expression in GC cells [51]. MiR-141 is a tumor suppressor and a member of the miR-200 family, which was found to be downregulated in GC cells, and associated with cell proliferation in MGC-803, HGC-27, SGC-7901, and BGC-823 cell lines [52]. Recently, miR-141 was also reported to target the STAT4 gene, which is involved in the transformation of CAFs from normal fibroblasts in AGS cells [53].
Angiogenesis/neovascularization
The process of tumor cell angiogenesis and neovascularization is a well-known mechanism by which tumor cells are able to grow, progress, and eventually develop the means for metastatic spread. The development of newly formed blood vessels by the tumor itself has been clearly established as an important mechanism for tumor cell survival, with antiangiogenic treatment strategies integrated into the current cancer treatment regimens. MiRNAs, such as miR-29c, are stimulated by the treatment with insulin-like growth factor 1 (IGF1) within the endothelium. In turn, direct targeting of miR-29c promotes tube network formation by human umbilical vein endothelial cells (HUVECs) in vitro [54]. The gene expression of vascular endothelial growth factor (VEGF) is upregulated in GC and is directly targeted by miR-29a/c, with its overexpression shown to suppress angiogenesis within the TME. MiR-29a/c delivered by microvesicles (MVs) effectively suppressed the proliferation and ring formation of HUVECs. The blood vessel density was markedly reduced by MV-delivered miR-29a/c in vivo, as clearly indicated by the downregulation of CD31, known as one of the vascular markers [55].
VEGF-C is a putative target of miR-27b, which may function as a tumor suppressor in human GC development by inducing apoptosis. It has been recently reported that the overexpression of miR-27b suppresses GC cell proliferation and inhibits the expression of VEGF-C [56]. In addition, miR-132 was reported to facilitate pathological angiogenesis by targeting p120RasGAP and activating the endothelium [57]. After delivering anti-miR-132 to the tumor endothelium of mice utilizing the nanoparticles targeting integrin αVβ3, Anand et al. found that the tumor angiogenesis induced by a VEGF-secreting ovarian carcinoma could be ultimately blocked in vivo, which was further validated in a xenograft tumor model of breast cancer with MDA-MB-231 cells. These results support the notion that miRNA modification can regulate pathological neovascularization in vivo [57]. MiR-130a was identified in GC cell-derived exosomes, noted to have the capacity to invade HUVECs and target c-Myb in order to drive angiogenesis [58]. By harnessing the vascular-modulatory functions of miRNAs, we may be able to manipulate the antiangiogenic effect on tumor cells, possibly developing a more effective treatment approach.
Immune cells
The TME consists of a variety of immune cells, which have a dominant influence on and control of tumorigenesis, immune tolerance, and immune escape. Multiple immune cell types, including neutrophils, macrophages, dendritic cells, natural killer (NK) cells, and T and B lymphocytes, have been shown to infiltrate the tumor and actively participate in the modulation of the TME [59]. MiRNAs are recognized as dynamic regulators of immune cell functions in human cancers, and tumor-derived miRNAs can significantly influence the TME and specifically target immune cells to facilitate immune surveillance [60]. There has been a scarcity of publications that have detailed the true impact of miRNAs upon immune cells in GC.
Various levels of miRNA expression will have differential effects on NK cell and invariant NKT (iNKT) cell development. In the thymus and peripheral lymphoid organs, miR-150 negatively regulates iNKT cells [61]. Exosomal miR-451 was associated with increased Th17 cell differentiation and the redistribution of miR-451 from GC cells to infiltrating T cells [62]. MiR-155 regulates interferon γ (IFN-γ) production in NK cells by IL-12, IL-18, or CD16 stimulation [63]. In the GC TME, a novel mechanism was identified by which the downregulation of the miR-155-5p drives the switch of bone marrow mesenchymal stem cells (MSCs) to a more aggressive GC tissue-derived MSC-like phenotype. The mechanism was dependent upon the activation of nuclear factor kappa B (NF-κB) p65, revealing a potential meaningful approach for GC therapy within the TME [64].
MiRNAs and GC chemoresistance
It is readily apparent that miRNA expression contributes to tumor growth by modulating the functional expression of critical genes and signaling pathways that are important for tumor cell proliferation or survival. In addition, we have discussed a number of specific miRNAs related to the regulation of GC growth, as well as their responses to chemotherapy and targeted therapy. Although the molecular mechanisms accounting for the chemoresistance in GC cells are not fully understood yet, miR-21 [65, 66], miR-99a and miR-491 [67], miR-132 [68], miR-147 [69], miR-214 [70], miR-590-5p [71], and miR-3174 [24], have been identified as contributing to the resistance to chemotherapy in GC cells. For example, miR-99a and miR-491 were identified to be upregulated in GC cell lines with resistance to the DDP treatment, the gene calpain small subunit 1 (CAPNS1) was demonstrated to be targeted by both miRNAs. Inhibiting miR-99a and miR-491, or overexpressing CAPNS1 could robustly improve the sensitivity of these resistant GC cells to DDP [67]. In addition, anti-miR-21 combined with 5-fluorouracil (5-FU) can induce the sensitivity of receptor tyrosine-protein kinase erbB-2 (HER2)-positive GC cells to the anti-HER2 antibody trastuzumab, repressing GC cell proliferation and slowing disease progression [66].
