Abstract
MicroRNAs (miRNAs) are a class of non-coding RNAs (ncRNAs) with a short length of 19–22 nucleotides. miRNAs are posttranscriptional regulators of gene expression involved in various biological processes like cell growth, apoptosis, and angiogenesis. miR-184 is a well-studied miRNA, for which most studies report its downregulation in cancer cells and tissues and experiments support its role as a tumor suppressor inhibiting malignant biological behaviors of cancer cells in vitro and in vivo. To exert its functions, miR-184 affects some signaling pathways involved in tumorigenesis like Wnt and β-catenin, and AKT/mTORC1 pathway, oncogenic factors (e.g., c-Myc) or apoptotic proteins, such as Bcl-2. Interestingly, clinical investigations have shown miR-184 with good performance as a prognostic/diagnostic biomarker for various cancers. Additionally, exogenous miR-184 in cell and xenograft animal studies suggest it as a therapeutic anticancer target. In this review, we outline the studies that evaluated the roles of miR-184 in tumorigenesis as well as its clinical significance.
Similar content being viewed by others
Facts
-
miR-184 shows downregulation in a majority of cell and clinical studies of cancer
-
Experimental investigations document an anticancer role for miR-184 suppressing proliferation, migration, invasion, and metastasis of tumor cells
-
Ectopic expression of miR-184 demonstrates therapeutic benefits in cell and animal studies
-
miR-184 can act as a diagnostic/prognostic biomarker for cancer patients
Open questions
-
Does miR-184 show antitumor role for all human cancers?
-
How can be interpreted some controversial findings on the role of miR-184?
-
What are the targets of miR-184 and how they can be manipulated for cancer therapy?
-
Do large clinical studies support the role and significance of miR-184 in human cancers?
Introduction
MicroRNAs (miRNAs) are a class of endogenous small (containing 19-25 nucleotides) group of non-coding RNAs (ncRNAs) that are widely found in eukaryotic cells [1]. Although, the precise roles of ncRNAs are not well elucidated, based on a developing extent of research evidence, they are suggested to function as posttranscriptional gene expression regulators [2]. In addition to transcription regulation, through interaction with other subclasses like sponging miRNAs by circular RNAs (circRNAs), interaction with RNA-binding proteins (RBPs), and translation into proteins, ncRNAs are involved in regulating the translation of protein-coding mRNAs as well as process of various RNA molecules [3, 4]. Estimations show that miRNAs may control an approximately one-third of the human genome [5]. The main mechanism of action identified for miRNAs is through degradation or translational inhibition and decreasing the stability of specific target messenger RNAs (mRNAs) via binding to their 3′ untranslated regions (UTRs) [6]. By development of high throughput technologies like reverse transcription polymerase chain reaction (qRT-PCR), chips, and miRNA profiling using the next generation sequencing (NGS), the count of identified miRNAs shows constant increasing and accordingly several databases (e.g., miRBase) are created for depositing and providing access for researchers and scientists [7,8,9]. A growing body of studies has indicated that miRNAs may exert critical roles in regulating various fundamental biological processes, such as inflammation, metabolism, cell growth, differentiation, development, apoptosis, and migration [10,11,12]. Accordingly, a large number of clinically significant diseases are related to the abnormal expression level of miRNAs, including cancer, cardiovascular diseases (heart failure), autoimmune diseases, neurodevelopmental conditions (Down syndrome, Alzheimer’s disease), asthma, frailty, liver disease (viral hepatitis), skeletal muscle, and skin disorders (psoriasis) [13,14,15,16]. Accumulating evidence has identified that an alteration in miRNAs level occurs in various human cancers [17, 18]. Therefore, miRNAs are associated with tumorigenic processes like cell growth and differentiation, angiogenesis, metastasis, and apoptosis [19]. According to the putative function of miRNAs in cancer studies, they are classified into two groups of oncogenic (oncomiRs) and tumor suppressor miRNAs. As their names suggest, oncomiRs are upregulated miRNAs in cancer cells and tissues, which functionally promote the oncogenic phenotype of tumor cells, including their proliferation as well as migratory and invasive capacities, while suppressing apoptosis [20]. Tumor suppressor miRNAs unlike the first category show downregulation in cancers and are represented with antitumor effects that negatively impact the potential of cancer development, progression and metastasis [21, 22]. The miRNA expression profile is documented for many types of cancer [5]. Accordingly, miRNAs are increasingly suggested with diagnostic, prognostic, and therapeutic targets in malignancies [23, 24]. Among a huge number of miRNAs being studied in human cancers, miRNA-184 (miR-184) has been identified as an evolutionarily conserved miRNA with abnormal expression in many malignant tumors. It is documented that miR-184 shows widely deregulation in numerous cancers with controversial functions either as an oncomiR in some cancers or dominantly a tumor suppressor miRNA [25]. Interestingly, miR-184 is also found with clinical potential in diagnosis, prediction of prognosis, and as a therapeutic target for some cancers. Thus, in this study, we discuss the roles and clinical significance of miR-184 in different types of cancers.
miR-184ʼs biogenesis
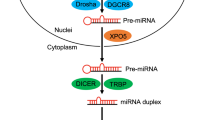
As shown in Fig. 1, the biogenesis of miR-184, same to other miRNAs, involves a series of intricate steps that culminate in the production of a fully functional miRNA molecule. This process for all miRNAs can be broadly categorized into two stages: transcriptional and posttranscriptional, each comprising multiple pivotal steps. RNA polymerase II initiates the transcription of the miRNA gene, resulting in the synthesis of a primary miRNA transcript (pri-miRNA) in the nucleus. The pri-miRNA adopts a hairpin-like structure and undergoes processing within the nucleus facilitated by the Microprocessor protein complex, which comprises the RNase III enzyme Drosha in addition to DGCR8. This complex recognizes and cleaves the hairpin structure of the pri-miRNA, liberating a precursor miRNA (pre-miRNA) with an ~70–100 nucleotides length and a stem-loop structure. After cleavage, a complex composed of exportin-5-Ran-GTP is responsible for the transportation of pre-miRNA to the cytoplasm. Upon reaching the cytoplasm, pre-miRNA transcript undergoes further processing mediated by Dicer, another RNase III enzyme. Dicer recognizes the structural characteristics of the pre-miRNA and cleaves it, generating a short double-stranded RNA duplex. This duplex consists of a mature guide strand, and a passenger strand on the opposite direction. To ensure the functional activity of miR-184, the guide strand selectively incorporates itself into the RNA-induced silencing complex (RISC). The RISC complex, composed of Argonaute proteins and associated factors, facilitates the binding of the guide strand and subsequent unwinding of the passenger strand. The unwound passenger strand is then degraded, and the guide strand remains within the RISC, poised to exert its regulatory effects [26, 27].
miR-184 is synthesized and processed according to a common mechanism known for miRNAs (details within the text). In cancer, the overexpression of LIN28, MYCN, and Oct4 inhibits DGCR8 expression. Dysregulation of c-Myc, LIN28, and DDX5 directly suppresses Drosha. Abnormalities in NPM1, RAN, and CRM1 disrupt Exportin-5. Mutations in RAS genes, dysregulation of the mTOR pathway, and anomalies in IMPDH and GART disturb GTP levels. Furthermore, dysregulation of NPM1, TPX2, CRM1 inhibit Ran, and overexpression of Lin-28B, and miR-103/107 affects nucleocytoplasmic transport and suppresses Dicer expression.
Within the context of cancer, numerous molecules can exert effects on the genes involved in the synthesis of miRNAs. Overexpression of LIN28 and MYCN, as well as Oct4, a transcription factor associated with pluripotency, in cancer, can impede the expression or function of DGCR8, leading to a diminished production of miRNAs [28]. Additionally, the overexpression of c-Myc and LIN28, coupled with the dysregulation of DDX5 in cancer, can directly suppress the expression of Drosha, resulting in reduced levels of miRNAs. Furthermore, activating mutations in RAS genes, dysregulation of the mTOR pathway can disturb GTP levels and nucleotide metabolism, thereby impacting the synthesis of miRNA. The overexpression of Lin-28B and the action of miR-103/107 can suppress Dicer expression, leading to a reduction in miRNA levels [29,30,31,32,33] (Fig. 1).
The physiologic functions of miR-184
The encoding gene of miR-184, as a conserved, newly identified miRNA, is located on human chromosome human 15q25.1. It encodes a small transcript with 84 bp length, while no other clustered miRNAs are found near miR-184-encoding region [34]. This miRNA demonstrates uncommon properties compared to many other intergenic miRNAs [34]. It has been proved that miR-184 shows a tissue-specific expression pattern and its abnormal function may result in tissue-specific diseases [35]. In the fly Drosophila, miR-184 is found with involvement in numerous biological functions, particularly female germline stem cell differentiation [36]. It is also documented with crucial roles regulating the differentiation of other types of stem cells like embryonic stem (ES) cells and periodontal ligament stem cells as well as physiological functions of granulosa cells [37,38,39]. Particularly, its function in the regulation of cell physiologic processes like neural cells’ growth and differentiation may suggest therapeutic potential in human diseases [40]. The methyl-CpG binding protein 1 (MBD1) is a regulatory protein functions in modulating gene expression by an epigenetic mechanism based on DNA methylation. In adult neural stem/progenitor cells (aNSCs), MBD1 is shown to target several miRNAs, among them miR-184 is specifically repressed [41]. miR-184 at high levels enhanced the proliferation, while suppressed the differentiation of aNSCs. Mechanistically, miR-184 was found to exert its role through targeting Numblike (Numbl) that is discovered in brain development [41]. Moreover, miR-184 was reported to involve in the eye development, migration of keratinocytes, and corneal epithelial differentiation [42, 43]. The expression levels of miR-184 are documented to be high in the committed epidermal and corneal epithelium cells and regulated the balance between epidermal cell proliferation and differentiation [44]. A body of studies report involvement of miR-184 in the development mechanisms of human diseases, which is the subject of the current study.