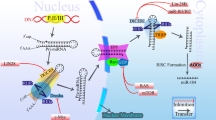
Furthermore, the sensitivity of GC cells to chemotherapy drugs can be enhanced by the overexpression of miR-7 [72], miR-23b-3p [73], miR-30a [26], miR-33b-5p [74], miR-34a [75], miR-101 [76], miR-204 [77], miR-375 [78], miR-647 [79], and miR-939 [80]. For example, miR-101 was identified to be downregulated in GC tissues and chemoresistant GC cells, showing an inverse correlation to the gene expression of annexin A2 (ANXA2). Forced expression of miR-101 could enhance the response of GC cells to DDP and vincristine (VCR) [76]. In addition, the GC chemoresistant cell line, SGC-7901/VCR, not only showed the resistance to VCR, but also to 5-FU and DDP. Of significance, the mechanistic studies demonstrated that a long noncoding RNA, metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), was involved in the development of chemoresistance in SGC-7901/VCR cells, interacting with autophagy-related 12 (ATG12). Intriguingly, miR-23b-3p was identified as the “linker” between MALAT1 and ATG12, because it could suppress the expression of ATG12 and was also targeted by MALAT1 directly. In the in vivo studies, the drug resistance caused by MALAT1 overexpression could be compromised by the ectopic expression of miR-23b-3p [73]. Figure 2 illustrates the mechanistic involvement of these miRNAs and their putative target genes in GC chemoresistance.
5-FU 5-fluorouracil, DTX docetaxel, DDP/CDDP cisplatin, PTX paclitaxel, VCR vincristine, ABCG2 ATP binding cassette subfamily G member 2, Akt protein kinase B, ANK2 ankyrin 2, ANXA2 annexin A2, ARHGAP10 rho GTPase activating protein 10, ATG12 autophagy-related 12, CAPNS1 calpain small subunit 1, E2F5 E2F transcription factor 5, ERBB2 erb-b2 receptor tyrosine kinase 2, HMGA2 high mobility group AT-Hook 2, MALAT1 metastasis-associated lung adenocarcinoma transcript 1, MDR1 multidrug resistance mutation 1, mTOR mammalian target of rapamycin, PI3K phosphoinositide 3-kinases, PTEN phosphatase and tensin homolog, RECK reversion-inducing cysteine-rich protein with Kazal motifs, SIRT1 sirtuin 1, SLC34A2 solute carrier family 34 member 2, TGFBR2 transforming growth factor beta receptor 2.
Conclusion
Our group has been focused on researching the central importance of the interactions of miRNAs with several human cancers for well over a decade [81,82,83,84,85,86,87,88,89,90]. We have learned quite a bit about the role of miRNAs, with much more to understand about their involvement with the TME and the host immune system. In this review, we have summarized the latest literature on this topic, focusing on GC and the related genes involved in tumor development, progression, and chemoresistance. In doing so, we expect to identify homologous target genes and the associated signaling pathways involved in the clinically aggressive behavior of GC. Although the effects of particular miRNAs on GC have been identified, the true function of miRNA remains quite enigmatic, with further research needed examining the specific impact on GC development and progression.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. https://doi.org/10.3322/caac.21338.
Lordick F, Shitara K, Janjigian YY. New agents on the horizon in gastric cancer. Ann Oncol. 2017;28:1767–75. https://doi.org/10.1093/annonc/mdx051.
He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. https://doi.org/10.1038/nrg1379.
Yang J, Song H, Cao K, Song J, Zhou J. Comprehensive analysis of Helicobacter pylori infection-associated diseases based on miRNA-mRNA interaction network. Brief Bioinform. 2019;20:1492–501. https://doi.org/10.1093/bib/bby018.
Oertli M, Engler DB, Kohler E, Koch M, Meyer TF, Muller A. MicroRNA-155 is essential for the T cell-mediated control of Helicobacter pylori infection and for the induction of chronic gastritis and colitis. J Immunol. 2011;187:3578–86. https://doi.org/10.4049/jimmunol.1101772.
Tsai MM, Huang HW, Wang CS, Lee KF, Tsai CY, Lu PH, et al. MicroRNA-26b inhibits tumor metastasis by targeting the KPNA2/c-jun pathway in human gastric cancer. Oncotarget. 2016;7:39511–26. https://doi.org/10.18632/oncotarget.8629.
Wang F, Liu J, Zou Y, Jiao Y, Huang Y, Fan L, et al. MicroRNA-143-3p, up-regulated in H. pylori-positive gastric cancer, suppresses tumor growth, migration and invasion by directly targeting AKT2. Oncotarget. 2017;8:28711–24. https://doi.org/10.18632/oncotarget.15646.
Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916–25. https://doi.org/10.1086/605443.
Li L, Wang X, Li W, Yang L, Liu R, Zeng R, et al. miR-21 modulates prostaglandin signaling and promotes gastric tumorigenesis by targeting 15-PGDH. Biochem Biophys Res Commun. 2018;495:928–34. https://doi.org/10.1016/j.bbrc.2017.09.137.
Wang P, Guan Q, Zhou D, Yu Z, Song Y, Qiu W. miR-21 Inhibitors modulate biological functions of gastric cancer cells via PTEN/PI3K/mTOR pathway. DNA Cell Biol. 2018;37:38–45. https://doi.org/10.1089/dna.2017.3922.
Zhang BG, Li JF, Yu BQ, Zhu ZG, Liu BY, Yan M. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep. 2012;27:1019–26. https://doi.org/10.3892/or.2012.1645.