The role of miR-184 in human diseases
In 2006, expression of miR-184 was reported in a mouse model, according to which the suprabasal cells of the corneal epithelium exhibited strong expression [45]. miR-184 is identified as the miRNA with the highest expression in the cornea and lens and it can partly explain association between this miRNA dysregulation and eye diseases. In 2011, a report demonstrated that a miR-184 mutation causes familial keratoconus with cataracts [46]. In the corneal tissue, miR-184 can alter Akt and vascular endothelial growth factor (VEGF) pathways, resulting in angiostatic properties. miR-184 is shown to negatively affect angiogenesis by regulating proangiogenic secreted factors, such as VEGF. This process is vital for the functional light passing to the lens and helps the visual sense, while miR-184 is also involved in the early development of the eye [47]. Moreover, miR-184 is significantly expressed in the human retinal pigment epithelium (RPE) and its downregulation is reported in several eye conditions like age-related macular degeneration (AMD) [48]. Point mutations at miR-184 are found in association with the pathogenesis of lens/corneal dystrophy and blindness [43, 49,50,51]. Downregulation of this miRNA alters the levels of EZR-bound LAMP-1 protein and affects the process of phagocytosis, which further interferes with the normal function of PRE [48]. A single base mutation in miR-184 is identified in a rare genetic eye disorder called EDICT syndrome, which is presented with several ocular manifestations, such as dystrophy of epithelium cells, hypoplastic changes in iris, congenital cataract, and thinning of the stromal layer [49]. It is also reported as a contributor of secondary cataract (SC) that is developed due to differentiation of remaining lens epithelial cells after extracapsular cataract extraction. By applying negative regulatory impact on the proliferative, migratory and cell cycle progression capacities of the corneal epithelial cells, miR-184 is found to delay the corneal epithelial wound healing (CEWH) process via targeting CDC25A, CARM1, and LASP1 genes in mice [52]. A complex, competitive RNA network, including miR-184, regulates the formation of SC in mice [53]. Moreover, miR-184 modulated Wnt signaling pathway by directly targeting frizzled-7 suggested its decreased expression can function in the pathogenesis of oxygen-induced retinopathy (OIR) via aberrant activation of Wnt signaling [54]. Ischemic stroke causes brain damage in affected patients. Various elements, such as oxidative stress and inflammation contribute to the process of ischemic stroke [55]. Evidence has shown that in rodents and humans, altered expression of many miRNAs is an important biomarker following ischemic stroke [56]. Among them, miR-184 is reported with downregulated expression following ischemic stroke in male rats. However, high levels of miR-184 can reduce subsequent brain damage [57]. Seizure preconditioning (brain exposure to a sub-threshold stimulant) can reduce the harmful effects of prolonged seizures (epileptic tolerance). The mouse model results revealed that seizure preconditioning upregulated miR-184 in the neurons, while its in vivo inhibition interfered with the protective mechanism of epileptic tolerance and resulted in neural death and brain damage after severe seizures [58]. Autism spectrum disorders (ASDs), including the fragile X syndrome and Rett syndrome are associated with synaptic plasticity defects. Both miRNA and DNA methylation pathways are involved in this process. miR-184, as a brain-specific miRNA, is discovered to exert pathogenic roles in Rett syndrome [59], which the disease is characterized by neuropsychiatric abnormalities [60]. miR-184 plays a substantial role in differentiating ES cells to some cardiac cells like mesoderm and cardiomyocytes by regulating Wnt3 expression [37]. The results of a cell model study have shown that inhibition of miR-184 suppressed inflammation and oxidative stress process, and as a result prevented apoptosis in cardiomyocytes [61]. miR-184 also constitutes an abundant number of miRNAs in pancreatic β-cells, which affects the cellular pathways of insulin secretion and impacts glucose metabolism as an insulin release suppressor [62]. Those findings have suggested miR-184 as a crucial role player in fine-tuning and regulation of pancreatic β-cell function. Downregulation of miR-184 is documented in human pancreatic islets of patients with type 2 diabetes relative to healthy donors [63]. Importantly, miR-184 inhibition in human β-cells protected them against palmitate-induced apoptosis as well as metabolic and inflammatory stress conditions. Those effects were found to be exerted through targeting the CREB transcriptional coactivator 1 (CRTC1), while CRTC1 overexpression showed reverse effects compared to miR-184 inhibition. Importantly, the transcription factor NKX6.1 with specific expression in β-cells regulated miR-184 expression via binding to its promoter sequence in human and in murine β-cells [63]. This miRNA is also found to play a role in the pathogenesis of cyanotic congenital heart diseases (CHD) via regulating the proliferation and apoptosis of cardiomyocytes [64]. Furthermore, miR-184 is found to function in the development of several other human diseases like Kawasaki disease [65], Recurrent spontaneous abortion (RSA) [66], and sepsis [67], while evaluation of its expression levels has suggested it as a potential diagnostic and predictive biomarker for conditions like polycystic ovary syndrome (PCOS) [68], and Anderson-Fabry disease (AFD) [69].
miR-184 in cancers
The carcinogenesis process is composed of several steps involving two classes of genes, protooncogenes and tumor suppressor genes [70]. Genetic alterations drive the normal cells to progress from the pre-malignant initiative status to malignant phenotype [71]. According to the global cancer statistics 2020, 19.3 million new cases in addition to 10.0 million deaths were reported for 2020 [72]. Early detection of cancer patients might improve the survival of patients by providing real-time access to therapies. Imaging techniques, laboratory tests, and tumor biomarkers constitute the main diagnostic approaches used for cancer detection, which still lack sufficient performance for real-time screening and diagnosis of cancer patients [73]. Current therapeutic strategies include chemotherapy, and radiation therapy, in addition to immunotherapy as a novel category of anticancer therapy [74, 75]. Although considerable achievements have been in improving the efficacy of anticancer therapeutics like hopeful findings reported for anticancer vaccines and immune checkpoint inhibitors (ICIs) [76, 77]; however, resistance to anticancer agents and late diagnosis might negatively affect survival among patients [78]. Resistance to anticancer therapies is mainly considered due to the tumor microenvironment (TME) and existence of some elements like cancer stem cells (CSCs) that in turn can affect the survival of cancer patients [79].
Several years of research have demonstrated that miRNAs might play crucial roles in all known physiologic activities similar to normal cells as well as pathogenic mechanisms in cancer cells, including cell growth and death, aggressiveness behaviors, metabolism, and survival [80]. Dysregulation of miRNA expression might affect the development, progression, and dissemination of human tumors, which are mechanistically conducted by regulating the expression of specific target genes. Both oncomiRs and tumor suppressor miRNAs are reported with substantial functions in regulating the malignant behaviors of tumor cells. Enhanced expression of oncomiRs or downregulation of tumor suppressor miRNAs suggests therapeutic potential, particularly in cancers. To achieve that, both miRNA mimics and antagonists have emerged as potential therapeutic agents that act by interfering with various cancer molecular pathways [80]. Some potential therapeutics include antisense oligonucleotide (ASO) strategy to inhibit the function of oncomiRs, exogenous miRNAs for augmentation of the tumor suppressor miRNAs expression, and artificial miRNA for targeting malignant tumor phenotype-related genes [71]. Also, the clustered regulatory interspaced short palindromic repeat (CRISPR)-associated nucleases (Cas) technology that uses a novel technology for manipulating genomes of various organisms by delivering Cas9 protein and a guide RNA to the target cells [81] may suggest potential for targeting and inhibition of oncomiRs [82]. Importantly, miRNAs have been found in increasing experimental data as biomarker candidates for various human diseases, including cancer [83]. This is not limited to miRNAs, while other classes of ncRNAs, including long transcripts (lncRNAs) and circRNAs, are also extensively studied as biomarkers to help diagnosis and prediction of prognosis among cancer patients [84,85,86,87,88,89,90]. Detection of miRNAs in the serum and other biological fluids (known as liquid biopsy) of cancer patients potentially can help non-invasive diagnosis of different cancers [91]. miR-184 is mainly reported with a tumor suppressor role suppressing the proliferation, migration, and invasion and induces apoptosis of cancer cells. Mechanistically, it is documented acting through targeting various genes to exert a suppressing role on the malignant behavior of cancer cells. Impacting some signaling pathways like Wnt and β-catenin, and AKT/mTORC1 pathway, oncogenic factors (e.g., c-Myc) or apoptotic proteins, such as Bcl-2 can partly explain some mechanisms through which miR-184 may exert its role as a tumor suppressor (Table 1). Xenograft animal studies also have supported the inhibiting impact of miR-184 on tumor growth and metastasis. Interestingly, aberrant expression of miR-184 is revealed with a prognostic and diagnostic performance for a number of human cancers.
Lung cancer
Lung cancer is responsible for the highest number of cancer-related mortalities globally [92]. Non-small-cell lung cancer (NSCLC) is one category of lung cancer with a poor prognosis and chemotherapy resistance [93]. Modern targeted therapies for NSCLC are more efficient and specific in comparison with conventional radio-chemotherapy [94]. Recent studies indicate that miRNAs expression profile is different between the resistant and sensitive tumors under the epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) therapy [92]. Results of a case-control study revealed that miR-184 expression was considerably higher in patients with NSCLC compared to those with benign lung diseases. It was also confirmed that after 3 years of follow-up, the levels of exosomal miR-184 in serum specimens of the NSCLC surviving patients were considerably lower compared to non-surviving group [95]. Bcl-2 family of apoptosis-regulating factors are already shown to affect the survival of NSCLC cells [96]. miR-184 downregulation by E6 oncoprotein can confer chemoresistance to cisplatin and negatively affect therapeutic responses of NSCLC patients via enhancing Bcl-2 expression [97]. In this study, Tung et al. assessed the expression level of miR-184 in the cervical cancer SiHa cells and human papillomavirus (HPV) 16-positive TL-1 cells. Results showed that miR-184 was downregulated in TL-1 and SiHa cells compared to controls, and miR-184 regulated the proliferation and survival of those cells through modulating the c-Myc and Bcl-2 expression. Also, they discovered that expression levels of Bcl-2 and miR184 was high in E6-positive tumor samples than in E6-negative tumor samples, which finally it can be concluded that the presence of E6 oncoprotein reduced miR-184 expression, while this miRNA affected cell growth and apoptosis via exerting a suppressing impact on the target genes [97]. miR-184 is documented to act as a tumor suppressor with inhibitory effects on cell survival and invasive capability of NSCLC cells by modulating the expression of CDC25A and c-Myc expression. Interestingly, miR-184 downregulation was suggested as a contributor to unfavorable survival among NSCLC patients [98]. Small-cell lung cell (SCLC), as a highly malignant type of neuroendocrine tumor, constitutes 15–20% of lung cancer cases and has poor clinical prognosis due to fatal metastasis [99]. Results of a study revealed that miR-184 suppressed migration and invasion of NSCLC cells functioned as a tumor suppressor. miR-184 could significantly attenuate metastasis of SCLC through participating in β-catenin signaling and downregulating the endothelial PAS domain protein 1 (EPAS1), which is a transcription factor functioning in various tumors including SCLC [100]. Moreover, miR-184 is documented to be involved in inhibiting the proliferation and epithelial-to-mesenchymal transition (EMT) process of airway epithelial cells contributing to suppressing the pathogenesis of idiopathic pulmonary fibrosis (IPF) [101].