Wang J, Feng W, Dong Y, Mao X, Guo F, Luo F. MicroRNA495 regulates human gastric cancer cell apoptosis and migration through Akt and mTOR signaling. Oncol Rep. 2018;40:3654–62. https://doi.org/10.3892/or.2018.6722.
Eun JW, Kim HS, Shen Q, Yang HD, Kim SY, Yoon JH, et al. MicroRNA-495-3p functions as a tumor suppressor by regulating multiple epigenetic modifiers in gastric carcinogenesis. J Pathol. 2018;244:107–19. https://doi.org/10.1002/path.4994.
Fang Z, Zhang L, Liao Q, Wang Y, Yu F, Feng M, et al. Regulation of TRIM24 by miR-511 modulates cell proliferation in gastric cancer. J Exp Clin Cancer Res. 2017;36:17. https://doi.org/10.1186/s13046-017-0489-1.
Ren W, Zhang X, Li W, Feng Q, Feng H, Tong Y, et al. Exosomal miRNA-107 induces myeloid-derived suppressor cell expansion in gastric cancer. Cancer Manag Res. 2019;11:4023–40. https://doi.org/10.2147/CMAR.S198886.
Song J, Guan Z, Li M, Sha S, Song C, Gao Z, et al. MicroRNA-154 inhibits the growth and invasion of gastric cancer cells by targeting DIXDC1/WNT signaling. Oncol Res. 2018;26:847–56. https://doi.org/10.3727/096504017X15016337254632.
Zheng JJ, Que QY, Xu HT, Luo DS, Sun Z, Ni JS, et al. Hypoxia activates SOX5/Wnt/beta-catenin signaling by suppressing MiR-338-3p in gastric cancer. Technol Cancer Res Treat. 2020;19:1–9. https://doi.org/10.1177/1533033820905825.
Chen JQ, Huang ZP, Li HF, Ou YL, Huo F, Hu LK. MicroRNA-520f-3p inhibits proliferation of gastric cancer cells via targeting SOX9 and thereby inactivating Wnt signaling. Sci Rep. 2020;10:6197. https://doi.org/10.1038/s41598-020-63279-y.
Lin L, Xiao J, Shi L, Chen W, Ge Y, Jiang M, et al. STRA6 exerts oncogenic role in gastric tumorigenesis by acting as a crucial target of miR-873. J Exp Clin Cancer Res. 2019;38:452. https://doi.org/10.1186/s13046-019-1450-2.
Wu F, Li J, Guo N, Wang XH, Liao YQ. MiRNA-27a promotes the proliferation and invasion of human gastric cancer MGC803 cells by targeting SFRP1 via Wnt/beta-catenin signaling pathway. Am J Cancer Res. 2017;7:405–16.
Deng S, Zhang X, Qin Y, Chen W, Fan H, Feng X, et al. miRNA-192 and -215 activate Wnt/beta-catenin signaling pathway in gastric cancer via APC. J Cell Physiol. 2020;235:6218–29. https://doi.org/10.1002/jcp.29550.
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao Y, et al. MiRNA-194 activates the Wnt/beta-catenin signaling pathway in gastric cancer by targeting the negative Wnt regulator, SUFU. Cancer Lett. 2017;385:117–27. https://doi.org/10.1016/j.canlet.2016.10.035.
Li B, Wang L, Li Z, Wang W, Zhi X, Huang X, et al. miR-3174 contributes to apoptosis and autophagic cell death defects in gastric cancer cells by targeting ARHGAP10. Mol Ther Nucleic Acids. 2017;9:294–311. https://doi.org/10.1016/j.omtn.2017.10.008.
Cao W, Wei W, Zhan Z, Xie Y, Xiao Q. MiR-1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol Rep. 2016;35:2583–91. https://doi.org/10.3892/or.2016.4643.
Du X, Liu B, Luan X, Cui Q, Li L. miR-30 decreases multidrug resistance in human gastric cancer cells by modulating cell autophagy. Exp Ther Med. 2018;15:599–605. https://doi.org/10.3892/etm.2017.5354.
Wang H, Zhang X, Liu Y, Ni Z, Lin Y, Duan Z, et al. Downregulated miR-31 level associates with poor prognosis of gastric cancer and its restoration suppresses tumor cell malignant phenotypes by inhibiting E2F2. Oncotarget. 2016;7:36577–89. https://doi.org/10.18632/oncotarget.9288.
Cai L, Chen Q, Fang S, Lian M, Cai M. MicroRNA-329 inhibits cell proliferation and tumor growth while facilitates apoptosis via negative regulation of KDM1A in gastric cancer. J Cell Biochem. 2018;119:3338–51. https://doi.org/10.1002/jcb.26497.
Wang Z, Liu M, Zhu H, Zhang W, He S, Hu C, et al. miR-106a is frequently upregulated in gastric cancer and inhibits the extrinsic apoptotic pathway by targeting FAS. Mol Carcinog. 2013;52:634–46. https://doi.org/10.1002/mc.21899.
Imamura T, Komatsu S, Ichikawa D, Miyamae M, Okajima W, Ohashi T, et al. Low plasma levels of miR-101 are associated with tumor progression in gastric cancer. Oncotarget. 2017;8:106538–50. https://doi.org/10.18632/oncotarget.20860.