Glioma
Glioma is the most malignant and common form of primary brain tumors in adults with an extremely bad prognosis of approximately one year [102]. The aggressive progression and invasive nature of glioma cells, facilitate the recurrence of the disease after therapies of surgery, chemotherapy, or radiation [103,104,105]. Recent studies provided evidence that cellular and molecular biological processes including abnormal gene expression and regulation of factors involved in tumor growth, play a significant role in glioma [106]. Thus, more attention has been paid to biological target therapy of glioma. In glioma, the expression of a handful of miRNAs, such as miR-21, miR-145, miR-940, and miR-101 are reported with downregulation while a number of other miRNAs like miR-191, miR-640, and miR-155-3p are upregulated in tumor tissues and cell lines [72]. Studies report miR-184 downregulation both in cell and tissue investigations. Exogenous expression of miR-184 decreased the proliferation and invasion of glioma cells by regulating SND1 expression. SND1 is known to be upregulated in human glioma with a regulatory role in glioma progression. Thus, miR-184/SND1 axis can be used as a target for diagnosis and therapy of malignant glioma [107]. Another study indicated that proliferation of glioma cells can be promoted by miR-184 overexpression through regulating FOXO3 [108]. Cheng et al. conducted a study, which demonstrated that miR-184 inhibited glioma progression through targeting TNFAIP2 expression and affecting its translation in glioma [35]. Results of a clinical experiment showed that miR-184 was overexpressed in glioma tissue in comparison to normal brain tissue [109]. Functional analyses revealed that miR-184 was suggested to promote the occurrence and development of gliomas. Furthermore, in higher pathological grades of glioma, miR-184 expression increased, but it did not show any correlation with the pathological type of glioma cells. However, a negative correlation was found between miR-184 expression and survival time of patients [109]. Collectively, in addition to witness supported the tumor suppressor role of miR-184, these findings suggested this miRNA as a potential prognostic and diagnostic biomarker for and a therapeutic target for glioma patients. This conclusion; however, is not supported at least by a study indicating upregulation of miR-184 and its oncogenic role enhanced the malignant phenotype and activities of A172 cells [110].
Endometrial cancer
Endometrial cancer (EC) is a common gynecologic malignancy in females in developed countries [111]. A recent study provides evidence that decreased miR-184 expression inhibited EC cell proliferation and invasion by targeting CDC25A-dependent Notch pathway [112]. It has been also suggested that miR-184 expression correlated with lymph node metastasis and survival of EC patients [113]. miRNA expression profile was used to predict the lymph node metastasis in a group of female patients diagnosed with grade 1-2 EC. Among dysregulated miRNAs, miR-184 showed downregulation in primary tumor specimen retrieved from women with lymph node metastases (positive LN) relative to those samples from patients with negative LN [113]. Additionally, miR-184 downregulation is also reported to correlate with pelvic LN metastasis and/or recurrence among EC patients [114].
Prostate cancer
Prostate cancer (PC) is the most commonly diagnosed cancer among men that is responsible for 27% of newly diagnosed cases and 11% of all cancer‑related deaths worldwide [115]. DLX1 is a protein that binds to β‑catenin and enhances viability and migratory capacities of cancer cells through activation of the β‑catenin/TCF signaling pathway [116]. miR‑184 is reported with downregulated expression in PC tissues and cell lines (C4-2, PC-3, Du145, and LNCaP) relative to normal prostate tissues and RWPE-2 cell lines, respectively [117]. miR-184 overexpression suppressed the growth and malignant activities of PC cells, while exogenous expression of DLX1 showed reverse impact. Mechanistically, miR-184 was found on bioinformatic investigations and dual luciferase reporter assay to directly target DLX1. This study suggested miR-184 as a tumor suppressor miRNA and a target for finding a molecular-based therapy for PC patients.
Breast cancer
Breast cancer (BC) is the most frequent malignancy for women and a high incidence globally, with an estimated 2,300,000 cases in addition to 685,000 deaths in 2020, which is predicted to reach 4,400,000 lives in 2070 [118]. Based on the tumor type and the clinicopathological stage, monotherapy or combinational therapy using conventional agents is recommended for BC patients [119]. According to various studies, many miRNAs are effective in regulation of initiation and progression in BC subtypes by mediating vital cellular processes, including cell proliferation, differentiation, and apoptosis [120]. Studies indicated that miR-184 is a tumor suppressor, inhibiting cell growth, self-renewal, and modulating the development of metastatic lesions to function in the BC pathogenesis [121, 122]. Phua et al. evaluated the role of miR-184 on tumorigenesis and distant metastasis in the orthotopic xenografts of BC cells. For this purpose, they investigated the expression of miR-184 and gene targets by qRT-PCR, microarray, in situ assessments, and luciferase reporter assay. To determine the miR-184 function, they transfected miR-184 into MDA-MB-436 and HS578T BC cells. Results showed that miR-184 overexpression suppressed the proliferative and self-renewal capacities of the control cells, delayed primary tumor development, and attenuated metastatic potential of BC cell lines. Findings also demonstrated that miR-184 suppressed the AKT/mTORC1 pathway, which functions in tumor invasion and metastasis. miR-184 is also reported to may target PRAS40 and TSC2, mTORC1 inhibitors, and it can suppress AKT2, in breast cancer [122] (see Fig. 2). In another study, Wang et al. evaluated the influence of miR-184 and tripterine on BC progression. They assessed the impact of miR-184 and tripterine on the human BC cell lines (MCF-7 and BT-474) using BrdU, CCK-8, Transwell, and wound healing investigations. This research discovered that cells’ viability, proliferation, migration, and invasion capacity were attenuated in BC cell lines treated with miR-184 and tripterine, while those agents had a synergistic effect in the development of BC [121]. Studies already have demonstrated that tripterine acts effectively against many types of cancer [121, 123, 124].
A Notch Signaling Pathway. The binding of DLL or JAGGED to Notch receptors leads to the liberation of the NICD, which is subsequently translocated to the nucleus where it interacts with transcription factors. In Nasopharyngeal Carcinoma, miR-184 suppresses Notch2 receptor. B PI3K/AKT/mTOR Pathway: PI3K generates phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which activates AKT and it directly targets mTOR, existing in mTORC1 and mTORC2 complexes. PTEN functions by dephosphorylating PIP3. In pancreatic ductal adenocarcinoma, miR-184 directly targets PI3K and in breast cancer, it may target mTORC1 inhibitors PRAS40 and TSC2 to eventually suppress AKT pathway. C Wnt/β-catenin pathway: In the absence of Wnt ligands, β-catenin is targeted for degradation by a complex comprising Axin, APC, GSK-3β, and CK1. Wnt binding to Frizzled receptors activates the LRP5/6 co-receptor, inhibiting the degrading complex and allows β-catenin to accumulate. In endometrial cancer, miR-184 inhibits Wnt, Frizzled, and DVL, whereas in cervical cancer, it downregulates Wnt. In small-cell lung cancer, miR-184 inhibits EPAS1, β-catenin phosphorylation enhancer. In osteosarcoma, miR-184 increases Wnt and enhances β-catenin levels and phosphorylation status. D TGF-B/Smad pathway: Ligands bind to TGF-B receptors, activating the receptor complex and phosphorylating R-Smads, which then develop complexes with Smad4, translocating into the nucleus to regulate gene expression. The effect of miR-184 on this pathway is unclear. DLL delta-like ligand, PI3K phosphoinositide 3-kinase, PIP3 phosphatidylinositol (3,4,5)-trisphosphate, mTOR mammalian target of rapamycin, mTORC mammalian target of rapamycin complexes, PTEN phosphatase and tensin homolog, PRAS40 proline-rich Akt substrate of 40 kDa, EPAS1 endothelial PAS domain protein 1, TGF-B transforming growth factor-beta.
Colorectal cancer
Colorectal cancer (CRC) is already known as the third most common malignancy and also the third cause of cancer-related deaths in both sexes accounting for 10% of all newly diagnosed cases of cancers and 9.4% of cancer mortality [72]. Numerous factors and molecular mechanisms, including genetic background and environmental agents, are involved in CRC progression [125]. miRNAs have a significant role in the initiation and development of CRC (tumor development, progression, and metastasis) [126]. As shown in Fig. 3, miR-184 can act as a tumor suppressor in CRC by directly targeting insulin-like growth factor 1 receptor (IGF-1R) [127]. Expression of IGF-1R, a transmembrane tyrosine kinase receptor of the insulin receptor family, is related to a malignant phenotype, tumor progression, chemoresistance, and unfavorable survival in CRC [128]. To analyze the function of miR-184 in CRC, Wu et al. transfected human CRC cell lines (HCT116, HT29, and SW620) and human normal colon epithelium cell line with miR-184 mimic and then evaluated the impact of mi-184 expression on the aggressive phenotype of those cells [127]. Analysis of tested groups showed that miR-184 was downregulated in CRC tissues and cell lines. Following overexpression of miR-184, 2,5-diphenyl-2H-tetrazolium bromide (MTT) and Transwell assays revealed that proliferation, migration, and invasion of CRC cells were inhibited in vitro. To understand the precise mechanism of miR-184 in CRC, western blotting (WB) analysis demonstrated that the expression of IGF-1R protein was significantly diminished in miR-184-overexpressing HCT116 and SW620 cells relative to control cells. The authors concluded that miR-184 inhibited aggressive phenotype of CRC cells by targeting IGF-1R [127].