Feng R, Chen X, Yu Y, Su L, Yu B, Li J, et al. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. https://doi.org/10.1016/j.canlet.2010.06.004.
Wang J, Zhou Y, Fei X, Chen X, Yan J, Liu B, et al. ADAM9 functions as a promoter of gastric cancer growth which is negatively and post-transcriptionally regulated by miR-126. Oncol Rep. 2017;37:2033–40. https://doi.org/10.3892/or.2017.5460.
Xiong X, Ren HZ, Li MH, Mei JH, Wen JF, Zheng CL. Down-regulated miRNA-214 induces a cell cycle G1 arrest in gastric cancer cells by up-regulating the PTEN protein. Pathol Oncol Res. 2011;17:931–7. https://doi.org/10.1007/s12253-011-9406-7.
Zhu C, Huang Q, Zhu H. miR-383 inhibited the cell cycle progression of gastric cancer cells via targeting cyclin E2. DNA Cell Biol. 2019;38:849–56. https://doi.org/10.1089/dna.2019.4624.
Pastushenko I, Blanpain C. EMT transition states during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–26. https://doi.org/10.1016/j.tcb.2018.12.001.
Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–58.
Tamilzhalagan S, Rathinam D, Ganesan K. Amplified 7q21-22 gene MCM7 and its intronic miR-25 suppress COL1A2 associated genes to sustain intestinal gastric cancer features. Mol Carcinog. 2017;56:1590–602. https://doi.org/10.1002/mc.22614.
Li C, Zou J, Zheng G, Chu J. MiR-30a decreases multidrug resistance (MDR) of gastric cancer cells. Med Sci Monit. 2016;22:4509–15.
Wang LL, Zhang XH, Zhang X, Chu JK. MiR-30a increases cisplatin sensitivity of gastric cancer cells through suppressing epithelial-to-mesenchymal transition (EMT). Eur Rev Med Pharmacol Sci. 2016;20:1733–9.
Tao Y, Yang S, Wu Y, Fang X, Wang Y, Song Y, et al. MicroRNA-216a inhibits the metastasis of gastric cancer cells by targeting JAK2/STAT3-mediated EMT process. Oncotarget. 2017;8:88870–81. https://doi.org/10.18632/oncotarget.21488.
Mi Y, Zhang D, Jiang W, Weng J, Zhou C, Huang K, et al. miR-181a-5p promotes the progression of gastric cancer via RASSF6-mediated MAPK signalling activation. Cancer Lett. 2017;389:11–22. https://doi.org/10.1016/j.canlet.2016.12.033.
Huang J, He Y, McLeod HL, Xie Y, Xiao D, Hu H, et al. miR-302b inhibits tumorigenesis by targeting EphA2 via Wnt/ beta-catenin/EMT signaling cascade in gastric cancer. BMC Cancer. 2017;17:886. https://doi.org/10.1186/s12885-017-3875-3.
Hu J, Shan Z, Hu K, Ren F, Zhang W, Han M, et al. miRNA-223 inhibits epithelial-mesenchymal transition in gastric carcinoma cells via Sp1. Int J Oncol. 2016;49:325–35. https://doi.org/10.3892/ijo.2016.3533.
Zhou Q, Zheng X, Chen L, Xu B, Yang X, Jiang J, et al. Smad2/3/4 pathway contributes to TGF-beta-induced MiRNA-181b expression to promote gastric cancer metastasis by targeting Timp3. Cell Physiol Biochem. 2016;39:453–66. https://doi.org/10.1159/000445638.
Pan Y, Shu X, Sun L, Yu L, Sun L, Yang Z, et al. miR196a5p modulates gastric cancer stem cell characteristics by targeting Smad4. Int J Oncol. 2017;50:1965–76. https://doi.org/10.3892/ijo.2017.3965.
Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8:761–73. https://doi.org/10.7150/jca.17648.
Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18:70. https://doi.org/10.1186/s12943-019-0994-2.
Li P, Shan JX, Chen XH, Zhang D, Su LP, Huang XY, et al. Epigenetic silencing of microRNA-149 in cancer-associated fibroblasts mediates prostaglandin E2/interleukin-6 signaling in the tumor microenvironment. Cell Res. 2015;25:588–603. https://doi.org/10.1038/cr.2015.51.
Yang TS, Yang XH, Chen X, Wang XD, Hua J, Zhou DL, et al. MicroRNA-106b in cancer-associated fibroblasts from gastric cancer promotes cell migration and invasion by targeting PTEN. FEBS Lett. 2014;588:2162–9. https://doi.org/10.1016/j.febslet.2014.04.050.
Tang H, Deng M, Tang Y, Xie X, Guo J, Kong Y, et al. miR-200b and miR-200c as prognostic factors and mediators of gastric cancer cell progression. Clin Cancer Res. 2013;19:5602–12. https://doi.org/10.1158/1078-0432.CCR-13-1326.
Kurashige J, Mima K, Sawada G, Takahashi Y, Eguchi H, Sugimachi K, et al. Epigenetic modulation and repression of miR-200b by cancer-associated fibroblasts contribute to cancer invasion and peritoneal dissemination in gastric cancer. Carcinogenesis. 2015;36:133–41. https://doi.org/10.1093/carcin/bgu232.
Du Y, Xu Y, Ding L, Yao H, Yu H, Zhou T, et al. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J Gastroenterol. 2009;44:556–61. https://doi.org/10.1007/s00535-009-0037-7.