In colorectal cancer, miR-184 can act as a tumor suppressor by directly targeting IGF-1R. In gastric cancer, miR-184 inhibits STC2, thereby suppressing cell proliferation, invasion, and metastasis. However, there is controversy surrounding the role of circ_0021087, which has been reported to exert a tumor suppressor function in gastric cancer by targeting miR-184 and upregulating FOSB. In hepatocellular carcinoma, miR-184 targets INPPL1 and caspase 3/7, leading to the inhibition of cellular proliferation and promotion of apoptosis, respectively. Additionally, miR-184 inhibits C-Myc, C-Jun, and Bcl-2, contributing to the suppression of cell growth and apoptosis induction in pancreatic adenocarcinoma. STC2 stanniocalcin 2, IGF-1R insulin-like growth factor 1 receptor, INPPL1 inositol polyphosphate phosphatase-like 1.
Gastric cancer
Gastric cancer (GC) is among the most common types of cancer, which causes high mortality worldwide [72]. The abnormal expression of miRNA could significantly affect biological characteristics of GC like cell growth, migration, invasion, and apoptosis [129]. The clinical significance of Stanniocalcin 2 (STC2) in GC was discovered by several studies [130,131,132]. STC2 is a glycoprotein with high expression in various tumor cells. The expression level of STC2 is upregulated in gastric tissues, and STC2 as an independent factor in cancer elevates lymphatic metastasis and venous invasion in GC [130, 133]. Qiao et al. evaluated miR-184 expression levels in MGC803 GC cell lines and human normal gastric epithelial cell lines. They also investigated the role of miR-184 as a tumor suppressor in blocking GC progression through targeting STC2, as shown in Fig. 3 [132]. The results showed downregulation of miR-184 and STC2 upregulation in GC cell lines. Ectopic expression of miR-184 in MGC803 cells significantly inhibited the expression of STC2. Consistently, STC2 inhibition suppressed the growth and invasion of GC cells indicating that miR-184 may play a tumor suppressor role [132]. Inconsistent with this study, Yu et al. reported that miR-184 reversed the inhibitory role of circ_0021087 on proliferation, EMT, migration, and invasion of GC cells [134]. Circ_0021087 exerted a tumor suppressor role in GC through targeting miR-184 and upregulating FOSB (Fig. 3). Opposite results mandate further investigations to shed light on the precise mechanism and therapeutic significance.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) constitutes a majority (75–85%) of primary liver cancer cases [72]. Serum miRNAs are suggested as biomarkers for the early diagnosis and prediction of prognosis of HCC patients [135]. Among various miRNAs, miR-184 is considered as a candidate tumor suppressor inhibiting the development and progression of HCC [136, 137]. In a study by the Gao et al., the effect of miR-184 inhibition on HCC development and progression was evaluated in HepG2 cells [137]. Bioinformatics and luciferase reporter assay identified inositol polyphosphate phosphatase-like 1 (INPPL1) as the target of miR-184. Results revealed that the upregulation of miR-184 expression was significant in HCC tissues compared to normal tissue. Furthermore, expression analysis demonstrated a reverse relationship between INPPL1 and miR184 in HCC cells. For elucidating the role of miR-184 on apoptosis, researchers investigated the impact of anti-miR-184 on caspase 3/7 activity in HepG2 cells. Caspase 3/7 activity in anti-miR-184-treated HepG2 cells was higher than untreated cells, which they concluded that miR-184 silencing induced HepG2 apoptosis by caspase 3/7. Therefore miR-184 silencing can induce HepG2 apoptosis by caspase 3/7 and inhibit cellular proliferation by INPPL1, as shown in Fig. 3 and Fig. 4 [137].
The extrinsic pathway of apoptosis starts by the binding of death ligands (TNF, FasL, and TRAIL) to corresponding receptors, causing recruitment of FADD to the intracellular domain of the death receptor. This triggers the formation of DISC, which itself in a series of reactions activates initial caspase-8 or -10, and then activation of effector caspases-3, -6, and -7 leading to cell death. Caspase-8 or caspase-10 can activate Bid that in turn causes activation of pro-apoptotic proteins Bax and Bak while inhibiting anti-apoptotic members of the Bcl-2 family. In the intrinsic pathway, cellular stress signals trigger conformational changes in Bax and Bak, resulting in release of cytochrome c into the cytoplasm, where it forms the apoptosome complex with Apaf-1 and dATP. Apoptosome causes serial activation of caspases-9, -3 and -7 to initiate cell death. In cancers, miR-184 exhibits dual effects on apoptosis. It acts as an inhibitor of apoptosis by downregulating caspase-3, and caspase-6, in hepatocellular carcinoma. Conversely, in pancreatic ductal adenocarcinoma, miR-184 induces apoptosis by inhibiting anti-apoptotic Bcl-2 and increasing caspase-3 and caspace 9. TNF tumor necrosis factor, FasL Fas ligand, TRAIL TNF-related apoptosis-inducing ligand, FADD Fas-associated death domain, DISC Death-inducing signaling complex, Bid BH3-interacting domain death agonist, Bcl-2 B-cell lymphoma 2, Apaf-1 Apoptotic protease-activating factor 1, dATP Deoxyadenosine triphosphate, TRAIL-R TNF-related apoptosis-inducing ligand receptor 1, XIAP X-linked inhibitor of apoptosis.
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma (HNSCC) is a malignancy with high rate of incidence and mortality that occurs in oral, oropharynx, larynx, or hypopharynx [138]. Aberrant expression of miRNAs is indicated in HNSCC specimens compared with the non-malignant samples [139]. Wong et al. evaluated the plasma levels of miR-184 in the tongue squamous cell carcinoma (SCC) tissues. miR-184 expression was significantly higher in isolated tongue SCC tissues compared with control group. Also, suppressing miR-184 induced apoptosis in studied cell lines (Cal27 and HN21B and HN96). After transfection of miR-184 inhibitor into tongue SCC cell lines, a decrease in proliferation rate and downregulation of c-Myc was found. Those results demonstrated that miR-184 could act as an oncogenic factor through inhibition of apoptosis and promotion of cell proliferation in tongue SCC [140].
Osteosarcoma
Osteosarcoma (OS) is a primary malignant cancer of bone structures that is mostly seen in children and younger adults [141]. A handful of ncRNAs including miRNAs as regulatory players are linked to tumor initiation, invasion, occurrence, and metastasis of OS [142, 143]. The impact of miR-184 in enhancing the proliferation and invasion of OS is already documented [144]. In the research by Lin et al., the role of miR-184 as a mediator of chemoresistance was investigated in OS [145]. For this purpose, miR-184 expression was evaluated following transfection of miR-184 agomir or miR-184 antagomir into OS cell lines U-2 OS and MG-63 under doxorubicin treatment. They found that miR-184 expression in transfected cells was time-dependent and inducible by doxorubicin. The luciferase reporter assay detected BCL2L1 as the direct target of miR-184. Also, in doxorubicin-treated OS cells, overexpression of miR-184 caused significant elevation in BCL2L1 expression. Ectopic expression of miR-184 agomir and miR-184 antagomir following transfection caused a decrease and promotion of doxorubicin-induced cell apoptosis of OS cells, respectively. These results showed upregulation of miR-184 occurred in doxorubicin-treated OS patients, and increasing expression of miR-184 by targeting BCL2L1 caused doxorubicin resistance in OS cells [145]. The Wnt/β-catenin pathway regulates cell renewal, cell proliferation, and differentiation in OS cells through intercellular communication by extracellular signals [146, 147]. miR-184 is already identified as one of the important miRNAs functioning in regulating the Wnt/β-catenin signaling pathway [11]. Du et al. evaluated the impact and the associated mechanism of miR-184 on the proliferation, invasion and metastasis of OS cells using in vitro and in vivo experiments [25]. Cell studies revealed upregulation of miR-184 and Wnt/β-catenin, and an increase in the proliferative and invasive potentials of miR-184-overexpressing U-2OS and 143B cells relative to un-transfected cells. In the xenograft animal study, tumor model was established in the mic. Results revealed that significant upregulation of miR-184 and Wnt and increase in phosphorylated β-catenin level. Also, miR-184 could impact the OS development and metastasis through modulating the Wnt/β-catenin signaling pathway [25]. Consistently, upregulation of miR-184 and its impact on the aggressive phenotype of SOSP-9607 OS cells is documented in another study by Tao et al. [148].
Nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is a rare type of head and neck malignant and is the most common in Southeast Asia and southern China [149]. Evidence has demonstrated that miRNAs via regulating target gene expression are associated with malignant progression of NPC [150]. The programmed cell death 4 (PDCD4), a tumor suppressor with downregulation in various tumor types, induces cell apoptosis and inhibits the aggressive phenotype of cancer cells [151]. Inhibition of BCL2 and C-MYC translation by PDCD4 suppressed cell growth and survival in NPC [152]. Zhen et al. revealed that PDCD4 protein expression was significantly reduced in NPC samples. PDCD4 suppressed the cell proliferation and cell survival in NPC through regulating the PI3K/AKT and JNK/C-Jun pathways by directly targeting C-MYC, BCL-2, and the oncogenic transcription factor C-JUN. They also showed miR-184 directly targeted the BCL2 and C-MYC and affected cell proliferation and survival in NPC [152]. In another study, Zhu et al. found downregulation of miR-184 in NPC cell lines. miR-184 suppressed the aggressive phenotype of NPC cells via regulation of EMT process and targeting Notch2 [153]. The EMT process is already known to play crucial roles during cancer progression and permits solid tumors to become more malignant [154], and thus miR-184 may function in the pathogenesis of NPC by regulating EMT.