Zhou Y, Zhong JH, Gong FS, Xiao J. MiR-141-3p suppresses gastric cancer induced transition of normal fibroblast and BMSC to cancer-associated fibroblasts via targeting STAT4. Exp Mol Pathol. 2019;107:85–94. https://doi.org/10.1016/j.yexmp.2018.11.014.
Hu Y, Deng F, Song J, Lin J, Li X, Tang Y, et al. Evaluation of miR-29c inhibits endotheliocyte migration and angiogenesis of human endothelial cells by suppressing the insulin like growth factor 1. Am J Transl Res. 2015;7:866–77.
Zhang H, Bai M, Deng T, Liu R, Wang X, Qu Y, et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016;375:331–9. https://doi.org/10.1016/j.canlet.2016.03.026.
Chen X, Cui Y, Xie X, Xing Y, Yuan Z, Wei Y. Functional role of miR-27b in the development of gastric cancer. Mol Med Rep. 2018;17:5081–7. https://doi.org/10.3892/mmr.2018.8538.
Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–14. https://doi.org/10.1038/nm.2186.
Yang H, Zhang H, Ge S, Ning T, Bai M, Li J, et al. Exosome-derived miR-130a activates angiogenesis in gastric cancer by targeting c-myb in vascular endothelial cells. Mol Ther. 2018;26:2466–75. https://doi.org/10.1016/j.ymthe.2018.07.023.
Ma R, Yi B, Riker AI, Xi Y. Metformin and cancer immunity. Acta Pharmacol Sin. 2020; 41: in press. https://doi.org/10.1038/s41401-020-00508-0.
Hirschberger S, Hinske LC, Kreth S. MiRNAs: dynamic regulators of immune cell functions in inflammation and cancer. Cancer Lett. 2018;431:11–21. https://doi.org/10.1016/j.canlet.2018.05.020.
Bezman NA, Chakraborty T, Bender T, Lanier LL. miR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011;208:2717–31. https://doi.org/10.1084/jem.20111386.
Liu F, Bu Z, Zhao F, Xiao D. Increased T-helper 17 cell differentiation mediated by exosome-mediated microRNA-451 redistribution in gastric cancer infiltrated T cells. Cancer Sci. 2018;109:65–73. https://doi.org/10.1111/cas.13429.
Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, et al. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–85. https://doi.org/10.1182/blood-2011-12-398099.
Zhu M, Wang M, Yang F, Tian Y, Cai J, Yang H, et al. miR-155-5p inhibition promotes the transition of bone marrow mesenchymal stem cells to gastric cancer tissue derived MSC-like cells via NF-kappaB p65 activation. Oncotarget. 2016;7:16567–80. https://doi.org/10.18632/oncotarget.7767.
Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. https://doi.org/10.1186/s13046-017-0528-y.
Hu N, Yin JF, Ji Z, Hong Y, Wu P, Bian B. et al. Strengthening gastric cancer therapy by trastuzumab-conjugated nanoparticles with simultaneous encapsulation of anti-MiR-21 and 5-fluorouridine. Cell Physiol Biochem. 2017;44:2158–73. https://doi.org/10.1159/000485955.
Zhang Y, Xu W, Ni P, Li A, Zhou J. Xu S. MiR-99a and MiR-491 regulate cisplatin resistance in human gastric cancer cells by targeting CAPNS1. Int J Biol Sci. 2016;12:1437–47. https://doi.org/10.7150/ijbs.16529.
Zhang L, Guo X, Zhang D, Fan Y, Qin L, Dong S, et al. Upregulated miR-132 in Lgr5(+) gastric cancer stem cell-like cells contributes to cisplatin-resistance via SIRT1/CREB/ABCG2 signaling pathway. Mol Carcinog. 2017;56:2022–34. https://doi.org/10.1002/mc.22656.
Shen J, Niu W, Zhang H, Jun M, Zhang H. Downregulation of MicroRNA-147 inhibits cell proliferation and increases the chemosensitivity of gastric cancer cells to 5-fluorouracil by directly targeting PTEN. Oncol Res. 2018;26:901–11. https://doi.org/10.3727/096504017X15061902533715.
Wang X, Zhang H, Bai M, Ning T, Ge S, Deng T, et al. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol Ther. 2018;26:774–83. https://doi.org/10.1016/j.ymthe.2018.01.001.
Shen B, Yu S, Zhang Y, Yuan Y, Li X, Zhong J, et al. miR-590-5p regulates gastric cancer cell growth and chemosensitivity through RECK and the AKT/ERK pathway. Onco Targets Ther. 2016;9:6009–19. https://doi.org/10.2147/OTT.S110923.
Xu N, Lian YJ, Dai X, Wang YJ. miR-7 increases cisplatin sensitivity of gastric cancer cells through suppressing mTOR. Technol Cancer Res Treat. 2017;16:1022–30. https://doi.org/10.1177/1533034617717863.
YiRen H, YingCong Y, Sunwu Y, Keqin L, Xiaochun T, Senrui C, et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer. Mol Cancer. 2017;16:174. https://doi.org/10.1186/s12943-017-0743-3.
Yang X, Zhao Q, Yin H, Lei X, Gan R. MiR-33b-5p sensitizes gastric cancer cells to chemotherapy drugs via inhibiting HMGA2 expression. J Drug Target. 2017;25:653–60. https://doi.org/10.1080/1061186X.2017.1323220.