Ovarian cancer
Ovarian cancer is among the most prevalent causes of cancer-related deaths in female, with over 230,000 new cases and 140,000 deaths yearly [155]. Abnormal miRNAs expression is associated with ovarian cancer and can be employed as biomarkers for prediction of therapy outcomes in ovarian cancer. miR-184 is shown to downregulate in epithelial ovarian cancer (EOC) in evaluations using a high-throughput microarray study compared with immortalized ovarian surface epithelium (IOSE) cell lines. Qin et al. analyzed the expression of miR-184 in clinical EOC tissues and EOC cell lines, also the role of miR-184 in the proliferation, apoptosis, and inflammation was investigated [156]. miR‑184 expression was found to be significantly reduced in EOC tissues and cell lines relative to paired non-cancerous tissues and IOSE cell line, respectively. They also showed that miR-184 overexpression in transfected cells inhibited EOC cell proliferation and suppressed inflammation by providing lower levels of several cytokines, such as the tumor necrosis factor alpha (TNF-α), and interleukins IL-6, IL-8, and IL-10. In vitro induction of apoptosis was detected by analysis of apoptosis-related genes, which showed decrease in the expression of Bcl-2 and increased expression of Bax and caspase-3 activity in EOC cells compared with respective controls. Notably, investigation of survival among EOC patients using Kaplan-Meier survival analysis revealed worse overall survival compared to those with high expression of this miRNA. Additionally, Cox regression multivariate analysis suggested miR-184 as an independent biomarker for EOC patients. These findings suggest miR-184 as a prognostic biomarker for OC patients along with its significance in the disease pathogenesis [156].
Other cancers
In a number of other cancers dysregulation of miR-184 as well as its significance in the carcinogenesis is documented. For instance, in clear cell renal cell carcinoma (ccRCC), miR-184-5p is reported with downregulation in A-498 and 786-O cell lines and its overexpression showed a tumor suppressor role suppressing the proliferation, and invasion and induction of apoptosis by directly targeting the NUS1 dehydrodolichyl diphosphate synthase subunit (NUS1) [157]. In melanoma, among a number of candidate miRNAs with potential role in regulating the aggressive features of cancer cells, miR-184 was shown to markedly inhibit the invasive capability and tube formation activity of HAG cells [158]. The tumor-suppressing role of miR-184 is also documented in human central nervous system lymphoma (HCNSL) cells by inhibiting the cell survival and invasion of HCNSL cells via regulating the PI3K/Akt signaling pathway [159]. Although an increasing number of ncRNAs are studied in retinoblastoma [160], miR-184 is documented in a single study with a tumor suppressing function, enhancing chemosensitivity through targeting SLC7A5 [161].
Clinical significance of miR-184 in human cancers
A biomarker is defined as a biological compound, which is detectable in body specimens and represents a specific physiologic process, a pathologic condition/outcome, or response to a pharmacologic intervention or exposure [162,163,164]. In a general classification, tumor biomarkers are divided into prognostic, used for prediction of survival and response to treatment, and diagnostic with potentials in differentiating cancer patients from healthy individuals or help diagnose a specific type of cancer [165]. Aberrant expression of ncRNAs, including miRNAs, may be used as a potential in the prediction of prognosis and diagnosis of cancer patients [166,167,168,169,170]. Showing a correlation with worse clinicopathological features as well as shorter survival rates among cancer patients, low levels of miR-184 may act as a prognostic biomarker for some cancers (see Table 1). In glioma, for example, miR-184 expression is shown to negatively correlate with survival time of those patients [109]. Moreover, miR-184 has shown acceptable diagnostic performance in the area under curve (AUC), Cox regression as well as univariate and multivariate analyses indicating that it may be efficient in diagnosis of cancer patients. For instance, survival analysis using Kaplan–Meier plot in EOC patients showed that patients with low expression levels of miR-184 had worse overall survival compared to those with high expression. Further analyses using Cox regression multivariate analysis suggested miR-184 as an independent biomarker for EOC patients [156]. Beyond the prognostic/diagnostic performance of miR-184, its putative roles in the carcinogenesis of various human cancers and contributions to enhanced chemosensitivity indicate its potentials in cancer therapeutics [161]. miRNA-targeted therapy is suggested as a therapeutic strategy for human cancers [19]. The development of novel approaches for effective and safe delivery of miRNAs to restore their tumor suppressor function in targeted tumor tissues is a research hot spot for cancer scientists. Delivery approaches including viral vectors, nanoparticles, and extracellular vesicles (EVs) have been tested for miRNA-targeted therapy [171]. Owing to their potential in loading various biological compounds, such as protein, miRNA, and DNA as well as drugs [172], EVs are being increasingly studied as ideal drug delivery vehicles for cancer therapeutics [173]. In cell experiments, viral vectors are mainly used for the delivery of miRNA constructs to the target cells for whom results mainly demonstrated that exogenous expression of miR-184 may suppress the malignant transformation of cancer cells and thus, suggest a therapeutic potential (Table 1).
Concluding remarks
miRNAs play crucial roles in posttranscriptional modulation of gene expression and regulate various biological functions and critical processes. Accordingly, their aberrant expression is associated with many pathologic conditions, particularly cancer. miR-184 is a well-studied miRNA, which is mainly reported to play a tumor suppressor role by inhibiting the malignant phenotype of cancer cells, although some controversial reports claim opposite effects. Affecting signaling pathways, oncogenic factors or apoptotic proteins are some discovered mechanisms through which miR-184 might function as a tumor suppressor. The aberrant expression of miR-184 is frequently reported in tumor tissues retrieved from cancer patients and an association between its expression levels and clinicopathological characteristics among cancer patients suggests it is a reliable prognostic biomarker. Moreover, miR-184 might be employed for the detection of cancer patients as a potential diagnostic biomarker. Additionally, experiments using exogenous miR-184 mainly support its role as a tumor suppressor inhibiting tumor growth and metastasis in vitro and in vivo highlighting its significance as a therapeutic target for cancer patients.
Data availability
No data were used for the manuscript
References
Rezaee D, Saadatpour F, Akbari N, Zoghi A, Najafi S, Beyranvand P, et al. The role of microRNAs in the pathophysiology of human central nervous system: a focus on neurodegenerative diseases. Ageing Res Rev. 2023;92:102090.
Sayad A, Najafi S, Kashi AH, Hosseini SJ, Akrami SM, Taheri M, et al. Circular RNAs in renal cell carcinoma: Functions in tumorigenesis and diagnostic and prognostic potentials. Pathol Res Pract. 2022;229:153720.
Najafi S. Circular RNAs as emerging players in cervical cancer tumorigenesis; A review to roles and biomarker potentials. Int J Biol Macromol. 2022;206:939–53.
Najafi S, Aghaei Zarch SM, Majidpoor J, Pordel S, Aghamiri S, Fatih Rasul M, et al. Recent insights into the roles of circular RNAs in human brain development and neurologic diseases. Int J Biol Macromol. 2023;225:1038–48.
Ardekani AM, Naeini MM. The role of MicroRNAs in human diseases. Avicenna J Med Biotechnol. 2010;2:161–79.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402.
Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–69.
Tam S, de Borja R, Tsao M-S, McPherson JD. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab Invest. 2014;94:350–8.
Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–7.
Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451–65.
Annese T, Tamma R, De Giorgis M, Ribatti D. microRNAs biogenesis, functions and role in tumor angiogenesis. Front Oncol. 2020;10:581007.
Ivanisenko NV, Seyrek K, Hillert-Richter LK, König C, Espe J, Bose K, et al. Regulation of extrinsic apoptotic signaling by c-FLIP: towards targeting cancer networks. Trends Cancer. 2022;8:190–209.
Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteom. Bioinform. 2012;10:246–53.
Paul P, Chakraborty A, Sarkar D, Langthasa M, Rahman M, Bari M, et al. Interplay between miRNAs and human diseases. J Cell Physiol. 2018;233:2007–18.
Davarinejad O, Najafi S, Zhaleh H, Golmohammadi F, Radmehr F, Alikhani M, et al. MiR-574-5P, miR-1827, and miR-4429 as potential biomarkers for schizophrenia. J Mol Neurosci. 2022;72:226–38.
Pordel S, Khorrami M, Saadatpour F, Rezaee D, Cho WC, Jahani S, et al. The role of microRNA-185 in the pathogenesis of human diseases: a focus on cancer. Pathol Res Pract. 2023;249:154729.
Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004.
Faramin Lashkarian M, Hashemipour N, Niaraki N, Soghala S, Moradi A, Sarhangi S, et al. MicroRNA-122 in human cancers: from mechanistic to clinical perspectives. Cancer Cell Int. 2023;23:29.
Bahari Khasraghi L, Nouri M, Vazirzadeh M, Hashemipour N, Talebi M, Aghaei Zarch F, et al. MicroRNA-206 in human cancer: mechanistic and clinical perspectives. Cell Signal. 2023;101:110525.
Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of microRNAs in cancer. Cancer Res. 2016;76:3666–70.
Otmani K, Lewalle P. Tumor suppressor miRNA in cancer cells and the tumor microenvironment: mechanism of deregulation and clinical implications. Front Oncol. 2021;11:708765.
Fattahi M, Shahrabi S, Saadatpour F, Rezaee D, Beyglu Z, Delavari S, et al. MicroRNA-382 as a tumor suppressor? Roles in tumorigenesis and clinical significance. Int J Biol Macromol. 2023;250:125863.
Paranjape T, Slack FJ, Weidhaas JB. MicroRNAs: tools for cancer diagnostics. Gut. 2009;58:1546–54.
Sempere LF, Azmi AS, Moore A. microRNA-based diagnostic and therapeutic applications in cancer medicine. Wiley Interdiscip Rev RNA. 2021;12:e1662.
Du Z, Li F, Wang L, Huang H, Xu S. Regulatory effects of microRNA184 on osteosarcoma via the Wnt/betacatenin signaling pathway. Mol Med Rep. 2018;18:1917–24.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. cell. 2004;116:281–97.
Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci. 2008;13:2537.
Piskounova E, Viswanathan SR, Janas M, LaPierre RJ, Daley GQ, Sliz P, et al. Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J Biol Chem. 2008;283:21310–4.
Wang X, Zhao X, Gao P, Wu M. c-Myc modulates microRNA processing via the transcriptional regulation of Drosha. Sci Rep. 2013;3:1942.
Muñoz-Maldonado C, Zimmer Y, Medová M. A comparative analysis of individual RAS mutations in cancer biology. Front Oncol. 2019;9:1088.
Krencz I, Sztankovics D, Danko T, Sebestyen A, Khoor A. Progression and metastasis of small cell lung carcinoma: the role of the PI3K/Akt/mTOR pathway and metabolic alterations. Cancer Metast Rev. 2021;40:1141–57.
Chang T-C, Zeitels LR, Hwang H-W, Chivukula RR, Wentzel EA, Dews M, et al. Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc Natl Acad Sci USA. 2009;106:3384–9.
Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–207.
Weitzel RP, Lesniewski ML, Greco NJ, Laughlin MJ. Reduced methyl-CpG protein binding contributing to miR-184 expression in umbilical cord blood CD4+ T-cells. Leukemia. 2011;25:169–72.
Cheng Z, Wang HZ, Li X, Wu Z, Han Y, Li Y, et al. MicroRNA-184 inhibits cell proliferation and invasion, and specifically targets TNFAIP2 in Glioma. J Exp Clin Cancer Res. 2015;34:27.
Iovino N, Pane A, Gaul U. miR-184 has multiple roles in drosophila female germline development. Dev Cell. 2009;17:123–33.
Liu X, Yang Y, Wang X, Guo X, Lu C, Kang J, et al. MiR-184 directly targets Wnt3 in cardiac mesoderm differentiation of embryonic stem cells. Stem Cells. 2020;38:1568–77.
Li C, Duan G, Feng Y. Downregulation of miR-184 facilitates osseous differentiation in periodontal ligament stem cells by modulating nuclear factor I-C. J Dent Sci. 2021;16:668–75.
Shi S, Hu Y, Song X, Huang L, Zhang L, Zhou X, et al. Totipotency of miR-184 in porcine granulosa cells. Mol Cell Endocrinol. 2022;558:111765.
Afrang N, Tavakoli R, Tasharrofi N, Alian A, Naderi Sohi A, Kabiri M, et al. A critical role for miR-184 in the fate determination of oligodendrocytes. Stem Cell Res Ther. 2019;10:112.
Liu C, Teng Z-Q, Santistevan NJ, Szulwach KE, Guo W, Jin P, et al. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6:433–44.
Yu J, Peng H, Ruan Q, Fatima A, Getsios S, Lavker RM. MicroRNA-205 promotes keratinocyte migration via the lipid phosphatase SHIP2. FASEB J. 2010;24:3950–9.
Shalom‐Feuerstein R, Serror L, De La Forest Divonne S, Petit I, Aberdam E, Camargo L, et al. Pluripotent stem cell model reveals essential roles for miR‐450b‐5p and miR‐184 in embryonic corneal lineage specification. Stem Cells. 2012;30:898–909.
Nagosa S, Leesch F, Putin D, Bhattacharya S, Altshuler A, Serror L, et al. microRNA-184 induces a commitment switch to epidermal differentiation. Stem Cell Rep. 2017;9:1991–2004.
Wong TS, Ho WK, Chan JY, Ng RW, Wei WI. Mature miR-184 and squamous cell carcinoma of the tongue. ScientificWorldJournal. 2009;9:130–2.
Hughes AE, Bradley DT, Campbell M, Lechner J, Dash DP, Simpson DA, et al. Mutation altering the miR-184 seed region causes familial keratoconus with cataract. Am J Hum Genet. 2011;89:628–33.
Park JK, Peng H, Yang W, Katsnelson J, Volpert O, Lavker RM. miR-184 exhibits angiostatic properties via regulation of Akt and VEGF signaling pathways. FASEB J. 2017;31:256–65.
Murad N, Kokkinaki M, Gunawardena N, Gunawan MS, Hathout Y, Janczura KJ, et al. miR-184 regulates ezrin, LAMP-1 expression, affects phagocytosis in human retinal pigment epithelium and is downregulated in age-related macular degeneration. FEBS J. 2014;281:5251–64.
Iliff BW, Riazuddin SA, Gottsch JD. A single-base substitution in the seed region of miR-184 causes EDICT syndrome. Invest Ophthalmol Vis Sci. 2012;53:348–53.
Lechner J, Bae HA, Guduric-Fuchs J, Rice A, Govindarajan G, Siddiqui S, et al. Mutational analysis of MIR184 in sporadic keratoconus and myopia. Invest Ophthalmol Vis Sci. 2013;54:5266–72.
Farzadfard A, Nassiri N, Moghadam TN, Paylakhi SH, Elahi E. Screening for MIR184 mutations in iranian patients with keratoconus. J Ophthalmic Vis Res. 2016;11:3–7.
Cao Q, Xu W, Chen W, Peng D, Liu Q, Dong J, et al. MicroRNA-184 negatively regulates corneal epithelial wound healing via targeting CDC25A, CARM1, and LASP1. Eye Vis (Lond). 2020;7:35.
Hoffmann A, Huang Y, Suetsugu-Maki R, Ringelberg CS, Tomlinson CR, Del Rio-Tsonis K, et al. Implication of the miR-184 and miR-204 competitive RNA network in control of mouse secondary cataract. Mol Med. 2012;18:528–38.
Takahashi Y, Chen Q, Rajala RVS, Ma J-x. MicroRNA-184 modulates canonical Wnt signaling through the regulation of frizzled-7 expression in the retina with ischemia-induced neovascularization. FEBS Lett. 2015;589:1143–9.
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97.
Rink C, Khanna S. MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics. 2011;43:521–8.
Yang H, Zhang Y, Chen H, Zhu Y, Li Y, Ouyang F, et al. Mir-184 contributes to brain injury through targeting PPAP2B following ischemic stroke in male rats. Front Mol Neurosci. 2021;14:613887.
McKiernan RC, Jimenez-Mateos EM, Sano T, Bray I, Stallings RL, Simon RP, et al. Expression profiling the microRNA response to epileptic preconditioning identifies miR-184 as a modulator of seizure-induced neuronal death. Exp Neurol. 2012;237:346–54.
Nomura T, Kimura M, Horii T, Morita S, Soejima H, Kudo S, et al. MeCP2-dependent repression of an imprinted miR-184 released by depolarization. Hum Mol Genet. 2008;17:1192–9.
Chahrour M, Zoghbi HY. The story of rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–37.
Zou JF, Wu XN, Shi RH, Sun YQ, Qin FJ, Yang YM. Inhibition of microRNA-184 reduces H2O2-mediated cardiomyocyte injury via targeting FBXO28. Eur Rev Med Pharmacol Sci. 2020;24:11251–8.
Tattikota SG, Rathjen T, Hausser J, Khedkar A, Kabra UD, Pandey V, et al. miR-184 regulates pancreatic beta-cell function according to glucose metabolism. J Biol Chem. 2015;290:20284–94.
Grieco GE, Brusco N, Fignani D, Nigi L, Formichi C, Licata G, et al. Reduced miR-184-3p expression protects pancreatic β-cells from lipotoxic and proinflammatory apoptosis in type 2 diabetes via CRTC1 upregulation. Cell Death Discov. 2022;8:340.
Huang J, Li X, Li H, Su Z, Wang J, Zhang H. Down-regulation of microRNA-184 contributes to the development of cyanotic congenital heart diseases. Int J Clin Exp Pathol. 2015;8:14221–7.
Liao J, Guo X, Fan X, Zhang X, Xu M. Upregulation of miR-184 and miR-19a-3p induces endothelial dysfunction by targeting AGO2 in Kawasaki disease. Cardiol Young. 2023;33:1962–66.
Zhang Y, Zhou J, Li M-Q, Xu J, Zhang J-P, Jin L-P. MicroRNA-184 promotes apoptosis of trophoblast cells via targeting WIG1 and induces early spontaneous abortion. Cell Death Dis. 2019;10:223.
Guo Z, Yi S. Bone marrow mesenchymal stem cells (BMSC) from exosome with high miR-184 level ameliorates sepsis. J Biomater Tissue Eng. 2023;13:24–30.
Gao C, Guo L, Jiang Z, Cao L. Evaluation of serum MiR-184 and MiR-326 expression in PCOS subjects: correlation with PCOS related parameters. Clin Lab.2022;68:1333–40.
Salamon I, Biagini E, Kunderfranco P, Roncarati R, Ferracin M, Taglieri N, et al. Circulating miR-184 is a potential predictive biomarker of cardiac damage in Anderson-Fabry disease. Cell Death Dis. 2021;12:1150.
Barrett JC, Wiseman RW. Cellular and molecular mechanisms of multistep carcinogenesis: relevance to carcinogen risk assessment. Environ Health Perspect. 1987;76:65–70.
Ji W, Sun B, Su C. Targeting microRNAs in cancer gene therapy. Genes (Basel). 2017;8:21.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Maruvada P, Wang W, Wagner PD, Srivastava S. Biomarkers in molecular medicine: cancer detection and diagnosis. Biotechniques. 2005;38:S9–S15.
Mortezaee K, Majidpoor J, Najafi S, Tasa D. Bypassing anti-PD-(L)1 therapy: Mechanisms and management strategies. Biomed Pharmacother. 2023;158:114150.
Najafi S, Majidpoor J, Mortezaee K. The impact of microbiota on PD-1/PD-L1 inhibitor therapy outcomes: a focus on solid tumors. Life Sci. 2022;310:121138.