Li L, Wu C, Zhao Y. miRNA-34a enhances the sensitivity of gastric cancer cells to treatment with paclitaxel by targeting E2F5. Oncol Lett. 2017;13:4837–42. https://doi.org/10.3892/ol.2017.6041.
Bao J, Xu Y, Wang Q, Zhang J, Li Z, Li D, et al. miR-101 a lleviates chemoresistance of gastric cancer cells by targeting ANXA2. Biomed Pharmacother. 2017;92:1030–7. https://doi.org/10.1016/j.biopha.2017.06.011.
Li LQ, Pan D, Chen Q, Zhang SW, Xie DY, Zheng XL, et al. Sensitization of gastric cancer cells to 5-FU by microRNA-204 through targeting the TGFBR2-mediated epithelial to mesenchymal transition. Cell Physiol Biochem. 2018;47:1533–45. https://doi.org/10.1159/000490871.
Zhou N, Qu Y, Xu C, Tang Y. Upregulation of microRNA-375 increases the cisplatin-sensitivity of human gastric cancer cells by regulating ERBB2. Exp Ther Med. 2016;11:625–30. https://doi.org/10.3892/etm.2015.2920.
Cao W, Wei W, Zhan Z, Xie D, Xie Y, Xiao Q. Regulation of drug resistance and metastasis of gastric cancer cells via the microRNA647-ANK2 axis. Int J Mol Med. 2018;41:1958–66. https://doi.org/10.3892/ijmm.2018.3381.
Zhang JX, Xu Y, Gao Y, Chen C, Zheng ZS, Yun M, et al. Decreased expression of miR-939 contributes to chemoresistance and metastasis of gastric cancer via dysregulation of SLC34A2 and Raf/MEK/ERK pathway. Mol Cancer. 2017;16:18. https://doi.org/10.1186/s12943-017-0586-y.
Xi Y, Shalgi R, Fodstad O, Pilpel Y, Ju J. Differentially regulated micro-RNAs and actively translated messenger RNA transcripts by tumor suppressor p53 in colon cancer. Clin Cancer Res. 2006;12:2014–24. https://doi.org/10.1158/1078-0432.CCR-05-1853.
Xi Y, Formentini A, Chien M, Weir DB, Russo JJ, Ju J, et al. Prognostic values of microRNAs in Colorectal cancer. Biomark Insights. 2006;2:113–21.
Kumar R, Xi Y. MicroRNA epigenetic machinery and lung cancer. Thorac Cancer. 2011;2:35–44. https://doi.org/10.1111/j.1759-7714.2011.00043.x.
Howell PM Jr., Li X, Riker AI, Xi Y. MicroRNA in melanoma. Ochsner J. 2010;10:83–92.
Li X, Wainscott C, Xi Y. MicroRNA provides insight into understanding esophageal cancer. Thorac Cancer. 2011;2:134–42. https://doi.org/10.1111/j.1759-7714.2011.00059.x.
Li X, Gao L, Cui Q, Gary BD, Dyess DL, Taylor W, et al. Sulindac inhibits tumor cell invasion by suppressing NF-kappaB-mediated transcription of microRNAs. Oncogene. 2012;31:4979–86. https://doi.org/10.1038/onc.2011.655.
Yi B, Chang H, Ma R, Feng X, Li W, Piazza GA, et al. Inhibition of breast cancer cell motility with a non-cyclooxygenase inhibitory derivative of sulindac by suppressing TGFbeta/miR-21 signaling. Oncotarget. 2016;7:7979–92. https://doi.org/10.18632/oncotarget.6888.
Ma R, Yi B, Piazza GA, Xi Y. Mechanistic role of microRNA in cancer chemoprevention by nonsteroidal anti-inflammatory drugs. Curr Pharmacol Rep.2015;1:154–60. https://doi.org/10.1007/s40495-014-0011-9.
Yi B, Piazza GA, Su X, Xi Y. MicroRNA and cancer chemoprevention. Cancer Prev Res (Philos). 2013;6:401–9. https://doi.org/10.1158/1940-6207.CAPR-13-0032.
Liang Z, Xi Y. MicroRNAs mediate therapeutic and preventive effects of natural agents in breast cancer. Chin J Nat Med. 2016;14:881–7. https://doi.org/10.1016/S1875-5364(17)30012-2.
Zheng J, Liu Y, Qiao Y, Zhang L, Lu S. miR-103 promotes proliferation and metastasis by targeting KLF4 in gastric cancer. Int J Mol Sci. 2017;18:910. https://doi.org/10.3390/ijms18050910.
Wang S, Ma G, Zhu H, Lv C, Chu H, Tong N, et al. miR-107 regulates tumor progression by targeting NF1 in gastric cancer. Sci Rep. 2016;6:36531. https://doi.org/10.1038/srep36531.
Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS. et al. Increased miR-146a in gastric cancer directly targets SMAD4 and is involved in modulating cell proliferation and apoptosis. Oncol Rep. 2012;27:559–66. https://doi.org/10.3892/or.2011.1514.
Hsu KW, Fang WL, Huang KH, Huang TT, Lee HC, Hsieh RH, et al. Notch1 pathway-mediated microRNA-151-5p promotes gastric cancer progression. Oncotarget. 2016;7:38036–51. https://doi.org/10.18632/oncotarget.9342.