Najafi S, Mortezaee K. Advances in dendritic cell vaccination therapy of cancer. Biomed Pharmacother. 2023;164:114954.
Mortezaee K, Majidpoor J, Najafi S. VISTA immune regulatory effects in bypassing cancer immunotherapy: updated. Life Sci. 2022;310:121083.
Wang X, Zhang H, Chen X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019;2:141–60.
Rouzbahani E, Majidpoor J, Najafi S, Mortezaee K. Cancer stem cells in immunoregulation and bypassing anti-checkpoint therapy. Biomed Pharmacother. 2022;156:113906.
Garofalo M, Croce CM. microRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43.
Najafi S, Tan SC, Aghamiri S, Raee P, Ebrahimi Z, Jahromi ZK, et al. Therapeutic potentials of CRISPR-Cas genome editing technology in human viral infections. Biomed Pharmacother. 2022;148:112743.
Wen D, Danquah M, Chaudhary AK, Mahato RI. Small molecules targeting microRNA for cancer therapy: promises and obstacles. J Control Release. 2015;219:237–47.
Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev Mol Diagn. 2010;10:297–308.
Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteom Bioinform. 2016;14:42–54.
Sanchez Calle A, Kawamura Y, Yamamoto Y, Takeshita F, Ochiya T. Emerging roles of long non‐coding RNA in cancer. Cancer Sci. 2018;109:2093–100.
Najafi S, Ghafouri-Fard S, Hussen BM, Jamal HH, Taheri M, Hallajnejad M. Oncogenic roles of small nucleolar RNA host gene 7 (SNHG7) long noncoding RNA in human cancers and potentials. Front Cell Dev Biol. 2022;9:809345.
Farzaneh M, Ghasemian M, Ghaedrahmati F, Poodineh J, Najafi S, Masoodi T, et al. Functional roles of lncRNA-TUG1 in hepatocellular carcinoma. Life Sci. 2022;308:120974.
Kristensen LS, Hansen TB, Venø MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–65.
Taheri M, Najafi S, Basiri A, Hussen BM, Baniahmad A, Jamali E, et al. The role and clinical potentials of circular RNAs in prostate cancer. Front Oncol. 2021;11:781414.
Najafi S. The emerging roles and potential applications of circular RNAs in ovarian cancer: a comprehensive review. J Cancer Res Clin Oncol. 2023;149:2211–34.
Wittmann J, Jack HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200–7.
Mizuno K, Mataki H, Seki N, Kumamoto T, Kamikawaji K, Inoue H. MicroRNAs in non-small cell lung cancer and idiopathic pulmonary fibrosis. J Hum Genet. 2017;62:57–65.
Hou J, Meng F, Chan LW, Cho W, Wong S. Circulating plasma MicroRNAs as diagnostic markers for NSCLC. Front Genet. 2016;7:193.
Wang X, Goldstein D, Crowe PJ, Yang J-L. Next-generation EGFR/HER tyrosine kinase inhibitors for the treatment of patients with non-small-cell lung cancer harboring EGFR mutations: a review of the evidence. Onco Targets Ther. 2016;9:5461.
Sayad A, Najafi S, Hussen BM, Abdullah ST, Movahedpour A, Taheri M, et al. The emerging roles of the β-secretase BACE1 and the long non-coding RNA BACE1-AS in human diseases: a focus on neurodegenerative diseases and cancer. Front Aging Neurosci. 2022;14:853180.
Zang H, Peng J, Wang W, Fan S. Roles of microRNAs in the resistance to platinum based chemotherapy in the non-small cell lung cancer. J Cancer. 2017;8:3856–61.
Tung MC, Lin PL, Cheng YW, Wu DW, Yeh SD, Chen CY, et al. Reduction of microRNA-184 by E6 oncoprotein confers cisplatin resistance in lung cancer via increasing Bcl-2. Oncotarget. 2016;7:32362–74.
Lin T-C, Lin P-L, Cheng Y-W, Wu T-C, Chou M-C, Chen C-Y, et al. MicroRNA-184 deregulated by the MicroRNA-21 promotes tumor malignancy and poor outcomes in non-small cell lung cancer via targeting CDC25A and c-Myc. Ann Surg Oncol. 2015;22:1532–9.
Riaz SP, Lüchtenborg M, Coupland VH, Spicer J, Peake MD, Møller H. Trends in incidence of small cell lung cancer and all lung cancer. Lung Cancer. 2012;75:280–4.
Zhou R, Zhou X, Yin Z, Guo J, Hu T, Jiang S, et al. Tumor invasion and metastasis regulated by microRNA-184 and microRNA-574-5p in small-cell lung cancer. Oncotarget. 2015;6:44609–22.
Li J, Pan C, Tang C, Tan W, Zhang W, Guan J. miR-184 targets TP63 to block idiopathic pulmonary fibrosis by inhibiting proliferation and epithelial-mesenchymal transition of airway epithelial cells. Lab Invest. 2021;101:142–54.
Wang Q, Li P, Li A, Jiang W, Wang H, Wang J, et al. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res. 2012;31(1):10.
Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507.
Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–22.
Alivand MR, Najafi S, Esmaeili S, Rahmanpour D, Zhaleh H, Rahmati Y. Integrative analysis of DNA methylation and gene expression profiles to identify biomarkers of glioblastoma. Cancer Genet. 2021;258-259:135–50.
Lam FC, Yaffe MB. Kicking genomic profiling to the curb: how re-wiring the phosphoproteome can explain treatment resistance in glioma. Cancer Cell. 2016;29:435–6.
Emdad L, Janjic A, Alzubi MA, Hu B, Santhekadur PK, Menezes ME, et al. Suppression of miR-184 in malignant gliomas upregulates SND1 and promotes tumor aggressiveness. Neuro Oncol. 2015;17:419–29.
Cui QK, Liu WD, Zhu JX, Wang YH, Wang ZG. MicroRNA-184 promotes proliferation ability of glioma cells by regulating FOXO3. Asian Pac J Trop Med. 2014;7:776–9.
Wu XB, Yang W, Fan G, Lin WR, Liu F, Lu ZM. Expression of microRNA-184 in glioma. Oncol Lett. 2018;15:727–30.
Yuan Q, Gao W, Liu B, Ye W. Upregulation of miR-184 enhances the malignant biological behavior of human glioma cell line A172 by targeting FIH-1. Cell Physiol Biochem. 2014;34:1125–36.
Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491–505.
Chen Z, Zhu Y, Fan X, Liu Y, Feng Q. Decreased expression of miR-184 restrains the growth and invasion of endometrial carcinoma cells through CDC25A-dependent Notch signaling pathway. Am J Transl Res. 2019;11:755–64.
Canlorbe G, Wang Z, Laas E, Bendifallah S, Castela M, Lefevre M, et al. Identification of microRNA expression profile related to lymph node status in women with early-stage grade 1-2 endometrial cancer. Mod Pathol. 2016;29:391–401.
de Foucher T, Sbeih M, Uzan J, Bendifallah S, Lefevre M, Chabbert-Buffet N, et al. Identification of micro-RNA expression profile related to recurrence in women with ESMO low-risk endometrial cancer. J Transl Med. 2018;16:131.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Liang M, Sun Y, Yang HL, Zhang B, Wen J, Shi BK. DLX1, a binding protein of beta-catenin, promoted the growth and migration of prostate cancer cells. Exp Cell Res. 2018;363:26–32.
Tan GG, Xu C, Zhong WK, Wang CY. miR-184 delays cell proliferation, migration and invasion in prostate cancer by directly suppressing DLX1. Exp Ther Med. 2021;22:1163.
Lei S, Zheng R, Zhang S, Wang S, Chen R, Sun K, et al. Global patterns of breast cancer incidence and mortality: a population‐based cancer registry data analysis from 2000 to 2020. Cancer Commun (Lond). 2021;41:1183–94.
Aghamiri S, Zandsalimi F, Raee P, Abdollahifar M-A, Tan SC, Low TY, et al. Antimicrobial peptides as potential therapeutics for breast cancer. Pharmacol Res. 2021;171:105777.
Shi D, Li Y, Fan L, Zhao Q, Tan B, Cui G. Upregulation of miR-153 inhibits triple-negative breast cancer progression by targeting ZEB2-mediated EMT and contributes to better prognosis. Onco Targets Ther. 2019;12:9611.
Wang J. Tripterine and miR-184 show synergy to suppress breast cancer progression. Biochem Biophys Res Commun. 2021;561:19–25.
Phua YW, Nguyen A, Roden DL, Elsworth B, Deng N, Nikolic I, et al. MicroRNA profiling of the pubertal mouse mammary gland identifies miR-184 as a candidate breast tumour suppressor gene. Breast Cancer Res. 2015;17:83.
Li X, Wang H, Ding J, Nie S, Wang L, Zhang L, et al. Celastrol strongly inhibits proliferation, migration and cancer stem cell properties through suppression of Pin1 in ovarian cancer cells. Eur J Pharmacol. 2019;842:146–56.
Yao SS, Han L, Tian ZB, Yu YN, Zhang Q, Li XY, et al. Celastrol inhibits growth and metastasis of human gastric cancer cell MKN45 by down‐regulating microRNA‐21. Phytother Res. 2019;33:1706–16.
Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular mechanisms of colon cancer progression and metastasis: recent insights and advancements. Int J Mol Sci. 2020;22:130.
Zhang N, Hu X, Du Y, Du J. The role of miRNAs in colorectal cancer progression and chemoradiotherapy. Biomed Pharmacother. 2021;134:111099.
Wu G, Liu J, Wu Z, Wu X, Yao X. MicroRNA-184 inhibits cell proliferation and metastasis in human colorectal cancer by directly targeting IGF-1R. Oncol Lett. 2017;14:3215–22.
Shiratsuchi I, Akagi Y, Kawahara A, Kinugasa T, Romeo K, Yoshida T, et al. Expression of IGF-1 and IGF-1R and their relation to clinicopathological factors in colorectal cancer. Anticancer Res. 2011;31:2541–5.
Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–90.