Wang Y, Zeng J, Pan J, Geng X, Liu Y, Wu J, et al. MicroRNA-200c is involved in proliferation of gastric cancer by directly repressing p27(Kip1). Biochem Biophys Rep. 2016;8:227–33. https://doi.org/10.1016/j.bbrep.2016.09.007.
Zheng L, Jiao W, Song H, Qu H, Li D, Mei H, et al. miRNA-558 promotes gastric cancer progression through attenuating Smad4-mediated repression of heparanase expression. Cell Death Dis. 2016;7:e2382. https://doi.org/10.1038/cddis.2016.293.
Yin K, Liu M, Zhang M, Wang F, Fen M, Liu Z, et al. miR-208a-3p suppresses cell apoptosis by targeting PDCD4 in gastric cancer. Oncotarget. 2016;7:67321–32. https://doi.org/10.18632/oncotarget.12006.
Kong P, Zhu X, Geng Q, Xia L, Sun X, Chen Y, et al. The microRNA-423-3p-Bim axis promotes cancer progression and activates oncogenic autophagy in gastric cancer. Mol Ther. 2017;25:1027–37. https://doi.org/10.1016/j.ymthe.2017.01.013.
Xu G, Zhu H, Zhang M, Xu J. Histone deacetylase 3 is associated with gastric cancer cell growth via the miR-454-mediated targeting of CHD5. Int J Mol Med. 2018;41:155–63. https://doi.org/10.3892/ijmm.2017.3225.
Li YR, Wen LQ, Wang Y, Zhou TC, Ma N, Hou ZH, et al. MicroRNA-520c enhances cell proliferation, migration, and invasion by suppressing IRF2 in gastric cancer. FEBS Open Bio. 2016;6:1257–66. https://doi.org/10.1002/2211-5463.12142.
Wu C, Zheng X, Li X, Fesler A, Hu W, Chen L, et al. Reduction of gastric cancer proliferation and invasion by miR-15a mediated suppression of Bmi-1 translation. Oncotarget. 2016;7:14522–36. https://doi.org/10.18632/oncotarget.7392.
Wang T, Hou J, Li Z, Zheng Z, Wei J, Song D, et al. miR-15a-3p and miR-16-1-3p negatively regulate Twist1 to repress gastric cancer cell invasion and metastasis. Int J Biol Sci. 2017;13:122–34. https://doi.org/10.7150/ijbs.14770.
Zhu C, Huang Q, Zhu H. Melatonin inhibits the proliferation of gastric cancer cells through regulating the miR-16-5p-Smad3 pathway. DNA Cell Biol. 2018;37:244–252. https://doi.org/10.1089/dna.2017.4040.
Kong Y, Zou S, Yang F, Xu X, Bu W, Jia J, et al. RUNX3-mediated up-regulation of miR-29b suppresses the proliferation and migration of gastric cancer cells by targeting KDM2A. Cancer Lett. 2016;381:138–48. https://doi.org/10.1016/j.canlet.2016.07.038.
Wang L, Yu T, Li W, Li M, Zuo Q, Zou Q, et al. The miR-29c-KIAA1199 axis regulates gastric cancer migration by binding with WBP11 and PTP4A3. Oncogene. 2019;38:3134–50. https://doi.org/10.1038/s41388-018-0642-0.
Wei J, Wang Z, Wang Z, Yang Y, Fu C, Zhu J, et al. MicroRNA-31 function as a suppressor was regulated by epigenetic mechanisms in gastric cancer. Biomed Res Int. 2017;2017:5348490. https://doi.org/10.1155/2017/5348490.
Guo LH, Li H, Wang F, Yu J, He JS. The tumor suppressor roles of miR-433 and miR-127 in gastric cancer. Int J Mol Sci. 2013;14:14171–84. https://doi.org/10.3390/ijms140714171.
He L, Qu L, Wei L, Chen Y, Suo J. Reduction of miR1323p contributes to gastric cancer proliferation by targeting MUC13. Mol Med Rep. 2017;15:3055–61. https://doi.org/10.3892/mmr.2017.6347.
Zhang C, Chen X, Chen X, Wang X, Ji A, Jiang L, et al. miR-135a acts as a tumor suppressor in gastric cancer in part by targeting KIFC1. Onco Targets Ther. 2016;9:3555–63. https://doi.org/10.2147/OTT.S105736.
Bao J, Zou JH, Li CY, Zheng GQ. miR-194 inhibits gastric cancer cell proliferation and tumorigenesis by targeting KDM5B. Eur Rev Med Pharmacol Sci. 2016;20:4487–93.
Zeng B, Shi W, Tan G. MiR-199a/b-3p inhibits gastric cancer cell proliferation via down-regulating PAK4/MEK/ERK signaling pathway. BMC Cancer. 2018;18:34. https://doi.org/10.1186/s12885-017-3949-2.
Zhao Y, Li C, Wang M, Su L, Qu Y, Li J, et al. Decrease of miR-202-3p expression, a novel tumor suppressor, in gastric cancer. PLoS One. 2013;8:e69756. https://doi.org/10.1371/journal.pone.0069756.
Yang H, Wang L, Tang X, Bai W. miR-203a suppresses cell proliferation by targeting E2F transcription factor 3 in human gastric cancer. Oncol Lett. 2017;14:7687–90. https://doi.org/10.3892/ol.2017.7199.
Yang L, Liang H, Wang Y, Gao S, Yin K, Liu Z, et al. MiRNA-203 suppresses tumor cell proliferation, migration and invasion by targeting Slug in gastric cancer. Protein Cell. 2016;7:383–7. https://doi.org/10.1007/s13238-016-0259-4.
Shrestha S, Yang CD, Hong HC, Chou CH, Tai CS, Chiew MY, et al. Integrated microRNA-mRNA analysis reveals miR-204 inhibits cell proliferation in gastric cancer by targeting CKS1B, CXCL1 and GPRC5A. Int J Mol Sci. 2017;19:87. https://doi.org/10.3390/ijms19010087.
Zheng L, Jiao W, Mei H, Song H, Li D, Xiang X, et al. miRNA-337-3p inhibits gastric cancer progression through repressing myeloid zinc finger 1-facilitated expression of matrix metalloproteinase 14. Oncotarget. 2016;7:40314–28. https://doi.org/10.18632/oncotarget.9739.
Kang W, Huang T, Zhou Y, Zhang J, Lung RWM, Tong JHM, et al. miR-375 is involved in Hippo pathway by targeting YAP1/TEAD4-CTGF axis in gastric carcinogenesis. Cell Death Dis. 2018;9:92. https://doi.org/10.1038/s41419-017-0134-0.
Liu GH, Liu YH, Yang Z, Zhu AL, Zhao CL. MicroRNA-524-5p suppresses the growth and invasive abilities of gastric cancer cells. Oncol Lett. 2016;11:1926–32. https://doi.org/10.3892/ol.2016.4143.
Zheng L, Chen Y, Ye L, Jiao W, Song H, Mei H, et al. miRNA-584-3p inhibits gastric cancer progression by repressing Yin Yang 1- facilitated MMP-14 expression. Sci Rep. 2017;7:8967. https://doi.org/10.1038/s41598-017-09271-5.
Ye G, Huang K, Yu J, Zhao L, Zhu X, Yang Q, et al. MicroRNA-647 targets SRF-MYH9 axis to suppress invasion and metastasis of gastric cancer. Theranostics. 2017;7:3338–53. https://doi.org/10.7150/thno.20512.
Zhang Y, Wu YY, Jiang JN, Liu XS, Ji FJ, Fang XD. MiRNA-3978 regulates peritoneal gastric cancer metastasis by targeting legumain. Oncotarget. 2016;7:83223–30. https://doi.org/10.18632/oncotarget.12917.
Wang M, Gu H, Qian H, Zhu W, Zhao C, Zhang X, et al. miR-17-5p/20a are important markers for gastric cancer and murine double minute 2 participates in their functional regulation. Eur J Cancer. 2013;49:2010–21. https://doi.org/10.1016/j.ejca.2012.12.017.
Deng Y, Huang Z, Xu Y, Jin J, Zhuo W, Zhang C, et al. MiR-215 modulates gastric cancer cell proliferation by targeting RB1. Cancer Lett. 2014;342:27–35. https://doi.org/10.1016/j.canlet.2013.08.033.
Guoping M, Ran L, Yanru Q. miR-143 inhibits cell proliferation of gastric cancer cells through targeting GATA6. Oncol Res. 2018;26:1023–9. https://doi.org/10.3727/096504018X15151515028670.
Lian S, Park JS, Xia Y, Nguyen TT, Joo YE, Kim KK, et al. MicroRNA-375 functions as a tumor-suppressor gene in gastric cancer by targeting recepteur d’origine nantais. Int J Mol Sci. 2016;17:1633. https://doi.org/10.3390/ijms17101633.
Shen Y, Chen H, Gao L, Zhang W, He J, Yang X, et al. MiR-638 acts as a tumor suppressor gene in gastric cancer. Oncotarget. 2017;8:108170–80. https://doi.org/10.18632/oncotarget.22567.
Hu X, Zhang M, Miao J, Wang X, Huang C. miRNA-4317 suppresses human gastric cancer cell proliferation by targeting ZNF322. Cell Biol Int. 2018;42:923–30. https://doi.org/10.1002/cbin.10870.
Acknowledgements
The authors sincerely wish to apologize to the many colleagues who have contributed significantly to this field of research, but whose publications were not cited due to the space limitations imposed upon this review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests
Rights and permissions
About this article
Cite this article
Liu, X., Ma, R., Yi, B. et al. MicroRNAs are involved in the development and progression of gastric cancer. Acta Pharmacol Sin 42, 1018–1026 (2021). https://doi.org/10.1038/s41401-020-00540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-00540-0
Keywords
This article is cited by
-
Epigenetically regulated gene expression profiles decipher four molecular subtypes with prognostic and therapeutic implications in gastric cancer
Clinical Epigenetics (2023)
-
Therapeutic effect of microRNA-21 on differentially expressed hub genes in gastric cancer based on systems biology
Scientific Reports (2023)
-
miR-196a-5p Correlates with Chronic Atrophic Gastritis Progression to Gastric Cancer and Induces Malignant Biological Behaviors of Gastric Cancer Cells by Targeting ACER2
Molecular Biotechnology (2023)
-
Roles of microRNAs and exosomes in Helicobacter pylori associated gastric cancer
Molecular Biology Reports (2023)
-
Hierarchical drug release designed Au @PDA-PEG-MTX NPs for targeted delivery to breast cancer with combined photothermal-chemotherapy
Journal of Nanobiotechnology (2021)