Cordero AIH, Gonzales NM, Parker CC, Sokolof G, Vandenbergh DJ, Cheng R, et al. Genome-wide associations reveal human-mouse genetic convergence and modifiers of myogenesis, CPNE1 and STC2. Am J Hum Genet. 2019;105:1222–36.
Hu Q, Masuda T, Sato K, Tobo T, Nambara S, Kidogami S, et al. Identification of ARL4C as a peritoneal dissemination-associated gene and its clinical significance in gastric cancer. Ann Surg Oncol. 2018;25:745–53.
Qing Q, He JX, Wang K, Zhou S, Dang L, Zhang B, et al. MIR-184 inhibits proliferation and invasion of gastric cancer cells by targeting STC2. Acta Med Mediterr. 2021;37:1277–82.
Qie S, Sang N. Stanniocalcin 2 (STC2): a universal tumour biomarker and a potential therapeutical target. J Exp Clin Cancer Res. 2022;41:161.
Yu Y, Li H, Wu C, Li J. Circ_0021087 acts as a miR-184 sponge and represses gastric cancer progression by adsorbing miR-184 and elevating FOSB expression. Eur J Clin Invest. 2021;51:e13605.
An Y, Gao S, Zhao W-C, Qiu B-A, Xia N-X, Zhang P-J, et al. Novel serum microRNAs panel on the diagnostic and prognostic implications of hepatocellular carcinoma. World J Gastroenterol. 2018;24:2596–2604.
Wu G-G, Li W-H, He W-G, Jiang N, Zhang G-X, Chen W, et al. Mir-184 post-transcriptionally regulates SOX7 expression and promotes cell proliferation in human hepatocellular carcinoma. PLoS ONE. 2014;9:e88796.
Gao B, Gao K, Li L, Huang Z, Lin L. miR-184 functions as an oncogenic regulator in hepatocellular carcinoma (HCC). Biomed Pharmacother. 2014;68:143–8.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92.
Shiiba M, Uzawa K, Tanzawa H. MicroRNAs in head and neck squamous cell carcinoma (HNSCC) and oral squamous cell carcinoma (OSCC). Cancers (Basel). 2010;2:653–69.
Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–92.
Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. 2017;4:25–43.
Lei Y, Junxin C, Yongcan H, Xiaoguang L, Binsheng Y. Role of microRNAs in the crosstalk between osteosarcoma cells and the tumour microenvironment. J Bone Oncol. 2020;25:100322.
Farzaneh M, Najafi S, Anbiyaee O, Azizidoost S, Khoshnam SE. LncRNA MALAT1-related signaling pathways in osteosarcoma. Clin Transl Oncol. 2023;25:21–32.
Yin G, Wang Q, Zhang X, Wang S. Regulatory role of microRNA184 in osteosarcoma cells. Genet Mol Res. 2015;14:14246–52.
Lin BC, Huang D, Yu CQ, Mou Y, Liu YH, Zhang DW, et al. MicroRNA-184 modulates doxorubicin resistance in osteosarcoma cells by targeting BCL2L1. Med Sci Monit. 2016;22:1761–5.
Rubin EM, Guo Y, Tu K, Xie J, Zi X, Hoang BH. Wnt inhibitory factor 1 decreases tumorigenesis and metastasis in osteosarcomawif-1 modulates tumor progression in osteosarcoma. Mol Cancer Ther. 2010;9:731–41.
An JH, Yang J-Y, Ahn BY, Cho SW, Jung JY, Cho HY, et al. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010;47:140–50.
Tao P, Feng J, Li Q, Liu W, Yang L, Zhao X, et al. Expression of miR-664 and miR-184 on proliferation, apoptosis and migration of osteosarcoma cells. Oncol Lett. 2019;17:1791–7.
Fang W, Li X, Jiang Q, Liu Z, Yang H, Wang S, et al. Transcriptional patterns, biomarkers and pathways characterizing nasopharyngeal carcinoma of Southern China. J Transl Med. 2008;6:1–13.
Wang S, Claret F-X, Wu W. MicroRNAs as therapeutic targets in nasopharyngeal carcinoma. Front Oncol. 2019;9:756.
Wang Q, Yang HS. The role of Pdcd4 in tumour suppression and protein translation. Biol Cell. 2018;110:169–177.
Zhen Y, Liu Z, Yang H, Yu X, Wu Q, Hua S, et al. Tumor suppressor PDCD4 modulates miR-184-mediated direct suppression of C-MYC and BCL2 blocking cell growth and survival in nasopharyngeal carcinoma. Cell Death Dis. 2013;4:e872-e.
Zhu HM, Jiang XS, Li HZ, Qian LX, Du MY, Lu ZW, et al. miR-184 inhibits tumor invasion, migration and metastasis in nasopharyngeal carcinoma by targeting Notch2. Cell Physiol Biochem. 2018;49:1564–76.
Lai X, Li Q, Wu F, Lin J, Chen J, Zheng H, et al. Epithelial-mesenchymal transition and metabolic switching in cancer: lessons from somatic cell reprogramming. Front Cell Dev Biol. 2020;8:760.
Dahiya N, Morin PJ. MicroRNAs in ovarian carcinomas. Endocr Relat Cancer. 2010;17:F77–F89.
Qin C-Z, Lou X-Y, Lv Q-L, Cheng L, Wu N-Y, Hu L, et al. MicroRNA-184 acts as a potential diagnostic and prognostic marker in epithelial ovarian cancer and regulates cell proliferation, apoptosis and inflammation. Pharmazie 2015;70:668–73.
Liu W, Ma C, Xu H, Wang L, Xu W, Zhang H, et al. miR-184-5p inhibits cell proliferation, invasion and predicts prognosis of clear cell renal cell carcinoma by targeting NUS1 dehydrodolichyl diphosphate synthase subunit: Results from large-scale comprehensive identification and validation. J Cancer. 2022;13:1398–409.
Greenberg E, Hershkovitz L, Itzhaki O, Hajdu S, Nemlich Y, Ortenberg R, et al. Regulation of cancer aggressive features in melanoma cells by microRNAs. PLoS ONE. 2011;6:e18936.
Liang X-g, Meng W-t, Hu L-j, Li L, Xing H, Xie G, et al. MicroRNA-184 modulates human central nervous system lymphoma cells growth and invasion by targeting iASPP. J Cell Biochem. 2017;118:2645–53.
Ahangar Davoodi N, Najafi S, Naderi Ghale-Noie Z, Piranviseh A, Mollazadeh S, Ahmadi Asouri S, et al. Role of non-coding RNAs and exosomal non-coding RNAs in retinoblastoma progression. Front Cell Dev Biol. 2022;10:1065837.
He TG, Xiao ZY, Xing YQ, Yang HJ, Qiu H, Chen JB. Tumor suppressor miR-184 enhances chemosensitivity by directly inhibiting SLC7A5 in retinoblastoma. Front Oncol. 2019;9:1163.
Biomarkers Definitions Working Group, Atkinson AJ Jr, Colburn WA, DeGruttola VG, DeMets DL, Downing GJ, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95.
Califf RM. Biomarker definitions and their applications. Exp Biol Med. 2018;243:213–21.
Rahmati Y, Asemani Y, Aghamiri S, Ezzatifar F, Najafi S. CiRS-7/CDR1as; an oncogenic circular RNA as a potential cancer biomarker. Pathol Res Pract. 2021;227:153639.
Sawyers CL. The cancer biomarker problem. Nature. 2008;452:548–52.
Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–9.
Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104.
Iaccarino I, Klapper W. LncRNA as cancer biomarkers. Methods Mol Biol. 2021;2348:27–41.
Sayad A, Najafi S, Hussen BM, Jamali E, Taheri M, Ghafouri-Fard S. The role of circular RNAs in pancreatic cancer: new players in tumorigenesis and potential biomarkers. Pathol Res Pract. 2022;232:153833.
Ghafouri-Fard S, Najafi S, Hussen BM, Basiri A, Hidayat HJ, Taheri M, et al. The role of circular rnas in the carcinogenesis of bladder cancer. Front Oncol. 2022;12:801842.
Shirvani H, Ghanavi J, Aliabadi A, Mousavinasab F, Talebi M, Majidpoor J, et al. MiR-211 plays a dual role in cancer development: from tumor suppressor to tumor enhancer. Cell Signal. 2023;101:110504.
Asemani Y, Najafi S, Ezzatifar F, Zolbanin NM, Jafari R. Recent highlights in the immunomodulatory aspects of Treg cell-derived extracellular vesicles: special emphasis on autoimmune diseases and transplantation. Cell Biosci. 2022;12:67.
Najafi S, Majidpoor J, Mortezaee K. Extracellular vesicle–based drug delivery in cancer immunotherapy. Drug Deliv Transl Res. 2023;13:2790–806.
Su Z, Chen D, Li Y, Zhang E, Yu Z, Chen T, et al. microRNA-184 functions as tumor suppressor in renal cell carcinoma. Exp Ther Med. 2015;9:961–6.
Li S, Li H, Ge W, Song K, Yuan C, Yin R. Effect of miR-184 on proliferation and apoptosis of pancreatic ductal adenocarcinoma and its mechanism. Technol Cancer Res Treat. 2020;19:1533033820943237.
Author information
Authors and Affiliations
Contributions
SN conceptualized and supervised the study, all authors contributed to collection of the information and composing the primary draft, FZR illustrated the figures, and SN revised and finalized the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fattahi, M., Rezaee, D., Fakhari, F. et al. microRNA-184 in the landscape of human malignancies: a review to roles and clinical significance. Cell Death Discov. 9, 423 (2023). https://doi.org/10.1038/s41420-023-01718-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41420-023-01718-1
This article is cited by
-
Redox proteomics of PANC-1 cells reveals the significance of HIF-1 signaling protein oxidation in pancreatic ductal adenocarcinoma pathogenesis
Journal of Translational Medicine (2024)
-
Circulating vitamin D level before initiating chemotherapy impacts on the time-to-outcome in metastatic colorectal cancer patients: systematic review and meta-analysis
Journal of Translational Medicine (2024)